Professional Documents
Culture Documents
Mertens 2003 PDF
Mertens 2003 PDF
Uploaded by
Edgard MalaguezCopyright:
Available Formats
You might also like
- Articulo Ref-AOAC 2002.04Document24 pagesArticulo Ref-AOAC 2002.04ESTEBAN RESTREPONo ratings yet
- DRIS Concepts and Applications On Nutritional Diagnosis in Fruit CropsDocument11 pagesDRIS Concepts and Applications On Nutritional Diagnosis in Fruit CropsRómulo Del ValleNo ratings yet
- 3.protein QuantificationDocument12 pages3.protein Quantificationmc4207901No ratings yet
- Food Chemistry: Susanne Westenbrink, Kommer Brunt, Jan-Willem Van Der KampDocument6 pagesFood Chemistry: Susanne Westenbrink, Kommer Brunt, Jan-Willem Van Der KampChintong LylayNo ratings yet
- Revista Brasileira de Zootecnia: Mary Beth HallDocument9 pagesRevista Brasileira de Zootecnia: Mary Beth HallAdna AlineNo ratings yet
- Gayoso 2016Document11 pagesGayoso 2016marcela.gonzalezNo ratings yet
- 83a DeterminationofTotalDietaryFiberCODEXDefinitionbyEnzymatic-GravimetricMethodandLiquidChromatography CollaborativeStudyDocument14 pages83a DeterminationofTotalDietaryFiberCODEXDefinitionbyEnzymatic-GravimetricMethodandLiquidChromatography CollaborativeStudyValeriu MunteanuNo ratings yet
- J. Nutr.-2013-Schoenaker-392-8Document7 pagesJ. Nutr.-2013-Schoenaker-392-8Fadilla NurrahmaNo ratings yet
- Determination of Insoluble Soluble and T PDFDocument13 pagesDetermination of Insoluble Soluble and T PDFJoanuel QuinteroNo ratings yet
- A Note On The Evaluation of The Acid-Insoluble Ash Technique As A Method For Determining Apparent Diet Digestibility in Beef CattleDocument6 pagesA Note On The Evaluation of The Acid-Insoluble Ash Technique As A Method For Determining Apparent Diet Digestibility in Beef CattleAJ ManurungNo ratings yet
- PdcaasDocument8 pagesPdcaasTan Suk WanNo ratings yet
- Desjardins 2018Document11 pagesDesjardins 2018pseptinaNo ratings yet
- 2017 Consumo de FibrasDocument8 pages2017 Consumo de FibrasMariNo ratings yet
- Biochemical and Nutritional Analysis of The Leaf Extract of (L.) CorreaDocument5 pagesBiochemical and Nutritional Analysis of The Leaf Extract of (L.) Correarihana yadavNo ratings yet
- Studies Assessing Food Consumption by The Scores Method: A Systematic ReviewDocument16 pagesStudies Assessing Food Consumption by The Scores Method: A Systematic ReviewyutefupNo ratings yet
- Nut Esportiva 1 4Document29 pagesNut Esportiva 1 4Anderson AlvesNo ratings yet
- 2022 - Ibañez - Instrumental and Sensory Techniques To Characterize The Texture of FoodsDocument34 pages2022 - Ibañez - Instrumental and Sensory Techniques To Characterize The Texture of FoodsJuan P. CortésNo ratings yet
- Mccleary 2012Document21 pagesMccleary 2012frendystpNo ratings yet
- Feed EvaluationDocument17 pagesFeed EvaluationdagolddoyinsolaNo ratings yet
- Nutritional Metabolomics and The Classification of Dietary Biomarker Candidates: A Critical ReviewDocument25 pagesNutritional Metabolomics and The Classification of Dietary Biomarker Candidates: A Critical ReviewRum Afida RasfaNo ratings yet
- Ajcn 150847Document2 pagesAjcn 150847Betelhiem WoldemedhinNo ratings yet
- N. Thiex and C. R. Richardson: Challenges in Measuring Moisture Content of FeedsDocument14 pagesN. Thiex and C. R. Richardson: Challenges in Measuring Moisture Content of FeedsvratonNo ratings yet
- Van Soest 1979. Systems Analysis Evaluating Fibrous FeedDocument16 pagesVan Soest 1979. Systems Analysis Evaluating Fibrous FeedEugenia Campon100% (1)
- Calderon 2009Document7 pagesCalderon 2009Vero CastroNo ratings yet
- Dietary Assessment Toolkits An OverviewDocument16 pagesDietary Assessment Toolkits An OverviewKeneniNo ratings yet
- 2014 Fredin Et Al Fecal Starch Indicator Total Tract DigestibilityDocument11 pages2014 Fredin Et Al Fecal Starch Indicator Total Tract DigestibilityISAAC TORRES VELASCONo ratings yet
- 82 FullDocument17 pages82 FullBetelhiem WoldemedhinNo ratings yet
- Metodos de SeparacionDocument12 pagesMetodos de Separaciondan martinezNo ratings yet
- Ajcn/47 3 440Document8 pagesAjcn/47 3 440Hoàng Thị Thanh VânNo ratings yet
- Dietary FiberDocument5 pagesDietary FiberdeisifvNo ratings yet
- STROBE-nut Checklist TableDocument8 pagesSTROBE-nut Checklist TableChi MichNo ratings yet
- Firkins 1998Document20 pagesFirkins 1998YUSUF ADENIJINo ratings yet
- 15 3 16 - p.325 331 PDFDocument7 pages15 3 16 - p.325 331 PDFbeatcookNo ratings yet
- Aing93m Soybean OilDocument13 pagesAing93m Soybean OilMellysaNo ratings yet
- Accuracy of The Atwater Factors and Related Food Energy Conversion Factors With Low-Fat, High-Fiber Diets When Energy Intake Is Reduced SpontaneouslyDocument8 pagesAccuracy of The Atwater Factors and Related Food Energy Conversion Factors With Low-Fat, High-Fiber Diets When Energy Intake Is Reduced SpontaneouslyNatalia GutierrezNo ratings yet
- Laporan PBLDocument12 pagesLaporan PBLLuna MiraNo ratings yet
- Methods of Dietary and Nutritional Assessment and Intervention and Other Methods in The Multiple Risk Factor Intervention Trial1'2Document15 pagesMethods of Dietary and Nutritional Assessment and Intervention and Other Methods in The Multiple Risk Factor Intervention Trial1'2Daniel YirdawNo ratings yet
- AbsorcionDocument79 pagesAbsorcionDan Martínez SilvaNo ratings yet
- Spirulina ObesidadeDocument10 pagesSpirulina ObesidadeIsabela CastroNo ratings yet
- 1 s2.0 S2666833524000868 MainDocument11 pages1 s2.0 S2666833524000868 Maincristian.oglindaNo ratings yet
- 1 s2.0 S2001037014600477 MainDocument7 pages1 s2.0 S2001037014600477 MainBetelhiem WoldemedhinNo ratings yet
- Cost of The Diet A Method and Software To CalculatDocument18 pagesCost of The Diet A Method and Software To CalculatAbhi RajputNo ratings yet
- Article 5 - Dietary Assessment in Digital Age - McCullough M - AJCN 2018Document2 pagesArticle 5 - Dietary Assessment in Digital Age - McCullough M - AJCN 2018Prajna AdityaraniNo ratings yet
- Muscle DysmorphiaDocument6 pagesMuscle DysmorphiaKarla RojasNo ratings yet
- TehranDocument6 pagesTehranNadiah Umniati SyarifahNo ratings yet
- (Artigo) Digestibilidade ProteinaDocument7 pages(Artigo) Digestibilidade ProteinaKennedy LadeiaNo ratings yet
- Data in BriefDocument8 pagesData in BrieffadhilNo ratings yet
- Guía de Manejo Nutricional para La Enfermedad de La Orina Con Jarabe de Arce Un Enfoque Basado en La Evidencia y El ConsensoDocument8 pagesGuía de Manejo Nutricional para La Enfermedad de La Orina Con Jarabe de Arce Un Enfoque Basado en La Evidencia y El ConsensoPablo MorenoNo ratings yet
- International Society of Sports Nutrition Position Stand Diets and Body CompositionDocument19 pagesInternational Society of Sports Nutrition Position Stand Diets and Body CompositionLuis Ricardo Hernandez GarciaNo ratings yet
- Diabetes & Metabolic Syndrome: Clinical Research & ReviewsDocument7 pagesDiabetes & Metabolic Syndrome: Clinical Research & ReviewsTasha FarahNo ratings yet
- Original CommunicationDocument8 pagesOriginal CommunicationMohammedNo ratings yet
- Mango NectarDocument10 pagesMango NectarHoài Thương Nghiêm ThịNo ratings yet
- 2017 - Development and Validation of A Near Infrered Spectro Method To Determine Total Antioxidant in MilkDocument6 pages2017 - Development and Validation of A Near Infrered Spectro Method To Determine Total Antioxidant in MilklucianamartinezluqueNo ratings yet
- Biomarkers of Berry Intake: Systematic Review Update: AccessDocument17 pagesBiomarkers of Berry Intake: Systematic Review Update: AccessWILBERTH YOHEL PINEDO CALDASNo ratings yet
- IJFANS - Research Paper - RGST ProjectDocument8 pagesIJFANS - Research Paper - RGST ProjectDr. Neha Lohia SainiNo ratings yet
- Dietary Protein Quality Evaluation in Human NutritionFrom EverandDietary Protein Quality Evaluation in Human NutritionNo ratings yet
- Determination of Diphenylamine Residue in Fruit Samples Using Spectrofluorimetry and Multivariate AnalysisDocument7 pagesDetermination of Diphenylamine Residue in Fruit Samples Using Spectrofluorimetry and Multivariate AnalysisRodrigoNo ratings yet
- Journal of Fish Biology - 2019 - Amundsen - Feeding Studies Take Guts Critical Review and Recommendations of Methods ForDocument10 pagesJournal of Fish Biology - 2019 - Amundsen - Feeding Studies Take Guts Critical Review and Recommendations of Methods ForNiken Aura Dina SilangenNo ratings yet
- Iodine ICPMSDocument8 pagesIodine ICPMSYap Poh SiewNo ratings yet
- Inadequacy Food and SupplementsDocument7 pagesInadequacy Food and Supplementsgianluca.franceschini38No ratings yet
- Table Grape (Wiki 11 - 2 - 2020)Document4 pagesTable Grape (Wiki 11 - 2 - 2020)andi2rNo ratings yet
- Past Simple, Continuous, Present Perfect 13.12.23Document12 pagesPast Simple, Continuous, Present Perfect 13.12.23crowofdark455No ratings yet
- English Structure Basic PDFDocument120 pagesEnglish Structure Basic PDFdiyah fitri wulandariNo ratings yet
- FSSC 22000 v6 (FSMS) A-LA VILT TC - LG 17-08-2023Document373 pagesFSSC 22000 v6 (FSMS) A-LA VILT TC - LG 17-08-2023mohamed adel100% (1)
- Prof Ingles Ingreso2020 Introduccion Lengua InglesaDocument80 pagesProf Ingles Ingreso2020 Introduccion Lengua InglesaSalvador AcuñaNo ratings yet
- Oktoberfest Meeting Notes - July 28, 2010Document5 pagesOktoberfest Meeting Notes - July 28, 2010jberesNo ratings yet
- Used To/ Get Used To: Choose The Best Answer To Complete These Following SentencesDocument3 pagesUsed To/ Get Used To: Choose The Best Answer To Complete These Following SentencesJhon KeaNo ratings yet
- Punctuations QP 2Document3 pagesPunctuations QP 2Siti Nurain Mohd GhazaliNo ratings yet
- English 1 - Meeting 3 - Structure Present TenseDocument9 pagesEnglish 1 - Meeting 3 - Structure Present TenseRio HansNo ratings yet
- Ria Sweatha of Curry Leaf Aparna's BlogDocument36 pagesRia Sweatha of Curry Leaf Aparna's BlogcruciatusNo ratings yet
- Mueed Babar - ArticleDocument2 pagesMueed Babar - ArticleJunaid MalikNo ratings yet
- Project in EnglishDocument18 pagesProject in EnglishCrisanta BorbeNo ratings yet
- Chapter 1 and 3 Talabun Ak BriquettesDocument32 pagesChapter 1 and 3 Talabun Ak BriquettesROSELLE SIRUENo ratings yet
- Practice Book AnswersDocument6 pagesPractice Book Answersmohd razif ibrahimNo ratings yet
- New Magic Grammar 2BDocument34 pagesNew Magic Grammar 2BYume ZhuNo ratings yet
- GoutDocument16 pagesGoutfzee13No ratings yet
- Fish Nutrition PPT Title Defense 1Document17 pagesFish Nutrition PPT Title Defense 1Cristel Mae De GuzmanNo ratings yet
- The Typical Dishes From TarijaDocument4 pagesThe Typical Dishes From TarijaAlejandro RomeroNo ratings yet
- Consumer Affairs and Customer Care-1Document47 pagesConsumer Affairs and Customer Care-1Aditya PrakashNo ratings yet
- Spring Creek Sun December 3 2021Document24 pagesSpring Creek Sun December 3 2021amoses88No ratings yet
- Design and Development of Fresh Soybean Grinding and Filtration MachineDocument8 pagesDesign and Development of Fresh Soybean Grinding and Filtration Machinejenixson tamondongNo ratings yet
- Vanstiphout 1992 The Banquet Scene in The Mesopotamian Debate Poems ResOr 4Document8 pagesVanstiphout 1992 The Banquet Scene in The Mesopotamian Debate Poems ResOr 4Deniz ılgazNo ratings yet
- Borkar Super StoreDocument7 pagesBorkar Super StoreAshutosh SharmaNo ratings yet
- DW7 FoodDocument8 pagesDW7 FoodAlexander PeraltaNo ratings yet
- List Bolde Februari 2022Document6 pagesList Bolde Februari 2022Richard LiongNo ratings yet
- Ratios & ProportionsDocument11 pagesRatios & Proportionsmahesh.aptitudeNo ratings yet
- PEEEEDocument4 pagesPEEEEHaziel Joy GallegoNo ratings yet
- Đề 1Document5 pagesĐề 1PlumCatNo ratings yet
- Customer Behavior and Customer TrendDocument2 pagesCustomer Behavior and Customer TrendÁnh NgọcNo ratings yet
- Module 1 - CH 7 Gluten & MillingDocument25 pagesModule 1 - CH 7 Gluten & Millingaadityachandra1987No ratings yet
Mertens 2003 PDF
Mertens 2003 PDF
Uploaded by
Edgard MalaguezOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mertens 2003 PDF
Mertens 2003 PDF
Uploaded by
Edgard MalaguezCopyright:
Available Formats
Challenges in measuring insoluble dietary fiber
D. R. Mertens
J Anim Sci 2003. 81:3233-3249.
The online version of this article, along with updated information and services, is located on
the World Wide Web at:
http://jas.fass.org/cgi/content/full/81/12/3233
www.asas.org
Downloaded from jas.fass.org by on January 3, 2011.
Challenges in measuring insoluble dietary fiber
D. R. Mertens1
USDA, ARS, U.S. Dairy Forage Research Center, Madison, WI 53706-1108
ABSTRACT: Objectives of this review are to define amylase-treated neutral detergent fiber (aNDF) and
criteria for evaluating insoluble dietary fiber (IDF) enzymatic-gravimetric methods, are relevant for mea-
methods, discuss their relevance in meeting the nutri- suring IDF. In a collaborative study, aNDF obtained
tional needs of ruminants and herbivores, describe a standard deviation of reproducibility (SDR) of 1.3%.
problems with empirical IDF methods, and assess their Enzymatic-gravimetric methods of measuring IDF
relative merits. The challenge for the researcher, nutri- have been evaluated using too few feed materials to
tionist, and analyst is to select fiber methods that are make statistically valid conclusions, but the SDR of
relevant and reproducible. Without relevance, there is IDF, for the few feeds evaluated, were similar to aNDF
no reason to measure IDF, and without reproducibility, (0.9 to 2.4%). The enzymatic-chemical method of mea-
there is no value in doing so. Insoluble dietary fiber is suring IDF as the sum of insoluble nonstarch polysac-
a complex matrix of chemical components, and there charides and lignin agrees with NDF, but the SDR of
neutral sugar analysis using acid hydrolysis and chro-
are no primary standards that can be used to establish
matography is greater (3.2%) than other dietary fiber
the validity of methods. Thus, the definition of fiber is
methods. Empirical methods—such as those used to
crucial in determining method relevance. For rumi-
measure IDF, although based on nutritional concepts—
nants and nonruminant herbivores, the appropriate actually define the fraction being measured and must
physiological definition for selecting IDF methods may be followed exactly, without modification. The selection
be as follows: the organic fraction of the diet that is of a suitable method for IDF depends on the purpose
indigestible or slowly digesting and occupies space in of analysis. Analysis of sugars in insoluble polysaccha-
the gastrointestinal tract. Crude fiber does not match rides provides more information but is less reproducible
this definition, and its use should be abandoned. Acid and more expensive to obtain. For routine nutritive
detergent fiber does not measure all IDF but is useful evaluation of feeds and formulation of rations, aNDF
when included with other dietary fiber methods to de- seems to be a reasonable choice for measuring IDF
scribe some feeds. Several current methods, including based on relevance and reproducibility.
Key Words: Analytical Methods, Detergent Fiber, Dietary Fiber, Fiber Analysis, Repeatability, Reproducibility
2003 American Society of Animal Science. All rights reserved. J. Anim. Sci. 2003. 81:3233–3249
Introduction without reproducibility among laboratories, there is no
value in reporting dietary fiber results. To be relevant,
Fiber concentration has been a useful measure for IDF methods must provide useful nutritional informa-
describing feeds and estimating energy value for nearly tion and must have utility as either a quantitative de-
150 yr (Dougall, 1956; Sullivan, 1964; Van Soest, 1964; scription of a feed or a means of evaluating feeds or
Tyler, 1975). Hipsley (1953) was the first to use the formulating rations. Reproducibility of IDF methods is
term dietary fiber to describe a nutritional property of crucial to have confidence that results are accurate and
diets. This generic term will be used to indicate the comparable among research, regulatory, and feed-test-
general concept of fiber as it relates to nutrition. The ing laboratories.
main challenges facing insoluble dietary fiber (IDF) Dietary fiber is unique among feed constituents be-
methods are relevance and reproducibility. Without rel- cause it is defined only on a nutritional basis (that is,
evance, there is no reason for measuring dietary fiber; in terms of the digestive and physiological effects that
it elicits) but must be measured chemically. Thus, the
nutritional definition for dietary fiber is key to method
1
relevance. The usefulness of dietary fiber results vary
Correspondence: 1925 Linden Drive, West (phone: 608-264-5228;
fax: 608-264-5147; E-mail: davem@dfrc.wisc.edu).
from its value as an indicator of physiological health
Received December 5, 2002. benefits to its value as a predictor of digestibility and
Accepted September 2, 2003. energy value of feeds. Furthermore, the relevance of
3233
Downloaded from jas.fass.org by on January 3, 2011.
3234 Mertens
dietary fiber data may be different between research was 985.29—Total dietary fiber in food, enzymatic-
and practical use, and vary within each use. gravimetric method, which did not allow separation of
Numerous methods have been proposed for measur- dietary fiber into soluble and insoluble fractions. Insolu-
ing dietary fiber, and some have become routine analy- ble dietary fiber can be determined using AOAC Official
ses for research and practical use. The scope of this Method 991.42—Insoluble dietary fiber in foods and
review will be limited to the official methods of fiber food products, enzymatic-gravimetric method (phos-
analysis as described by the Association of Official Ana- phate buffer) and SDF by Method 993.16—Soluble di-
lytical Chemists (AOAC) International. These methods etary fiber in food and food products, enzymatic-gravi-
can be used in situations in which accuracy and preci- metric method (phosphate buffer). These methods for
sion are required and often are the ones routinely used measuring TDF, IDF, and SDF have been superseded
in research and practical applications to describe feed by Official Method 991.43—Total, soluble, and insolu-
characteristics. Objectives of this review are to define ble dietary fiber in foods, enzymatic-gravimetric
the criteria needed to evaluate IDF methods, discuss method (MES-Tris buffer). Official Method 992.16—To-
the relevance of each method in meeting nutritional tal dietary fiber, enzymatic-gravimetric method, uses
needs, describe analytical problems in reproducing em- neutral detergent extraction with amylase treatment
pirical dietary fiber results, and assess the relative mer- and measurement of SDF to determine TDF. More de-
its of IDF methods. tailed analysis of TDF can be determined using Official
Method 994.13—Total dietary fiber (determined as neu-
Methods tral sugar residues, uronic acid residues, and Klason
lignin), gas chromatographic, colorimetric, gravimetric
Throughout this discussion, the term materials will method, which is based on acid hydrolysis and chro-
be used to describe individual feeds or lots of feed, sam- matographic analysis of sugar residues.
ple will be defined as the portion of a material that is
prepared for analysis, and test sample will be used to Collaborative Studies
describe the part of the sample that is actually an-
alyzed. One of the primary purposes of the AOAC is to spon-
sor collaborative studies for evaluating analytical meth-
AOAC Official Methods for Dietary Fiber ods under actual laboratory conditions with a diversity
of materials, personnel, environments, equipment, and
With a few exceptions, dietary fiber is determined so on (AOAC, 1993). Under these conditions, the total
gravimetrically as the difference in weights of a test precision of a method (reproducibility) can be deter-
sample before and after extraction in a solution(s). mined (Steiner, 1975), which the AOAC uses to make
There are two AOAC official methods for crude fiber an informed decision about the acceptability of the
(CF) in animal feeds: 962.09—Crude fiber in animal method as official. The total precision of an analytical
feed and pet foods, ceramic fiber filter method, or result is the sum of variability among laboratories and
978.10—Crude fiber in animal feed and pet foods, frit- within laboratories. Reproducibility of a method is de-
ted glass crucible method (AOAC, 2002). In the most fined as the variation among single results for the same
recent versions of Method 962.09, the precoating of the material when determined by different laboratories
Oklahoma filter screen or California Buchner funnel (different analyst, apparatus, environment, time, etc.).
with ceramic fiber when analyzing extremely fine sam- Repeatability of a method is defined as the variation
ples was clarified. Acid detergent fiber and acid deter- among results for the same material determined in sim-
gent lignin using sulfuric acid (ADSL) can be deter- ilar conditions within a laboratory (typically successive
mined using AOAC Official Method 973.18—Fiber (acid analyses within the same run: same analyst, apparatus,
detergent) and lignin in animal feed. Several clarifica- reagents, etc.).
tions have been included in the more recent version To assess the reproducibility and repeatability of a
of Method 973.18, such as 1) description for cleaning method requires replicated analyses of multiple materi-
crucibles, 2) specification of particle size for preparing als within multiple laboratories. Youden (1975) sug-
samples, 3) preextraction of test samples containing gested that the absolute minimum design for a collabo-
>10% fat with acetone or similar solvent, 4) time of rative study would be five laboratories analyzing three
soaking of residues after acid detergent extraction to pairs of materials (low, medium, and high concentra-
remove acid, and 5) addition of formula to report results tions of the analyte). He also suggested that matched
on a as-is or as-received basis (AOAC, 2002). Amylase- pairs of materials (Youden pairs) provide more statisti-
treated neutral detergent fiber (aNDF) can be mea- cal information than blind duplicates for the same num-
sured by AOAC Official Method 2002.04—Amylase- ber of analyses. The minimum design provides 30 obser-
treated neutral detergent fiber in feeds using refluxing vations, which is the minimum number needed to ob-
in beakers or crucibles. tain an acceptable estimate of standard errors
There are several AOAC official methods for measur- (Wernimont and Spendley, 1985). Typically, the AOAC
ing total dietary fiber (TDF), IDF, and soluble dietary requests at least eight laboratories and five materials
fiber (SDF). The first AOAC official method for TDF (duplicate analyses) for most collaborative studies
Downloaded from jas.fass.org by on January 3, 2011.
Measuring insoluble dietary fiber 3235
(AOAC, 1993). The materials should represent the full tive standard deviation of reproducibility (RSDR = SDR/
range of composition matrices to which the method will mean) was related to the concentration of the analyte
be applied. A study with more than one material would across a wide variety of methods and generated a for-
result in the following statistical model to obtain esti- mula for the Horwitz relative standard deviation of
mates of variation pooled across materials (Wernimont reproducibility (HRSDR):
and Spendley, 1985):
HRSDR (% of the mean) = 2e(1−0.5ⴢlog10C)
Yn = + Li + Mij + Li ⴢMij + rijk
where C is the fractional concentration of the analyte.
where Thompson and Lowthian (1997) confirmed that the
HRSDR provides an expected standard deviation of re-
Yn = result for a single analysis of the nth material, producibility for any method, which varies from 4% of
= mean observation, the mean at 1% concentration to 2% of the mean at >90%
Li = ith laboratory, concentration. The HRSDR indicates that the expected
Mij = jth material analyzed in the ith laboratory, standard deviation of most feed analysis methods,
LiⴢMij = ith laboratory by jth material interaction, and which have means of 10 to 60% of DM, would be 2 to 3%
rijk = kth replicate of the jth material within the ith of the mean. Dividing the RSDR observed for a specific
laboratory. method by the HRSDR generates the Horwitz ratio
(HORRAT), which permits the comparison of reproduc-
An acceptable method must have a nonsignificant LiⴢMij ibility among diverse methods (Horwitz et al., 1990). A
interaction and variations within and among labora- HORRAT of 1.0 or less indicates that a method has
tories that are small relative to the variation among reproducibility similar to other methods approved by
materials. Assuming the sources of variation are inde- AOAC. With some exceptions, a HORRAT of >2.0 sug-
pendent, the variances can be partitioned so that the gests that a method is unacceptable with respect to the
variation for reproducibility of the method can be cal- reproducibility of other official methods.
culated:
Proficiency Testing of Laboratories
σR2 = σL2 + σr2
The ongoing validity of each individual laboratory’s
where ability to generate reliable results is accomplished by
proficiency testing or performance check programs. In
σR2 = variance of reproducibility of an individual mea- these programs, carefully prepared, homogeneous sam-
surement from any laboratory, ples are analyzed by participating laboratories to com-
σL2 = variance among laboratories, and pare results. The Association of American Feed Quality
σr2 = variance of replicated analysis within labora- Control Officials operates a check sample program in
tories and the standard deviation of reproducibil- which results of participating laboratories are summa-
ity (SDR) and of repeatability (SDr) are the rized and reported back to the participants for use in
square roots of the respective variances. monitoring their results. The National Forage Testing
Association (NFTA) was established under the aus-
This model applies to study designs in which replicates pices of the American Forage and Grassland Council,
are run successively or within the same run or day. the National Hay Association, and commercial forage
Because replicates are analyzed successively, this analysis laboratories to certify the proficiency of partici-
model assumes that repeatability within a laboratory pating laboratories. The results of each laboratory are
is primarily a function of random variation among repli- compared to a consensus reference value for each mate-
cate test samples and that techniques, equipment, re- rial and, if they fall within a specified range, the profi-
agents, and so on, within laboratories do not vary ciency of the laboratory is certified by the NFTA (Mer-
among runs or days. tens, 1998b). Results for other analyses are monitored
However, it is reasonable to assume that within-labo- by NFTA, but only DM, CP, ADF, and aNDF are used
ratory repeatability has two sources of variation: repli- for certification of proficiency.
cation within a run in which conditions are relatively Ideally, materials used for proficiency testing would
uniform with respect to environments, apparatus, re- have a known composition for each analyte. However,
agents, and so on, and variation among runs, which there are no primary standards for dietary fiber or dry
is associated with uncontrollable laboratory conditions matter, and it is necessary to establish reference values
over longer periods of time. These sources of repeatabil- for each material used in a proficiency-testing program.
ity within laboratories can be assessed by designing The NFTA program allows each laboratory to select
a collaborative study in which laboratories replicate the method used to measure each of the analytes, and
analyses in different days or runs. requires only that laboratories analyze the proficiency-
Horwitz (1982) compared the results of numerous testing samples with the same method they use in rou-
AOAC collaborative studies and observed that the rela- tine practice. The results of each laboratory are com-
Downloaded from jas.fass.org by on January 3, 2011.
3236 Mertens
pared to a consensus value, the reference method aver- of dietary fiber. In the most general terms, dietary fiber
age (RMA) for each analyte (Mertens et al., 1994). The is the coarse-textured portion of edible materials that
reference method for each analyte is either an AOAC is difficult to digest and adds bulk to digesta and feces.
official method or a method accepted by the NFTA (Un- Mertens (1985) proposed that dietary fiber for herbi-
dersander et al., 1993). The results used to calculate the vores be defined as the “indigestible or slowly digesting
RMA are selected based on each laboratory’s answers to portion of feeds that occupies space in the gastrointesti-
a questionnaire about the specific details of their rou- nal tract.” Perhaps to distinguish dietary fiber from
tine methods to determine whether they followed the indigestible ash, this definition should be modified to
reference method. Results of laboratories using refer- include only “indigestible or slowly digesting organic
ence methods are censured by selecting only those matter of feeds that occupies space in the gastrointesti-
within one standard deviation of the median; that is, nal tract.” These definitions of dietary fiber exclude
results are ranked and the top and bottom 15.8% are rapidly fermenting polysaccharides of plant cell walls
discarded. Typically, 10 to 30 laboratory results are (such as pectin) and soluble polysaccharides that do not
used to generate the RMA. Censuring ensures that occupy space in a liquid environment (such as fructans
anomalous results are not used to determine the RMA and gums), but would include slowly fermented, com-
to which all laboratories are compared for certification plex polysaccharides that are digested by fermentation
of proficiency. Six materials are analyzed each year, and in the alimentary tract of herbivores (such as cellulose
laboratories are certified as proficient if their results fall and hemicellulose). Essentially, this more restricted
within ±3ⴢHRSDR of the RMA for CP, ADF, and aNDF definition of dietary fiber describes IDF, which is the
and within a modified HRSD for DM. feed component that is variable in digestibility and af-
fects the total DM or OM digestibility of feeds or diets
Discussion by ruminants. It excludes the rapidly fermentable SDF
because they have true digestibilities similar to plant
Relevance of Dietary Fiber Methods cell contents. Although SDF may alter ruminal fermen-
tation, its effect on the health and performance of rumi-
Dietary fiber is a nutritional entity that can be truly nants are unknown. Insoluble dietary fiber affects the
measured only by the digestive process of the animal. digestibility and passage rate of feeds and diets in all
In the laboratory, chemical or enzymatic methods are animals. Due to their high intakes of dietary fiber, the
devised to measure dietary fiber, but accuracy and rele- space-occupying characteristics of IDF and its require-
vance of a method is based on how well the analytically ment for chewing to reduce particle size for passage
measured fiber matches its nutritional definition. Thus, through the alimentary tract may be factors that make
the development of fiber methods must be based on an IDF more important to herbivorous animals than SDF.
acceptable definition of fiber. The concept of dietary For a dietary fiber method to be practicable, it must
fiber for humans was developed initially by Burkitt et apply to all potential feed ingredients and compound
al. (1972) and Trowell (1974) to describe plant cell wall mixtures of feeds. Therefore, the restriction that dietary
components in the diet that were resistant to hydrolysis fiber comes only from plant sources is practically inap-
by mammalian digestive enzymes. Later, Trowell et propriate and nutritionally inconsistent with the defi-
al. (1976) broadened the definition of dietary fiber to nition of dietary fiber. The strictly physiological defini-
include all indigestible polysaccharides, such as gums tion does not require that dietary fiber originates from
and mucilages, whether or not they originate from plant plants or their cell walls, and even TDF as defined for
cell walls. This physiological-chemical definition of di- humans contains compounds that do not occur natu-
etary fiber as “polysaccharides and remnants of plant rally in plants. Although fiber has been linked to plant
materials that are resistant to hydrolysis (digestion) cell walls because they contain similar chemical compo-
by human alimentary enzymes” is the basis for AOAC nents in forages, fiber and cell walls are not synony-
official methods for TDF that have been accepted for mous terms. Insoluble dietary fiber is not cell walls
human food labeling (Cho et al., 1997). Because TDF because analytical methods often isolate insoluble com-
is based solely on resistance to digestion, it contains ponents in feeds other than plant cell walls, and cell
both SDF and IDF. Partitioning TDF into SDF and walls are not IDF because some plant cell wall compo-
IDF may provide important nutritional information for nents, such as pectin, are rapidly fermented and are
nonruminants because their recovery in the feces and solubilized by many fiber methods.
impact on the physiological processes of digestion (fer- The goal of dietary fiber methodology is to accurately
mentability, viscosity, water-holding capacity, disten- evaluate nutritive value and ultimately be useful in
sion, etc.) may be quite different. improving the nutritional quality of animal diets. Theo-
The definition of TDF for humans, which limits di- retically, dietary fiber methods should be developed to
etary fiber to components that cannot be digested by fit a nutritional definition and not vice versa. However,
mammalian enzymes, may be unduly restrictive for ru- it is unlikely that any chemical or enzymatic measure-
minants and herbivores, which have a symbiotic rela- ment will mimic all of the nutritional effects of fiber in
tionship with microorganisms and other adaptations of the animal. Although dietary fiber should be defined
digestive physiology that enable significant digestion by nutritional concepts and not analytical methodology,
Downloaded from jas.fass.org by on January 3, 2011.
Measuring insoluble dietary fiber 3237
practically, dietary fiber is an empirical measurement
that is defined by the method per se. In this situation,
consensus dietary fiber values can be generated for ref-
erence materials by averaging the results of several
laboratories that follow exactly a prescribed method.
To ensure the accuracy of results, efforts are needed
to generate reference materials that can be used to
document the accuracy of dietary fiber analyses among
research, regulatory, and feed-testing laboratories.
A practical method should not require exotic instru-
ments, reagents, equipment, laboratory environment,
and the like so that it is suitable for routine analyses
by feed-testing and regulatory laboratories. In addition,
the method should be rapid, convenient, and economical
to allow multiple samples of materials to be analyzed
in a timely manner. Given the variability in materials
and their sampling before being sent to the laboratory,
routine methods should be designed to allow multiple
determinations, rather than be so expensive or time
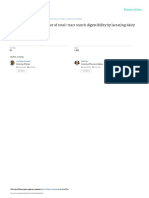
consuming that nutritional decisions must be based on Figure 1. Illustration of the concepts of accuracy and
a single sample. Specificity is required to ensure that precision: a) inaccurate and imprecise, b) accurate and
the method measures fiber accurately without interfer- imprecise, c) inaccurate and precise, and d) accurate
ence or artifacts that alter the fractions being mea- and precise.
sured. Finally, a method must have a clearly defined
limit of reliability that determines the method’s ability
to discriminate among analyte levels in materials and As illustrated in Figure 1, accuracy and precision are
detect concentrations different from zero. independent characteristics of a method’s reproducibil-
For research, specific methods that require sophisti- ity. The error associated with precision (scatter about
cated equipment and techniques may be needed to pro- the average value) tells us nothing about the error asso-
vide detailed analyses for comparisons among treat- ciated with accuracy (how close the average result
ments or to assess molecular or structural characteris- matches the true value). Yet we often hear that the
tics of dietary fiber components. However, if their results of a method used in a laboratory must be correct
conclusions are to be relevant to field applications, re- because duplicate variation (only a measure of preci-
searchers have a burden to provide routine analytical sion) was small. Precision does not guarantee accuracy
information as well as their detailed analyses to estab- because it is possible to precisely determine the wrong
lish a connection between research findings and field result (Figure 1c). No one would argue that an inaccu-
applications. In addition, researchers have a responsi- rate and imprecise method (Figure 1a) is useful; how-
bility to ensure that routine methods used in their labo- ever, it could be argued that precision is not particularly
ratory correspond to those used in the field. Therefore, relevant because the true value can be detected using
research laboratories should participate in proficiency- an imprecise method simply by averaging a large num-
testing programs with regulatory and feed-testing labo- ber of replications (Figure 1b). Conversely, researchers
ratories, and provide their performance statistics in sometimes argue that precision is more important than
reports and manuscripts. accuracy because the true value is unattainable or irrel-
evant when we are only concerned about detecting dif-
Reproducibility of Dietary Fiber Methods ferences among treatments or feeds. However, if data
are compared among institutions or laboratories, if new
After it has been established that a dietary fiber knowledge is built on one another’s results, or if re-
method is relevant because it matches the definition of search conclusions are to be applicable in the field, then
fiber, it must be established that it is reproducible. research analytical results must be both accurate and
Reproducibility is related to how well analytical results precise (Figure 1d) to allow others to reproduce and use
are measured (Steiner, 1975). It is a deceptively simple the information that is generated.
term that represents the sum of variation associated Statistical reproducibility is the total variation of an
with accuracy and precision. Accuracy is the ability to individual dietary fiber measurement from any labora-
measure the “true” value of a primary standard with tory. It is the sum of the variance of results among and
known composition or of the consensus concentration within laboratories. Variances among laboratories or
of a reference material that is determined by a group among days within laboratories are systematic because
of analysts exactly following a defined method. Preci- they represent a consistent pattern of deviations in
sion is the ability to repeat a measurement, or more mean results. These systematic biases are related to
quantitatively, the variation among repeated results. the ways methods are performed, equipment is adjusted
Downloaded from jas.fass.org by on January 3, 2011.
3238 Mertens
or calibrated, or reagents are prepared that cause con- A primary factor affecting gravimetric reproducibil-
sistent differences. For an ideal method, the variance ity is the accuracy and precision of the balance. Accu-
among laboratories, among days within laboratories, racy of a balance depends on its ability to report the
and among material × laboratory interactions would be true value when tested with a known weight and its
zero, and the variance of individual results from any smallest weight of detection. It is clear that balances
laboratory would be equal to the variance among repli- should be routinely maintained and standardized, and
cate analyses. This replication variance is due primarily should be calibrated or checked for accuracy at each
to random differences in test samples that are taken use. Even with daily calibration of balances, we have
from prepared samples that are not completely homoge- observed an unexplained systematic bias that is consis-
neous. To truly measure within-laboratory repeatabil- tent for all weights taken within weighing sessions.
ity, analyses must be performed in different runs or Correcting for blanks accounts for this systematic bias
days. This ensures that the results would be repeatable in weighing and has greatly improved the precision of
in that laboratory if measured at some future time; replicates and the accuracy of results for test samples
therefore, they are valid for making comparisons with that have small residue weights (such as lignin or di-
other results generated within that laboratory. Replica- etary fibers <10% of DM) in our laboratory. The im-
tion within a run, particularly if measured consecu- provement in the accuracy of results occurs because the
tively, does not provide such assurance. systematic bias is often a significant proportion of the
Unlike research laboratories, commercial feed analy- residue weight (Mertens, 2002). The problem of the
sis laboratories typically analyze only one test sample lowest weight of detection may be less obvious because
for each material received. Thus, the reproducibility laboratories occasionally weigh test samples or residues
(approximate 95% confidence interval) among single only to the nearest 0.01 g without recognizing that this
analyses performed by two laboratories on representa- negatively affects results. If test samples of 0.50 g are
tive samples of the same material is 2.8ⴢSDR. The repro- used, results can be reported legitimately to only two
ducibility of a method is a quantitative measure of its significant digits (nearest 1 percentage unit) because
robustness, or its power and sturdiness in measuring this is the limit of information in the original weight
the analyte in all types of materials using generally regardless of the number of digits generated during cal-
accepted practices within laboratories. Several perfor- culation.
mance characteristics of a method determine its repro- The reproducibility of results is also determined by
ducibility or robustness: ruggedness, practicality, speci- the precision of weighing the test sample, such as, if
ficity, and limit of reliability (Wernimont and Spendley, the precision of this balance is ±0.01, then the 95%
1985). Ruggedness refers to a method’s ability to gener- confidence interval for the test sample weight is 0.48
ate acceptable results when small, uncontrolled to 0.52 and the potential variation in weighing over-
changes in operating conditions occur. Ruggedness test- whelms the remaining factors associated with method
ing of a method (Youden, 1975) involves evaluating the variability. However, the converse of this situation is
impact of making small perturbations in the reagents not true. If the balance used weighs to the nearest
(concentrations, sources, etc.), conditions (temperature, 0.0001 g, this does not guarantee four significant digits
time, etc.), equipment (settings, models, etc.), and steps of precision because other characteristics of the method
(skipping or modifying). Ruggedness testing can be a can affect the precision of results. Sokal and Rohlf
daunting task when methods are complex and involve (1981) suggest that, in general, the number of decimal
sophisticated equipment, and typically these methods places for reporting results should be based on the stan-
are less thoroughly tested. Thus, complex methods that dard error of the mean using the following guideline:
are less rugged are more demanding in expertise and divide the standard error by 3 and use the decimal place
in exactness of following procedures than are simple of the first nonzero digit to determine the significant
solubility methods. digits to report. Because the standard error of most
dietary fiber methods is less than ±2.5, results should
Types of Dietary Fiber Methods typically be reported to the nearest 0.1%.
and Sources of Variation Cherney et al. (1985) demonstrated that the variation
in fiber results is also affected by the amount of test
Fiber methods are typically categorized into three sample. The effects of weighing error increased as the
types (chemical-gravimetric, enzymatic-gravimetric, or test sample amount decreased, especially when <0.3 g
enzymatic-chemical) based on the ways fibrous residues (for alfalfa containing about 26% NDF and 18% ADF).
are isolated and measured. Isolation of dietary fiber Goering and Van Soest (1970) reported that weighing
residues is done by extraction in chemical solutions, materials hot directly from the oven instead of transfer-
enzymatic hydrolysis of nonfibrous constituents, or a ring oven-dried materials to a desiccator before
combination of the two. After the fibrous residue is weighing is not only faster and more labor efficient, but
isolated, it is measured either gravimetrically also more accurate. The accuracy of the hot-weighing
(weighing the residue) or chemically (hydrolyzing the technique is better than when using desiccators be-
residue and measuring individual components, such as cause any change in the zero value of the balance is
sugars and lignin). recorded and subtracted from the hot weight to arrive
Downloaded from jas.fass.org by on January 3, 2011.
Measuring insoluble dietary fiber 3239
at the true weight of the material. In effect, this corrects Because much of the hemicellulose and lignin is in-
each weight for slight changes in the balance between cluded in the NFE fraction, the digestibility of NFE,
weighings. In addition, hot-weighing eliminates the which is supposed to contain the easily digested carbo-
variation due to the use or inadequate maintenance of hydrates, is less than the digestibility of CF for 25% of
desiccators. Both Horwitz et al. (1990) and Mertens the feeds listed by Morrison (1956). Currently, CF is
(2002) reported that the minimum SDR for gravimetric used only for quality control and specification of feeds
methods is about 0.3 to 0.4 percentage units when the (minimum CF) by regulatory agencies. Its lack of accu-
concentration of the analyte approaches zero. Thus, the racy in measuring dietary fiber and abandonment by
HORRAT may be an inadequate estimate of the ex- researchers and practicing nutritionists suggests that
pected reproducibility of gravimetric methods when fac- its use for feed regulation should be abolished.
tional concentrations are <0.1. In gravimetric methods,
the error associated with weighing does not approach Acid Detergent Fiber and Acid
zero as the analyte concentration approaches zero as Detergent Sulfuric Lignin
predicted by the HRSDR equation, which is based on
all methods including instrumental methods that have Like CF, ADF (AOAC Official Method 973.18) is an
small limits of detection (approximately parts per bil- empirical method that was designed to be a preparatory
lion or less). step for the determination of ADSL. The reason for
specifying that lignin was determined using sulfuric
Crude Fiber acid is that ADF can also be the preparatory step for
determining lignin using permanganate (ADPL);
Crude fiber was supposed to measure the indigestible therefore, the term acid detergent lignin is not adequate
ballast of feeds. The method was based on chemical to differentiate between the two correlated but different
extraction with alkali and acid solutions, which were measures of lignin (ADPL typically has higher values
the known characteristics of the digestion process be- than ADSL). In the ADF method, protein is removed
fore enzymes were discovered. The organic matter lost from the fibrous residue by the cationic detergent cetyl
during ashing is calculated as CF, which was initially trimethylammonium bromide to minimize the nitrogen
called wood fiber or crude woody fiber (Dougall, 1956). contamination of lignin. Acid extraction is used to re-
It was discovered that CF was digested as it passed move nonfibrous compounds while minimizing the
through the alimentary tract of ruminants (Henneberg losses of alkali-labile lignin. Acid detergent fiber does
and Stohman, 1860), but their method became a stan- not meet the nutritional definition of dietary fiber or
dard part of the proximate analysis scheme. The CF IDF for ruminants because acid-soluble hemicelluloses
method was first approved as an official method for the are removed and some rapidly fermented pectin is not.
AOAC in 1890 (Wiley, 1890), and it became the de facto The precipitation of pectins in strong acid may be the
definition of dietary fiber for over 100 yr. The current reason that some feeds containing high proportions of
AOAC Official Method 920.86 was adopted for flour in pectin (e.g., immature alfalfa, citrus pulp) may have
1920 and Official Method 962.09 was adopted for ani- ADF results that are higher than NDF.
mal feeds in 1962. The CF method is extremely robust The reproducibility of the ADF determined during an
in that it can be easily measured in all types of feeds AOAC collaborative study (Van Soest, 1973) was good,
and foods and can be reproduced within and among with a SDR of 1.13 and a HORRAT of 1.2 (Table 1). The
laboratories. Although the method is empirical (defined SDr was approximately one-third of the total SDR of
solely by the method used to measure it), CF has been individual analyses among laboratories. This indicates
useful, historically, in estimating digestibility or energy that the variation among laboratories is twice the varia-
value within feed types. tion within laboratories, which falls within the typical
The major deficiency of CF is its lack of relationship range for most methods (Horwitz, 1982). The SDR for
to any acceptable nutritional definition of dietary fiber ADSL was 0.62, but the HORRAT was 3.1, primarily
and its inability to advance understanding about physi- because the small mean of ADSL results in an unrealis-
ological responses to dietary fiber or its impact on di- tically small HRSDR for a gravimetric method (Mer-
gestibility. The proximate system partitions carbohy- tens, 2002).
drates and allied compounds into two fractions: CF, The keys to measuring ADF are to standardize the
which is measured analytically, and nitrogen-free ex- acid to 1 N, properly prepare samples for analysis, and
tract (NFE), which is calculated by difference (NFE = adequately soak fiber residues in hot water after extrac-
100 − CP − EE − CF − ash). Neither of these fractions tion to remove acid and acid detergent solubles. To
meets criteria for uniform nutritional availability or prepare samples for ADF analyses, they must be dried
for the definition of dietary fiber (Van Soest, 1967). at <60°C and ground through a cutter mill with a 1-
Depending on the feed material, CF may contain only mm screen. Cyclone mills tend to produce a finer grind
40 to 100% of the cellulose, 15 to 20% of the pentosans with the same apertures in the screen, so a 2-mm screen
from hemicellulose, and 5 to 90% of the lignin. Lignin is in a cyclone mill results in a particle size similar to
dissolved, especially in grasses, by the alkali extraction that of a 1-mm screen in a cutter mill. It has recently
step in the CF method that is used to remove protein. been discovered that samples with fat >10% can result
Downloaded from jas.fass.org by on January 3, 2011.
3240 Mertens
Table 1. Repeatability and reproducibility of the acid cellulose, hemicellulose, and lignin (Van Soest and
detergent fiber (ADF) and acid detergent sulfuric Wine, 1967). The significant nutritional attribute of
lignin (ADSL) AOAC Official Method 973.18 neutral detergent extraction is that it separates feeds
(Van Soest, 1973) into two major fractions that are distinctly different in
their digestibility and intake by ruminants and herbi-
Item ADF ADSL
vores, and in many cases by nonruminants as well (Mer-
Number of materials 6 6 tens, 1993). Whereas NDF has variable digestibility,
Number of laboratories 10 10 occupies space in the alimentary tract that can be a
Mean, % of DM 39.47 6.66 physical constraint on intake, and requires significant
SDra 0.38 0.29 chewing to reduce particle size, neutral detergent solu-
SDRb 1.13 0.62 bles (NDS), which represent the inverse of NDF (NDS
Repeatability within laboratoriesc 1.06 0.81
= 100 − NDF), have nearly constant true digestibilities
Reproducibility among laboratoriesd 3.16 1.74
HORRATe 1.24 3.10 near 100%, occupy little space because they are rapidly
a
solubilized, and require minimal chewing. Van Soest
Standard deviation of repeatability within laboratories.
b
Standard deviation of reproducibility among laboratories.
(1967) reported that NDS have a true digestibility of
c
2.8ⴢSDr, which is the approximate 95% confidence interval for about 98% and a relatively constant endogenous loss
duplicate analyses within a laboratory. of 12.9% when consumed by ruminants at maintenance
d
2.8ⴢSDR, which is the approximate 95% confidence interval for
single analyses between two laboratories.
levels of intake. The level of intake is important be-
e
Horwitz ratio, which is the SDR divided by the expected SDR based cause, at maintenance levels of intake, herbivores, espe-
on the equation of Horwitz (1982). cially sheep, chew feeds adequately, which allows com-
plete digestion of NDS.
Neutral detergent fiber is measured using a chemical
in ADF values that are artificially high. Therefore, the solubility-gravimetric method. Proteins are extracted
most recent version of the AOAC Official Method 973.18 using anionic detergent and sodium sulfite. Fats are
was modified to require preextraction of the test sample removed using hot detergent and acetone. Soluble di-
with acetone or other suitable solvents to remove fat etary fiber is removed primarily by hot detergent ex-
when the material contained >10% fat. The method was traction, and the solubility of easily fermented pectin
also modified to indicate that extracted fiber residues is enhanced by chelating calcium bound in pectin com-
must be soaked three times in 90 to 100°C water for at plexes using EDTA. In the original NDF method (Van
least 3 min to equilibrate acid from within particle
Soest and Wine, 1967), soluble carbohydrates and
pores. It is essential that all residual acid be removed
starch were extracted by hot solutions. It was discov-
from the fiber before it is dried. During drying of ADF,
ered that the original NDF method inadequately re-
any residual acid is wicked to the surface of particles
moved starch from some feeds and foods. Numerous
and concentrated as water evaporates. Residual concen-
modifications of the NDF method have been proposed
trated sulfuric acid will char the edges of particles,
since the original publication of the method (McQueen
especially when heated in a >100°C oven. Blackened
and Nicholson, 1979; Robertson and Van Soest, 1980;
or charred ADF residues indicate that acid was not
Mascarenhas Ferreira et al., 1983; Van Soest et al.,
completely removed during the residue-washing steps
1991). Of these modifications, the NDR method of Rob-
and that ADF results will be low.
ertson and Van Soest (1980), which uses a heat-stable
Although it is not an AOAC official method, there are
α-amylase to remove starch during detergent extraction
circumstances when it may be desirable to measure
and eliminated the use of sodium sulfite, became the
ADF sequentially (sADF) after neutral detergent ex-
de facto method for measuring NDF.
traction. Sequentially determined ADF is almost al-
The original NDF method of Van Soest and Wine
ways less than ADF determined by the official method
(1967) was never evaluated by a collaborative study.
because neutral detergent removes some components
However, a method for measuring IDF based on the
that are not removed as well by acid detergent, such
NDR modification of Roberson and Van Soest (1980)
as pectins and tannin or phenolic acid complexes. Hintz
was evaluated as a method for measuring the IDF por-
et al. (1996) determined ADF sequentially on NDF resi-
tion of TDF (Mongeau and Brassard, 1990). Although
dues that were isolated using heat-stable α-amylase
with (aNDF) or without sulfite (neutral detergent resi- most of the materials used in the collaborative study
due, NDR). When sADF was determined on NDR, val- were human foods, the comparison for wheat bran may
ues were 1 to 3 percentage units lower than ADF mea- represent results for high-fiber by-product feeds (Table
sured using the official method and this difference in- 3). The SDR for wheat brans (±1.92) was slightly higher
creased to 2 to 4 percentage units when sADF was than that observed for ADF (±1.13), and the RSDR was
determined on aNDF (Table 2). also slightly higher (4.7 vs. 2.9%). The SDR was higher
for TDF compared to IDF in wheat brans and foods,
Neutral Detergent Fiber suggesting that the SDF contained in TDF may be less
reproducible than IDF.
The NDF method was designed initially to isolate the The aNDF method was developed as an IDF method
insoluble dietary fiber components in plant cell walls: that could be used on all feeds, including forages,
Downloaded from jas.fass.org by on January 3, 2011.
Measuring insoluble dietary fiber 3241
Table 2. Comparison of acid detergent fiber (ADF) determined by AOAC Official
Method (973.18) to ADF determined sequentially after neutral detergent extraction
using heat-stable α-amylase with (sADFaNDF) or without sodium sulfite (sADFNDR)
Difference between ADF and:
sADFNDR sADFaNDF
Feed type ADF (−sulfite) (+sulfite) sADFNDR sADFaNDF
Heated by-products 14.1 11.5 10.2 2.6 3.9
Oilseed meals 17.6 16.5 15.2 1.1 2.4
Grass forages 45.2 42.4 41.1 2.8 4.1
Legume forages 32.9 29.7 28.7 3.2 4.2
grains, oilseeds, and plant and animal by-products used in fiber is not subtracted twice. It is often unclear
for animal feeds (Mertens, 2002). The method is a modi- whether NDF results reported in the literature are ash-
fication of the original NDF method, which included free organic matter, but neither the original method of
the use of sodium sulfite, but added the use of a heat- Van Soest and Wine (1967) or the handbook of Goering
stable α-amylase standardized to remove starch during and Van Soest (1970), which are often cited as sources
neutral detergent extraction and with specific modifi- of methods, indicate that NDF should be determined
cations (sand and other filter aids) that solve filtering as ash-free organic matter.
difficulties for all types of materials. The aNDF method Determination of aNDF has SDR among laboratories
allows results to be reported as either aNDF with fiber- (Table 4) similar to that reported for ADF (Table 1). It
associated ash or as ash-free aNDF organic matter (aN- is surprising that the SDr within laboratories, which is
DFom), and each of these results can be reported with due primarily to random variation in test samples, was
or without blank correction. It is anticipated that aNDF a much larger proportion of SDR for aNDF (79%) than
will be reported for routine feed analyses because it for ADF (34%). This suggests that either the variability
does not require an ashing step before reporting results. in aNDF among test samples is much larger than for
Although crucibles are routinely cleaned by ashing, ADF or that other sources of variation within labora-
that step represents an additional time delay in re- tories contributed to repeatability differences for aNDF.
porting results and most, if not all, commercial feed The repeatability value in Table 4 indicates that analy-
testing laboratories currently report aNDF. ses should be rerun if duplicates differ by more than
For the most accurate estimate of insoluble dietary about 2.9 percentage units. The reproducibility value
fiber, blank-corrected aNDFom is recommended. Blank indicates that results for 19 out of 20 laboratories per-
correction is especially important when fiber results forming a single analysis on a well-mixed material
are <25% aNDF because systematic weighing variation should be within 3.7 percentage units of each other.
can have substantial impact on these small residue The original NDR method (Robertson and Van Soest,
weights (Mertens, 2002). Reporting results as aNDFom 1980) differs from the aNDF method (Mertens, 2002) in
more accurately matches the definition of insoluble di- the use of sodium sulfite and type of amylase. However,
etary fiber as organic matter and improves the accuracy current implementations of the NDR method use amy-
of calculating nonfibrous carbohydrates because the ash lases similar to the aNDF method because the original
Table 3. Results of a collaborative study (Mongeau and Brassard, 1990) to evaluate
AOAC Official Method 992.16 for total dietary fiber (TDF) or insoluble dietary fiber
(IDF), which was determined using neutral detergent extraction with α-amylase (NDR)
Item IDF(NDR) IDF(NDR) TDF TDF
Material used Wheat bran Foods Wheat bran Foods
Number of materials 2 6 2 6
Number of laboratories 9/10 7/10 9/10 8/10
Mean, % of DM 40.48 6.95 46.80 10.38
SDra 0.71 0.45 0.86 0.70
b
SDR 1.92 0.70 2.80 1.02
Repeatability within laboratoriesc 1.99 1.26 2.39 1.96
Reproducibility among laboratoriesd 5.38 1.96 7.86 2.86
HORRATe 1.57 2.55 2.02 2.67
a
Standard deviation of repeatability within laboratories.
b
Standard deviation of reproducibility among laboratories.
c
2.8ⴢSDr, which is the approximate 95% confidence interval for duplicate analyses within a laboratory.
d
2.8ⴢSDR, which is the approximate 95% confidence interval for single analyses between two laboratories.
e
Horwitz ratio, which is the SDR divided by the expected SDR based on the equation of Horwitz (1982).
Downloaded from jas.fass.org by on January 3, 2011.
3242 Mertens
Table 4. Results of a collaborative study (Mertens, 2002) for amylase-treated neutral
detergent fiber (aNDF) when blank-corrected (aNDFbc) or when expressed
as ash-free fiber organic matter (aNDFom and aNDFombc)
Item aNDF aNDFbc aNDFom aNDFombc
Material used Feeds Feeds Feeds Feeds
Number of materials 11 11 11 11
Number of laboratories 11 11 11 11
Mean, % of DM 38.7 38.6 37.7 37.4
SDra 1.05 1.02 1.02 1.00
SDRb 1.33 1.28 1.28 1.24
Repeatability within laboratoriesc 2.94 2.86 2.85 2.80
Reproducibility among laboratoriesd 3.72 3.59 3.58 3.48
HORRATe 1.49 1.44 1.47 1.43
a
Standard deviation of repeatability within laboratories.
b
Standard deviation of reproducibility among laboratories.
c
2.8ⴢSDr, which is the approximate 95% confidence interval for duplicate analyses within a laboratory.
d
2.8ⴢSDR, which is the approximate 95% confidence interval for single analyses between two laboratories.
e
Horwitz ratio, which is the SDR divided by the expected SDR based on the equation of Horwitz (1982).
amylase is no longer available. Sulfite was eliminated 5). Although NDF, NDR, and aNDF can be corrected
when the NDR modification was developed because it for protein contamination by measuring the nitrogen
may extract lignin and phenolic complexes. Removing content of the respective fiber residues, this additional
sodium sulfite from the NDF procedure increases the step requires time and expense, which are large disad-
protein contamination of fibrous residues, which has vantages for a routine method and have little impact
been used to define a slowly degrading protein fraction on results in comparison to variation within and among
in feeds (Sniffen et al., 1992). However, protein contam- laboratories for IDF results. The aNDF method was
ination can greatly inflate the IDF values of feeds, espe- designed to improve the routine determination of IDF
cially heated feeds (Table 5). Using sodium sulfite re- in all feeds; therefore, sulfite was retained in the
duces aNDF values for forages and oilseed meals by method to remove most protein contamination. Sodium
only 1 to 4 percentage units; however, it reduces the sulfite has the additional benefit that it improves filtra-
fiber values of heated by-product feeds, such as brewer’s tion (and precision) during the analysis of some prob-
and distiller’s grains, by about 11 percentage units. lem materials.
Thus, the use of sodium sulfite in the aNDF method is a The NDF method has a reputation of being variable
compromise between losing a small amount of phenolic and difficult to accomplish. Most of the variability in
compounds in some feeds or having IDF contaminated NDF results among laboratories is related to differ-
by a large amount of protein that is apparently digested ences in the specific modification of the method that
in other feeds. With the exception of grass forages, was used. Much controversy about differences in NDF
which have the smallest difference in fiber due to the results among laboratories would be eliminated if labo-
use of sodium sulfite, most of the material extracted ratories would state exactly how they measured it and
from feeds by sulfite is crude protein equivalent (Table used specific nomenclature to identify it. Unfortu-
Table 5. Composition of the material extracted when sodium sulfite
is used during neutral detergent extraction
Composition of the difference
Feed type NDRa aNDFb Differencec CPEd sADSLe Otherf
(% of DM) (% of difference)
Heated by-products 45.5 34.4 11.1 67 8 25
Oilseed meals 26.9 22.8 4.1 54 6 40
Grass forages 73.1 71.1 2.0 24 76 0
Legume forages 40.3 38.9 1.4 75 25 0
a
Neutral detergent residue (Robertson and Van Soest, 1980) determined with heat-stable α-amylase and
without sodium sulfite.
b
Amylase-treated neutral detergent fiber (Mertens, 2002) determined with heat-stable α-amylase and
sodium sulfite.
c
NDR – aNDF.
d
Crude protein equivalent (nitrogen × 6.25).
e
Acid detergent lignin using sulfuric acid determined sequentially after neutral detergent extraction.
f
Undefined composition determined by difference.
Downloaded from jas.fass.org by on January 3, 2011.
Measuring insoluble dietary fiber 3243
Table 6. The effect of different dry matter adjustments trates that nutrient concentrations should not be com-
among laboratories on the concentration of nutrients pared among laboratories on a DM basis because these
with small magnitudes (crude protein and ether results combine the potential errors in both DM and
extract) or large magnitudes (neutral detergent nutrient determinations, making it impossible to deter-
fiber) when expressed on a dry matter basis mine which is the culprit when discrepancies occur.
Unfortunately, there is no AOAC official method for
Laboratory and As-is, DM DM-adjusted,
routine measurement of DM that is acceptable for all
nutrient % fractiona %
feeds and this situation needs to be rectified. The deter-
Laboratory 1 mination of DM is empirical and methods vary signifi-
Ether extract 2.2 0.88 2.5 cantly among laboratories. The 100 laboratories that
Crude protein 10.0 0.88 11.4
Neutral detergent fiber 65.0 0.88 73.9
responded to a 1993 NFTA questionnaire about drying
Laboratory 2 methods indicated that they used 47 different combina-
Ether extract 2.2 0.92 2.4 tions of time and temperature (Mertens, 1994). Twenty-
Crude protein 10.0 0.92 10.9 one temperatures (ranging from 57 to 140°C) and 16
Neutral detergent fiber 65.0 0.92 70.7 drying times (ranging from 2 to 48 h) were used for DM
a
The difference in DM between laboratories, which alters DM- determinations.
adjusted results, is not real, but due to differences in technique or
method for DM determination.
Total, Insoluble, and Soluble Dietary Fiber
Total dietary fiber in human foods has been measured
nately, it is not recognized that because NDF (like any by two major types of methods: enzymatic-gravimetric
IDF) is not a homogeneous chemical entity, its magni- or enzymatic-chemical. Enzymatic-gravimetric meth-
tude and properties are defined by the method used to ods can be used to measure TDF directly by AOAC
measure it. Thus, every modification measures some- Official Method 985.29 or by summing IDF (Official
thing slightly different from the original NDF method, Method 991.42) and SDF (Official Method 993.19).
and results should not be indiscriminately called NDF These three methods use essentially the same proce-
to avoid confusion when comparing results among ex- dure. Official Method 991.43, which supersedes these
periments or laboratories. Mertens (1998a) and Hintz earlier methods, is based on the same principle as Offi-
et al. (1996) reported the variation in dietary fiber con- cial Method 985.29 but uses an organic buffer instead
centration among the three major modifications of the of the phosphate buffer. The organic buffer used in
NDF method. In general, the NDR modification (Rob- Official Method 991.43 results in a method that is sim-
ertson and Van Soest, 1980), which uses heat-stable α- pler, uses fewer reagents, and generates lower and more
amylase, but not sulfite, results in slightly higher val- reproducible blanks; this method can be used to mea-
ues (approximately 0 to 1 percentage unit) for feeds sure TDF and its components IDF and SDF. All of these
with little starch and moderate protein and much lower methods involve the isolation of fiber residues by using
values for starch-containing feeds (approximately 1 to 5 α-amylase and amyloglucosidase to hydrolyze starch
percentage units) compared to the original NDF method and proteases to hydrolyze proteins. Insoluble dietary
(Van Soest and Wine, 1967). The aNDF modification fiber is the residue remaining after enzymatic hydroly-
(Mertens, 2002), which uses both sodium sulfite and sis. Soluble dietary fiber is precipitated from the hy-
heat-stable α-amylase, generates lower results than ei- drolyzed solution with ethyl alcohol at a final concentra-
ther NDF or NDR. tion of 78%. Ash and protein is determined on one of
One of the unappreciated sources of variation in NDF the duplicate IDF and SDF residues and used to correct
results is the effect of DM adjustment. Most chemical IDF and SDF for protein and ash.
entities are measured on a test sample that is not com- Although few materials were analyzed that are typi-
pletely dry; thus, the analysis is determined on an as- cal animal feeds, the results in Tables 7 and 8 suggest
is, or as-received, basis. Although feeds are bought, that enzymatic-gravimetric methods measure IDF with
sold, and regulated (feed tag specifications) on an as- reproducibility and repeatability similar to those of the
is basis, nutrients are also reported on a DM basis to aNDF method (Prosky et al., 1985, 1992, 1994; Lee et
allow a more direct comparison of nutrient densities al., 1992). The values for IDF using these methods are
without the confounding factor of moisture in the mate- within the range of aNDF values expected for these
rial. However, small apparent differences in DM deter- feed sources. However, these methods have not been
mination, which may be caused by poor technique or used on as wide a variety of feeds as the aNDF method
differences in methodology, can result in large artifact and it is difficult to draw firm conclusions about compar-
differences in nutrient concentration on a DM-adjusted isons between aNDF and enzymatic-gravimetric meth-
basis when the magnitude of analyte concentration is ods. Enzymatic-gravimetric methods are more complex
large (Table 6). The small discrepancy between these and require more intermediate analyses than aNDF;
two laboratories for EE or CP is inconsequential, but therefore, it is unlikely that reproducibility would be
the larger difference in NDF on a DM basis between better. The complexity and time required to conduct
these laboratories would be disconcerting. This illus- enzymatic fiber assays makes it difficult to envision
Downloaded from jas.fass.org by on January 3, 2011.
3244 Mertens
Table 7. Results of collaborative studies for enzymatic-gravimetric methods that use
phosphate buffers to measure total dietary fiber (TDF), insoluble
dietary fiber (IDF) and soluble dietary fiber (SDF)
AOAC Official Method: 985.29a 991.42b 993.19c 993.19 993.19
Item Dietary fiber: TDF IDF TDF IDF SDF
Material used Wheat bran Soy bran SB fiberd SB fiber SB fiber
Number of materials 1 1 1 1 1
Number of laboratories 9 13 10 10 10
Mean, % of DM 42.65 65.24 66.07 45.57 20.65
SDre 0.99 0.91 1.15 0.67 0.80
SDRf 1.21 2.40 1.59 0.98 1.35
Repeatability within laboratoriesg 2.78 2.55 3.22 1.88 2.24
Reproducibility among laboratoriesh 3.38 6.72 4.45 2.74 3.78
HORRATi 1.25 1.72 1.13 0.96 2.58
a
Prosky et al. (1985).
b
Prosky et al. (1992).
c
Prosky et al. (1994).
d
Sugar beet fiber.
e
Standard deviation of repeatability within laboratories.
f
Standard deviation of reproducibility among laboratories.
g
2.8ⴢSDr, which is the approximate 95% confidence interval for duplicate analyses within a laboratory.
h
2.8ⴢSDR, which is the approximate 95% confidence interval for single analyses between two laboratories.
i
Horwitz ratio, which is the SDR divided by the expected SDR based on the equation by Horwitz (1982).
that they will be adopted as routine methods for feed lian enzymes, whereas the latter is based on a chemical
analysis. Their appeal is based on the concept that the definition of dietary fiber as nonstarch carbohydrates
measurement of dietary fiber by enzymes mimics the and lignin (Theander et al., 1994; Englyst et al., 1994).
process of digestion; however, it is clear that neither The Uppsala method (Theander et al., 1995) is the only
the conditions nor the enzymes used for measurement enzymatic-chemical method for measuring TDF that is
approach the complexity of hydrolysis in the gastroin- an official method. Official Method 994.13 of the AOAC
testinal tract. Nonetheless, SDF measured by enzy- uses α-amylase and amyloglucosidase to hydrolyze and
matic methods appear to affect physiological processes remove starch, but no proteases are used to remove
in humans. protein. After starch hydrolysis, ethyl alcohol is used
An alternative to the enzymatic-gravimetric ap- to precipitate soluble polysaccharides. The combined
proach is to measure the monomeric composition of soluble and insoluble fiber residue is hydrolyzed to neu-
structural components of plants (cell walls) or of non- tral sugars and uronic acids using 12 M sulfuric acid
starch polysaccharides. The former is directly related at 30°C for 1 h followed by diluting the sulfuric acid to
to the physiological definition of dietary fiber as the 0.4 M and autoclaving at 125°C for 1 h. Klason lignin
dietary components resistant to hydrolysis by mamma- is determined as the loss of acid insoluble residue after
Table 8. Collaborative study results of AOAC Official Method 991.43, which used
MES-Tris buffer (Lee et al., 1992) to measure total dietary fiber (TDF),
insoluble dietary fiber (IDF), and soluble dietary fiber (SDF)
Dietary fiber TDFda TDFsb IDF SDF
Material used Bransc Brans Brans Brans
Number of materials 2 2 2 2
Number of laboratories 11 11 11 11
Mean, % of DM 42.03 42.23 35.13 7.04
SDrd 1.04 0.78 0.78 0.51
SDRe 1.56 1.22 0.94 0.87
Repeatability within laboratoriesf 2.90 2.17 2.17 1.43
Reproducibility among laboratoriesg 4.37 3.40 2.62 2.44
HORRATh 1.23 1.62 1.48 4.48
a
Total dietary fiber measured directly.
b
Total dietary fiber measured as the sum of IDF and SDF.
c
Soy and oat brans.
d
Standard deviation of repeatability within laboratories.
e
Standard deviation of reproducibility among laboratories.
f
2.8ⴢSDr, which is the approximate 95% confidence interval for duplicate analyses within a laboratory.
g
2.8ⴢSDR, which is the approximate 95% confidence interval for single analyses between two laboratories.
h
Horwitz ratio, which is the SDR divided by the expected SDR based on the equation by Horwitz (1982).
Downloaded from jas.fass.org by on January 3, 2011.
Measuring insoluble dietary fiber 3245
Table 9. Collaborative study results of Uppsala method (Theander et al., 1995) for
measuring total dietary fiber (TDF) as the sum of acid hydrolyzed uronic acids
and neutral sugars and Klason lignin (AOAC Official Method 994.13)
Dietary fiber TDF Uronic acids Neutral sugars Klason lignin
Material used Bransa Brans Brans Brans
Number of materials 2 2 2 2
Number of laboratories 9 9 9 9
Mean, % of DM 51.35 2.70 39.75 8.70
SDrb 1.28 0.24 1.31 0.44
SDRc 3.16 0.37 3.15 0.80
Repeatability within laboratoriesd 3.58 0.66 3.67 1.22
Reproducibility among laboratoriese 8.85 1.04 8.82 2.24
HORRATf 2.78 3.98 3.45 3.18
a
Wheat and oat brans.
b
Standard deviation of repeatability within laboratories.
c
Standard deviation of reproducibility among laboratories.
d
2.8ⴢSDr, which is the approximate 95% confidence interval for duplicate analyses within a laboratory.
e
2.8ⴢSDR, which is the approximate 95% confidence interval for single analyses between two laboratories.
f
Horwitz ratio, which is the SDR divided by the expected SDR based on the equation by Horwitz (1982).
ashing and the acid-hydrolyzed filtrate is analyzed for Knudsen (1997) reported detailed analysis of non-
neutral sugars and uronic acids. The neutral sugars starch polysaccharides and Klason lignin in feeds using
are measured as alditol acetates by gas chromatogra- modifications of the Uppsala method, that were re-
phy and uronic acids are measured colorimetrically. ported as soluble, insoluble, and total nonstarch poly-
The enzymatic-chemical chromatographic method is saccharide and lignin, which were summed to obtain
time consuming and expensive in terms of both labor TDF (Table 10). Although NDF was not measured by
and equipment. It requires highly skilled and trained Knudsen (1997), several samples of each feed were ana-
personnel to manage sensitive chemical reactions and lyzed, which can be compared to typical NDF values
to operate and maintain chromatographic instruments. reported by NRC (2001). The IDF of feeds based on
The method is currently used mostly in research labora- insoluble nonstarch polysaccharides plus lignin are
tories and is not used extensively by commercial or generally similar to NDF.
regulatory laboratories. Although the method is chemi-
cally sophisticated, the reproducibility of both TDF and Problems with Empirical Methods
total nonstarch polysaccharides (Table 9) indicates that
variability among laboratories is about twice that of The Codex Alimentarius Commission (1986) de-
gravimetric assays for dietary fiber (aNDF or en- scribes empirical methods as Type I Defining Methods
zymatic). because the values obtained can be generated only in
Combining quantification of sugar monomers with terms of the specific method—that is, defined by the
complementary chemical information can be used to method. This classification is not a negative reflection
describe secondary and tertiary structures that affect on the quality of a method or its repeatability within
the physicochemical properties of fiber that influence or reproducibility among laboratories. Because there
digestive physiology and digestibility. This is a valid are no primary reference standards for these methods,
research endeavor, but until the nutritional value of they cannot be validated for accuracy in determining
extensive monomer information is demonstrated and the “true” value for the constituent. To minimize sys-
the reproducibility of these methods is improved, it tematic errors (bias) among laboratories, empirical
seems premature to recommend these methods for the methods must be followed exactly because even slight
routine description of feeds. The relatively large SDR variations in methodology can result in the measure-
for TDF and neutral sugars (Table 9) suggests that ment of a different constituent. Systematic bias among
laboratories have difficulty reproducing monomeric in- laboratories for empirical methods can be determined
formation, which appears to be related to the empirical only using consensus values, which are the average
conditions used to isolate enzymatic residues and com- results of laboratories that follow the method exactly.
promises between maximum solubilization and mini- It might seem that dietary fiber can be defined more
mum degradation of sugars during acid hydrolysis. The accurately and precisely by specifying and quantifying
benefits of extensive carbohydrate analysis will be real- the chemical monomers in dietary fiber carbohydrates.
ized in the short term only if routine analyses are re- However, this assumes that sugar analysis is exact and
ported concomitantly to provide a bridge between cur- that detailed knowledge of polysaccharide analysis will
rent and future fiber analyses and to determine when lead to nutritional insights. Although the accuracy of
alternative methods of analysis provide similar or com- chromatographic measurement of sugars can be deter-
plementary information. mined using primary standards, the preparation of en-
Downloaded from jas.fass.org by on January 3, 2011.
3246 Mertens
Table 10. Enzymatic-chemical analysis of insoluble dietary fiber (IDF) determined as
the sum of insoluble nonstarch polysaccharides (NSP) and Klason lignin in feeds
as reported by Knudsen (1997) compared with neutral detergent fiber (NDF)
Soluble Insoluble Total Klason
Feed NSP NSP NSP lignin IDFa NDFb
Corn grain 0.9 8.8 9.7 1.1 9.9 9.5
Soybean meal 6.3 15.4 21.7 1.6 17.0 14.9
Wheat bran 1.5 35.9 37.4 7.5 43.4 42.5
Beet pulp 40.7 37.2 77.9 3.5 40.7 45.8
Grass meal 3.1 36.6 39.7 16.1 52.7 57.7
Alfalfa meal 7.7 25.2 32.9 12.8 38.0 41.6
a
Insoluble dietary fiber calculated as the sum of insoluble NSP and Klason lignin.
b
Reported by NRC (2001).
zymatic residues is relatively empirical and can affect geneous. Thus, it is difficult to prepare a homoge-
the quantities of sugars and lignin recovered (Marlett nous sample of most feeds because fiber compo-
et al., 1989). In addition, polysaccharides are converted nents tend to segregate. Assuming that technique
to monosaccharides, and monosaccharides are de- is relatively stable within a laboratory, most of the
graded at different rates depending on acid hydrolysis variation in repeatability within laboratories is re-
conditions and characteristics of the enzymatic resi- lated to random differences in the test samples.
dues. Because there is incomplete recovery of sugars, 2. Many dietary fiber methods are multistep pro-
correction factors (based on typical analyses) are cesses that often require corrections for ash or pro-
needed to correct results and generate adequate quanti- tein contamination and for component recoveries.
fication. Each of these steps or supplemental assays has
The nonuniform digestibility of fibrous carbohydrates associated random and systematic errors that con-
suggests that knowing their monomeric composition, tribute to the total variation of dietary fiber
no matter how accurately, provides little information methods.
about their availability to the animal. The physico- 3. Random and systematic errors in weighing consti-
chemical nature of fiber and its relationship to noncar- tute a significant source of error in gravimetric
bohydrate components, such as lignin, may have methods when fiber residues are small. If 1.0000-
greater nutritional significance than its intrinsic mono- g test samples are analyzed that contain <5% fiber,
saccharide composition. The consequence of this specu- the final fiber residue that is weighed is <50 mg.
lation is that even the most elegant analysis of carbohy- A balance precision of ±0.0001 would result in a
drate composition may add little to our ability to evalu- weighing error alone of 4% (±2ⴢSD). This error is
ate feeds nutritionally. The rationale for dietary fiber greatly magnified when the test sample or fiber
analysis is derived from its nutritional consequences. residue is smaller. In addition, small test sample
Caution should be exercised to ensure that research on amounts may make it difficult to obtain a represen-
dietary fiber is not shifted from an approach that uses tative test sample of heterogeneous materials.
the nutritional definition of dietary fiber to develop 4. Among-laboratory variation is large for empirical
methods for measuring it to an approach that develops methods because analysts often perform methods
a method that measures chemical constituents and at- in nonstandard ways that do not follow the official
tempts to define fiber on the basis of its composition. method. In addition, quality assurance programs
Dietary fiber methods are now, and may always be, instituted to verify results in laboratories often are
empirical because the result is dependent on the re- inadequate or even nonexistent. Many dietary fiber
agents and conditions used in each method to isolate methods have not been optimized to ensure ade-
fibrous residues. Horwitz et al. (1990) reevaluated the quate suitability for all types of material or to iden-
collaborative studies of all methods used for nutritional tify those steps that must be executed in detail.
labeling and concluded that all fiber methods had poor Often the limitations of methods and rationale for
reproducibility among laboratories when compared to specific steps in a method have not been published
the measurement of crude protein. They suggested that or have not been properly relayed to the analyst.
the lack of reproducibility was related to the empirical But perhaps most of the among-laboratories varia-
nature of these methods. The relatively poor reproduc- tion is associated with the desire of analysts to
ibility of dietary fiber methods compared with other improve efficiency by shortening times, eliminating
methods may be related to several factors. steps, or failing to follow the details of a method and
assuming that these deviations should or would not
1. The physical and chemical properties of dietary affect results. These sometimes well-intentioned
fiber are distinctly different from other components deviations ignore the fundamental property of em-
in feeds, which makes high-fiber feeds more hetero- pirical methods, such as dietary fiber, which re-
Downloaded from jas.fass.org by on January 3, 2011.
Measuring insoluble dietary fiber 3247
Table 11. Performance statistics of certified laboratories Table 12. Average regression parameters for the
participating in the proficiency-testing program relationship between digestibility and dietary
of the National Forage Testing Associationa fibers adapted from Giger-Reverdin (1995)
Year and Dry Crude Dietary fiber Intercept Slope R2 SErega
parameter matter protein ADF NDF
CF 89.7 −0.77 0.63 5.31
2000 (7.2)b (0.27) (0.16) (2.53)
RMAb 91.6 17.2 27.0 37.8 NDF 97.7 −0.54 0.67 5.92
HSDRc 0.24 0.44 0.66 0.87 (7.8) (0.17) (0.12) (3.28)
Nd 136 139 138 128 ADF 95.4 −0.71 0.68 4.95
SDLe 0.65 0.57 1.14 1.48 (9.1) (0.43) (0.16) (2.53)
2001 Lignin 83.1 −2.98 0.79 4.94
RMA 92.2 14.5 29.4 39.8 (3.7) (0.68) (0.08) (2.86)
HSDR 0.23 0.38 0.71 0.91 a
n 133 131 134 129 Standard error of regression.
b
Values in parentheses are standard deviations of the parameter
SDL 0.65 0.48 1.02 1.40
from 15 experiments.
a
Published with permission of the Board of Directors of the National
Forage Testing Association.
b
Reference method average of six materials to which all participat-
ing laboratories were compared.
Practical Utility of Empirical Methods
c
Horwitz standard deviation of reproducibility (Horwitz, 1982) cal-
culated from the RMA and used to determine certification of profi- One criterion for the usefulness of dietary fiber meth-
ciency (HSDR for dry matter was calculated from moisture concentra- ods is their ability to predict DM digestibility. Giger-
tion). Laboratories must have been within 4ⴢHSD of the RMA for
certification in 2000 and 3ⴢHSD of the RMA for certification in 2001.
Reverdin (1995) summarized a large number of pub-
d
Number of laboratories eligible for certification for each method. lished reports that provided independent regression
e
Standard deviation from the RMA among all certified laboratories equations for predicting digestibility based on various
for the mean of triplicate analyses.
fiber fractions, which were averaged to obtain the mean
and standard deviation of intercepts, slopes, R2, and
standard deviations of regression (Table 12). The high-
quires that they be followed to the utmost detail, est average and smallest SD of R2 indicates that ADSL
including calibration of equipment. had the best average relationship with digestibility.
However, the large SD for the slope of the regression
for ADSL suggests that its quantitative relationship
Proficiency of Laboratories in Using with digestibility is variable among populations of ma-
Empirical Methods terials. Although it has the highest and most variable
average standard error of regression, NDF obtained the
Although the standard deviation from the RMA
most robust relationship with digestibility as evidenced
among laboratories (SDL) eligible for NFTA certifica-
by the smallest variation in the slope. The average re-
tion for each method is not the same statistical parame-
gression parameters for NDF suggest that about half
ter as the SDR determined in AOAC collaborative stud-
of NDF is typically digested and that the digestibility
ies, it provides a similar indication of variation among
of NDS is about 97.7% (i.e., the digestibility of a feed
laboratories. The SDL differs from the SDR because it
containing no NDF and therefore 100% NDS).
does not include variation among replicates within labo-
ratories and is based on the average of triplicates rather Choice of Insoluble Dietary Fiber Methods
than single analyses; however, it includes systematic
bias because it is determined in relation to the RMA. There is no perfect method for measuring IDF be-
Observed SDL (Table 11) of ADF and NDF are larger cause the needs of each user of analytical information
than the HSDR (ranging from 144 to 173%), which may are unique. All dietary fiber methods are compromises
indicate that laboratories participating in the NFTA between nutritional relevance and analytical conve-
proficiency-testing program are not required to follow nience and reproducibility. Dietary fiber methods are
any specific method for their analyses (although they of variable and unequal value in measuring nutritional
are encouraged to do so). It may also indicate the vari- utility. Assuming that it is nutritionally relevant, the
ability of empirical fiber methods because the HORRAT selection of an IDF method depends on several factors:
for all of the dietary fiber methods are >1.0. Surpris- reproducibility, repeatability, labor efficiency, timeli-
ingly, the relative SDL for CP, ADF, and NDF were ness, personnel requirements, cost, and use of the re-
similar (3.3 to 4.2% of the mean), which disagrees with sults. Occasionally, nutritionists may benefit from frac-
the conclusion of Horwitz et al. (1990) that fiber results tionation of TDF into SDF and IDF when attempting
are less reproducible than protein results. The small to explain physiological responses of animals, and re-
relative SDL (<5%) for the routine methods of feed anal- searchers may want to expend the time and effort to
ysis suggests that analyses among laboratories certified obtain a detailed description of monomeric composition.
as proficient by the NFTA are comparable when repli- Even in these situations, reproducibility and rugged-
cated results are reported. ness of the IDF method are requisite so that results
Downloaded from jas.fass.org by on January 3, 2011.
3248 Mertens
can be interpreted reliably and applied universally. of routine and research analysis of feeds. More research
However, complete separation and quantification of is needed to evaluate IDF methods for their reproduc-
IDF polymers is impractical, expensive, and unneces- ibility when analyzing typical feeds and forages, and
sary for routine descriptions of feeds and formulation to directly compare their ability to provide both similar
of diets. Until the nutritional implications of detailed and complementary nutritional information.
dietary fiber analysis are understood and adapted to
ration formulation, it appears that simple empirical Literature Cited
methods of IDF analysis will provide the nutritional
information of interest. AOAC. 1993. AOAC Official Methods Program Manual on Develop-
ment, Study, Review, and Approval Process for AOAC Official
Methods. AOAC Int., Arlington, VA.
Conclusions AOAC. 2002. Official Methods of Analysis. 15th ed. Assoc. Offic. Anal.
Chem., Arlington, VA.
Methods for measuring insoluble dietary fiber are Burkitt, D. P., A. R. P. Walker, and N. S. Painter. 1972. Effect of
dietary fibre on stools and transit times, and its role in the
inherently more variable than methods for other nutri-
causation of disease. Lancet 2:1408–1412.
ents. Acceptability of a dietary fiber method is based Cherney, J. H., J. J. Volenec, and W. E. Nyquist. 1985. Sequential
on its ability to match the definition of fiber and its fiber analysis of forage as influenced by sample weight. Crop
reproducibility among laboratories. Several current Sci. 25:1113–1115.
methods, including aNDF and the enzymatic-gravimet- Cho, S., J. W. DeVries, and L. Prosky. 1997. Dietary Fiber Analysis
and Applications. AOAC Int., Gaithersburg, MD.
ric methods for measuring TDF, seem to be relevant Codex Alimentarius Commission. 1986. Procedural Manual. 6th ed.
for measuring IDF because they match the appropriate Food and Agricultural Organization. Rome, Italy.
definition of dietary fiber for herbivores. These methods Dougall, H. W. 1956. Crude fibre: Its determination and its place in
have acceptable reproducibility among laboratories the analysis of animal feeding-stuffs. East Afr. Agric. J.
when followed exactly. Variability in NDF among labo- 21:225–229.
Englyst, H. N., M. E. Quigley, and G. J. Hudson. 1994. Determination
ratories is related primarily to differences in modifica- of dietary fibre as non-starch polysaccharide with gas-liquid
tions of the method that are used, and laboratories chromatography, high-performance liquid chromatography or
need to be more exact in describing their methods and spectrophotometric measurements of constituent sugars. Ana-
naming their results. Official methods for measuring lyst 119:1497–1509.
Giger-Reverdin, S. 1995. Review of the main methods of cell wall
TDF using enzymatic hydrolysis and measurement of
determination: Interest and limits for ruminants. Anim. Feed
monomers and Klason lignin after acid hydrolysis do Sci. Tech. 55:295–334.
not report an IDF fraction but could be modified to do Goering, H. K., and P. J. Van Soest. 1970. Forage Fiber Analyses.
so. The sum of insoluble nonstarch polysaccharides and USDA Agric. Handbook No. 379. U.S. Gov. Printing Office,
Klason lignin agrees with IDF measured by NDF, but Washington, DC.
Henneberg, W., and F. Stohman. 1860. Begründung einer rationellen
the reproducibility of TDF when using acid hydrolysis
Ftterung der Wiederkäuer. Vol. 1. Schwetschtke u. Sohn,
is less than that of other dietary fiber methods. The Braunschweig.
selection of a suitable method for IDF depends on the Hintz, R. W., Mertens, D. R., and Albrecht, K. A. 1996. Effects of
purpose of analysis. Analysis of insoluble polysaccha- sodium sulfite on recovery and composition of detergent fiber
rides provides more chemical information but is less and lignin. J. AOAC Int. 79:16–22.
Hipsley, E. H. 1953. Dietary “fibre” and pregnancy toxemia. Br. Med.
reproducible among laboratories and more expensive J. 2:420–422.
to obtain. For routine nutritive evaluation of feeds and Horwitz, W. 1982. Evaluation of analytical methods used for regula-
formulation of diets, aNDF seems a reasonable choice tion of foods and drugs. Anal. Chem. 54:67A–76A.
for measuring IDF. To verify that their dietary fiber Horwitz, W., R. Albert, M. J. Deutsch, and J. N. Thompson. 1990.
results are comparable, research, regulatory, and com- Precision parameters of methods of analysis required for nutri-
tional labeling. Part 1. Major nutrients. J. AOAC Int. 73:661–
mercial feed-testing laboratories should participate in 680.
check-sample or proficiency-testing programs. Alterna- Knudsen, K. E. 1997. Carbohydrate and lignin contents of plant
tive methods of dietary fiber analysis may provide sup- materials used in animal feeding. Anim. Feed Sci. Tech.
plemental or complementary information, but routine 67:319–338.
Lee, S. C., L. Prosky, and J. DeVries. 1992. Determination of total
analyses should be reported for these experiments to
soluble and insoluble dietary fiber in foods. Enzymatic-gravimet-
provide a bridge between current and future dietary ric method, MES-TRIS buffer: collaborative study. J. AOAC Int.
fiber analyses. 75:395–416.
Marlett, J. A., J. G. Chesters, J. J. Longacre, and J. J. Bodganski.
Implications 1989. Recovery of soluble dietary fiber is dependent on the
method of analysis. Am. J. Clin. Nutr. 50:479–485.
Mascarenhas Ferreira, A., J. Kerstens, and C. H. Gast. 1983. The
All dietary fiber methods are empirical to some de- study of several modifications of the neutral detergent procedure.
gree. This places additional burden on the analyst to Anim. Feed Sci. Tech. 9:19–28.
avoid shortcuts and follow the details of the method McQueen, R. E., and W. G. Nicholson. 1979. Modification of the neu-
exactly, during every analysis. Although IDF analysis tral-detergent fiber procedure for cereals and vegetables by using
α-amylase. J. AOAC 62:676–680.
requires attention to detail and good laboratory tech- Mertens, D. R. 1985. Effects of fiber on feed quality for dairy cows.
niques, its practical utility in predicting digestibility, Pages 209–223 in Proc. 46th Minn. Nutr. Conf., Dept. of Anim.
intake, and nutritive value makes it a necessary part Sci., Univ. of Minn.
Downloaded from jas.fass.org by on January 3, 2011.
Measuring insoluble dietary fiber 3249
Mertens, D. R. 1993. Importance of the detergent system of feed ing cattle diets: II. Carbohydrate and protein availability. J.
analyses for improving animal nutrition. Pages 25–36 in Proc. Anim. Sci. 70:3562–3577.
Cornell Nutr. Conf., Anim. Sci. Dept., Cornell Univ., Ithaca. NY. Sokal, R. R., and F. J. Rohlf. 1981. Biometry. 2nd ed. W. H. Freeman
Mertens, D. R. 1994. Significant sources of variation in routine forages and Co., NY.
analyses identified from 1993 National Forage Testing Associa- Steiner, E. H. 1975. Planning and analysis of results of collaborative
tion questionnaire data. Pages 46–49 in the Proc. 1994 NFTA tests. Pages 65–88 in Statistical Manual of the Association of
Workshop, National Forage Testing Association, Omaha, NE. Official Analytical Chemists. AOAC Int., Arlington, VA.
Mertens, D. R. 1998a. Nutritional properties of carbohydrates in Sullivan, J. T. 1964. The chemical composition of forages in relation
feeds and their roles in dairy rations. Pages 85–99 in Proc. 19th to digestibility by ruminants. USDA-ARS Bulletin 34-62.
Western Canada Nutr. Conf., Univ. Saskatchewan, Saska- Theander, O., P. Aman, E. Westerlund, R. Anderson, and D. Pat-
toon, Canada. tersson. 1995. Measurement of dietary fiber using sugar analy-
Mertens, D. R. 1998b. Why Laboratories Fail NFTA Proficiency Tests. ses: collaborative study. J. AOAC Int. 78:1030–1044.
Section O1-O10 in the Proc. of the 1998 NFTA Workshop. Na- Theander, O., P. Aman, E. Westerlund, and H. Graham. 1994. Enzy-
tional Forage Testing Association, Omaha, NE. matic/chemical analysis of dietary fiber. J. AOAC Int. 77:703–
Mertens, D. R. 2002. Gravimetric determination of amylase-treated 709.
neutral detergent fiber in feeds using refluxing in beakers or Thompson, M. and P. J. Lowthian. 1997. The Horwitz function re-
crucibles: collaborative study. J. AOAC Int. 85:1217–1240. vised. J. AOAC Int. 80:676–679.
Mertens, D. R., N. Thiex, and N. P. Martin. 1994. Statistics and Trowell, H. 1974. Editorial: Definition of dietary fibre. Lancet 1:503.
standards proposed for 1995 NFTA certification. Pages 38–45 Trowell, H., D. A. T. Southgate, T. M. S. Wolever, A. R. Leeds, M.
in Proc. of the 1994 NFTA Workshop, National Forage Testing A. Gassull, and D. J. A. Jenkins. 1976. Letter: Dietary fiber
Association, Omaha, NE. redefined. Lancet 1:967.
Tyler, C. 1975. Albrecht Thaer’s hay equivalents: Fact or fiction. Nutr.
Mongeau, R., and R. Brassard. 1990. Determination of insoluble,
Abst. Rev. 45:1–11.
soluble and total dietary fiber: Collaborative study of a rapid
Undersander, D., D. R. Mertens, and N. Thiex. 1993. Forage Analyses
gravimetric method. Cereal Foods World 35:319–324.
Procedures. National Forage Testing Assoc., Omaha, NE.
Morrison, F. B. 1956. Feeds and Feeding. 22nd ed. Morrison Publish-
Van Soest, P. J. 1964. Symposium on nutrition and forage and pas-
ing Co., Ithaca, NY.
tures: New chemical procedures for evaluating forages. J. Anim.
NRC. 2001. Nutrient Requirements of Dairy Cattle. 7th ed. Natl.
Sci. 23:838–845.
Acad. Sci., Washington, DC.
Van Soest, P. J. 1967. Development of a comprehensive system of feed
Prosky, L., N. Asp, I. Furda, J. W. DeVries, T. F. Schweizer, and B.
analyses and its application to forages. J. Anim. Sci. 26:119–128.
F. Harland. 1985. Determination of total dietary fiber in foods
Van Soest, P. J. 1973. Collaborative study on acid-detergent fiber
and food products: Collaborative study. J. AOAC 68:677–679. and lignin. J. AOAC Int. 56:781–784.
Prosky, L., N. Asp, T. F. Schweizer, J. W. DeVries, and I. Furda. Van Soest, P. J., J. B. Roberson, and B. A. Lewis. 1991. Methods for
1992. Determination of insoluble and soluble dietary fiber in dietary fiber, neutral detergent fiber and nonstarch polysaccha-
foods and food products: Collaborative study. J. AOAC Int. rides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597.
75:360–367. Van Soest, P. J., and R. H. Wine. 1967. Use of detergents in the
Prosky, L., N. Asp, T. F. Schweizer, J. W. DeVries, I. Furda, and S. analysis of fibrous feeds. IV. Determination of plant cell-wall
C. Lee. 1994. Determination of soluble dietary fiber in foods and constituents. J. AOAC 50:50–55.
food products: Collaborative study. J. AOAC Int. 77:690–694. Wernimont, G. T., and W. Spendley. 1985. Use of statistics to develop
Robertson, J. B., and P. J. Van Soest. 1980. The detergent system of and evaluate analytical methods. AOAC Int., Arlington, VA.
analysis and its application to human foods. Pages 123–158 in Wiley, H. W. 1890. Official Methods. Page 212 in the Proc. 7th Conven-
the Analysis of Dietary Fiber in Foods. W. P. T. James and O. tion of AOAC. USDA. Div. of Chemistry. Bulletin No. 25.
Theander, ed. Marcel Dekker, Inc., NY. Youden, W. J. 1975. Statistical techniques for collaborative tests.
Sniffen, C. J., J. D. O’Connor, P. J. Van Soest, D. G. Fox, and J. B. Pages 1–64 in Statistical Manual of the Association of Official
Russell. 1992. A net carbohydrate and protein system of evaluat- Analytical Chemists. AOAC Int., Arlington, VA.
Downloaded from jas.fass.org by on January 3, 2011.
References This article cites 29 articles, 4 of which you can access for free at:
http://jas.fass.org/cgi/content/full/81/12/3233#BIBL
Citations This article has been cited by 1 HighWire-hosted articles:
http://jas.fass.org/cgi/content/full/81/12/3233#otherarticles
Downloaded from jas.fass.org by on January 3, 2011.
You might also like
- Articulo Ref-AOAC 2002.04Document24 pagesArticulo Ref-AOAC 2002.04ESTEBAN RESTREPONo ratings yet
- DRIS Concepts and Applications On Nutritional Diagnosis in Fruit CropsDocument11 pagesDRIS Concepts and Applications On Nutritional Diagnosis in Fruit CropsRómulo Del ValleNo ratings yet
- 3.protein QuantificationDocument12 pages3.protein Quantificationmc4207901No ratings yet
- Food Chemistry: Susanne Westenbrink, Kommer Brunt, Jan-Willem Van Der KampDocument6 pagesFood Chemistry: Susanne Westenbrink, Kommer Brunt, Jan-Willem Van Der KampChintong LylayNo ratings yet
- Revista Brasileira de Zootecnia: Mary Beth HallDocument9 pagesRevista Brasileira de Zootecnia: Mary Beth HallAdna AlineNo ratings yet
- Gayoso 2016Document11 pagesGayoso 2016marcela.gonzalezNo ratings yet
- 83a DeterminationofTotalDietaryFiberCODEXDefinitionbyEnzymatic-GravimetricMethodandLiquidChromatography CollaborativeStudyDocument14 pages83a DeterminationofTotalDietaryFiberCODEXDefinitionbyEnzymatic-GravimetricMethodandLiquidChromatography CollaborativeStudyValeriu MunteanuNo ratings yet
- J. Nutr.-2013-Schoenaker-392-8Document7 pagesJ. Nutr.-2013-Schoenaker-392-8Fadilla NurrahmaNo ratings yet
- Determination of Insoluble Soluble and T PDFDocument13 pagesDetermination of Insoluble Soluble and T PDFJoanuel QuinteroNo ratings yet
- A Note On The Evaluation of The Acid-Insoluble Ash Technique As A Method For Determining Apparent Diet Digestibility in Beef CattleDocument6 pagesA Note On The Evaluation of The Acid-Insoluble Ash Technique As A Method For Determining Apparent Diet Digestibility in Beef CattleAJ ManurungNo ratings yet
- PdcaasDocument8 pagesPdcaasTan Suk WanNo ratings yet
- Desjardins 2018Document11 pagesDesjardins 2018pseptinaNo ratings yet
- 2017 Consumo de FibrasDocument8 pages2017 Consumo de FibrasMariNo ratings yet
- Biochemical and Nutritional Analysis of The Leaf Extract of (L.) CorreaDocument5 pagesBiochemical and Nutritional Analysis of The Leaf Extract of (L.) Correarihana yadavNo ratings yet
- Studies Assessing Food Consumption by The Scores Method: A Systematic ReviewDocument16 pagesStudies Assessing Food Consumption by The Scores Method: A Systematic ReviewyutefupNo ratings yet
- Nut Esportiva 1 4Document29 pagesNut Esportiva 1 4Anderson AlvesNo ratings yet
- 2022 - Ibañez - Instrumental and Sensory Techniques To Characterize The Texture of FoodsDocument34 pages2022 - Ibañez - Instrumental and Sensory Techniques To Characterize The Texture of FoodsJuan P. CortésNo ratings yet
- Mccleary 2012Document21 pagesMccleary 2012frendystpNo ratings yet
- Feed EvaluationDocument17 pagesFeed EvaluationdagolddoyinsolaNo ratings yet
- Nutritional Metabolomics and The Classification of Dietary Biomarker Candidates: A Critical ReviewDocument25 pagesNutritional Metabolomics and The Classification of Dietary Biomarker Candidates: A Critical ReviewRum Afida RasfaNo ratings yet
- Ajcn 150847Document2 pagesAjcn 150847Betelhiem WoldemedhinNo ratings yet
- N. Thiex and C. R. Richardson: Challenges in Measuring Moisture Content of FeedsDocument14 pagesN. Thiex and C. R. Richardson: Challenges in Measuring Moisture Content of FeedsvratonNo ratings yet
- Van Soest 1979. Systems Analysis Evaluating Fibrous FeedDocument16 pagesVan Soest 1979. Systems Analysis Evaluating Fibrous FeedEugenia Campon100% (1)
- Calderon 2009Document7 pagesCalderon 2009Vero CastroNo ratings yet
- Dietary Assessment Toolkits An OverviewDocument16 pagesDietary Assessment Toolkits An OverviewKeneniNo ratings yet
- 2014 Fredin Et Al Fecal Starch Indicator Total Tract DigestibilityDocument11 pages2014 Fredin Et Al Fecal Starch Indicator Total Tract DigestibilityISAAC TORRES VELASCONo ratings yet
- 82 FullDocument17 pages82 FullBetelhiem WoldemedhinNo ratings yet
- Metodos de SeparacionDocument12 pagesMetodos de Separaciondan martinezNo ratings yet
- Ajcn/47 3 440Document8 pagesAjcn/47 3 440Hoàng Thị Thanh VânNo ratings yet
- Dietary FiberDocument5 pagesDietary FiberdeisifvNo ratings yet
- STROBE-nut Checklist TableDocument8 pagesSTROBE-nut Checklist TableChi MichNo ratings yet
- Firkins 1998Document20 pagesFirkins 1998YUSUF ADENIJINo ratings yet
- 15 3 16 - p.325 331 PDFDocument7 pages15 3 16 - p.325 331 PDFbeatcookNo ratings yet
- Aing93m Soybean OilDocument13 pagesAing93m Soybean OilMellysaNo ratings yet
- Accuracy of The Atwater Factors and Related Food Energy Conversion Factors With Low-Fat, High-Fiber Diets When Energy Intake Is Reduced SpontaneouslyDocument8 pagesAccuracy of The Atwater Factors and Related Food Energy Conversion Factors With Low-Fat, High-Fiber Diets When Energy Intake Is Reduced SpontaneouslyNatalia GutierrezNo ratings yet
- Laporan PBLDocument12 pagesLaporan PBLLuna MiraNo ratings yet
- Methods of Dietary and Nutritional Assessment and Intervention and Other Methods in The Multiple Risk Factor Intervention Trial1'2Document15 pagesMethods of Dietary and Nutritional Assessment and Intervention and Other Methods in The Multiple Risk Factor Intervention Trial1'2Daniel YirdawNo ratings yet
- AbsorcionDocument79 pagesAbsorcionDan Martínez SilvaNo ratings yet
- Spirulina ObesidadeDocument10 pagesSpirulina ObesidadeIsabela CastroNo ratings yet
- 1 s2.0 S2666833524000868 MainDocument11 pages1 s2.0 S2666833524000868 Maincristian.oglindaNo ratings yet
- 1 s2.0 S2001037014600477 MainDocument7 pages1 s2.0 S2001037014600477 MainBetelhiem WoldemedhinNo ratings yet
- Cost of The Diet A Method and Software To CalculatDocument18 pagesCost of The Diet A Method and Software To CalculatAbhi RajputNo ratings yet
- Article 5 - Dietary Assessment in Digital Age - McCullough M - AJCN 2018Document2 pagesArticle 5 - Dietary Assessment in Digital Age - McCullough M - AJCN 2018Prajna AdityaraniNo ratings yet
- Muscle DysmorphiaDocument6 pagesMuscle DysmorphiaKarla RojasNo ratings yet
- TehranDocument6 pagesTehranNadiah Umniati SyarifahNo ratings yet
- (Artigo) Digestibilidade ProteinaDocument7 pages(Artigo) Digestibilidade ProteinaKennedy LadeiaNo ratings yet
- Data in BriefDocument8 pagesData in BrieffadhilNo ratings yet
- Guía de Manejo Nutricional para La Enfermedad de La Orina Con Jarabe de Arce Un Enfoque Basado en La Evidencia y El ConsensoDocument8 pagesGuía de Manejo Nutricional para La Enfermedad de La Orina Con Jarabe de Arce Un Enfoque Basado en La Evidencia y El ConsensoPablo MorenoNo ratings yet
- International Society of Sports Nutrition Position Stand Diets and Body CompositionDocument19 pagesInternational Society of Sports Nutrition Position Stand Diets and Body CompositionLuis Ricardo Hernandez GarciaNo ratings yet
- Diabetes & Metabolic Syndrome: Clinical Research & ReviewsDocument7 pagesDiabetes & Metabolic Syndrome: Clinical Research & ReviewsTasha FarahNo ratings yet
- Original CommunicationDocument8 pagesOriginal CommunicationMohammedNo ratings yet
- Mango NectarDocument10 pagesMango NectarHoài Thương Nghiêm ThịNo ratings yet
- 2017 - Development and Validation of A Near Infrered Spectro Method To Determine Total Antioxidant in MilkDocument6 pages2017 - Development and Validation of A Near Infrered Spectro Method To Determine Total Antioxidant in MilklucianamartinezluqueNo ratings yet
- Biomarkers of Berry Intake: Systematic Review Update: AccessDocument17 pagesBiomarkers of Berry Intake: Systematic Review Update: AccessWILBERTH YOHEL PINEDO CALDASNo ratings yet
- IJFANS - Research Paper - RGST ProjectDocument8 pagesIJFANS - Research Paper - RGST ProjectDr. Neha Lohia SainiNo ratings yet
- Dietary Protein Quality Evaluation in Human NutritionFrom EverandDietary Protein Quality Evaluation in Human NutritionNo ratings yet
- Determination of Diphenylamine Residue in Fruit Samples Using Spectrofluorimetry and Multivariate AnalysisDocument7 pagesDetermination of Diphenylamine Residue in Fruit Samples Using Spectrofluorimetry and Multivariate AnalysisRodrigoNo ratings yet
- Journal of Fish Biology - 2019 - Amundsen - Feeding Studies Take Guts Critical Review and Recommendations of Methods ForDocument10 pagesJournal of Fish Biology - 2019 - Amundsen - Feeding Studies Take Guts Critical Review and Recommendations of Methods ForNiken Aura Dina SilangenNo ratings yet
- Iodine ICPMSDocument8 pagesIodine ICPMSYap Poh SiewNo ratings yet
- Inadequacy Food and SupplementsDocument7 pagesInadequacy Food and Supplementsgianluca.franceschini38No ratings yet
- Table Grape (Wiki 11 - 2 - 2020)Document4 pagesTable Grape (Wiki 11 - 2 - 2020)andi2rNo ratings yet
- Past Simple, Continuous, Present Perfect 13.12.23Document12 pagesPast Simple, Continuous, Present Perfect 13.12.23crowofdark455No ratings yet
- English Structure Basic PDFDocument120 pagesEnglish Structure Basic PDFdiyah fitri wulandariNo ratings yet
- FSSC 22000 v6 (FSMS) A-LA VILT TC - LG 17-08-2023Document373 pagesFSSC 22000 v6 (FSMS) A-LA VILT TC - LG 17-08-2023mohamed adel100% (1)
- Prof Ingles Ingreso2020 Introduccion Lengua InglesaDocument80 pagesProf Ingles Ingreso2020 Introduccion Lengua InglesaSalvador AcuñaNo ratings yet
- Oktoberfest Meeting Notes - July 28, 2010Document5 pagesOktoberfest Meeting Notes - July 28, 2010jberesNo ratings yet
- Used To/ Get Used To: Choose The Best Answer To Complete These Following SentencesDocument3 pagesUsed To/ Get Used To: Choose The Best Answer To Complete These Following SentencesJhon KeaNo ratings yet
- Punctuations QP 2Document3 pagesPunctuations QP 2Siti Nurain Mohd GhazaliNo ratings yet
- English 1 - Meeting 3 - Structure Present TenseDocument9 pagesEnglish 1 - Meeting 3 - Structure Present TenseRio HansNo ratings yet
- Ria Sweatha of Curry Leaf Aparna's BlogDocument36 pagesRia Sweatha of Curry Leaf Aparna's BlogcruciatusNo ratings yet
- Mueed Babar - ArticleDocument2 pagesMueed Babar - ArticleJunaid MalikNo ratings yet
- Project in EnglishDocument18 pagesProject in EnglishCrisanta BorbeNo ratings yet
- Chapter 1 and 3 Talabun Ak BriquettesDocument32 pagesChapter 1 and 3 Talabun Ak BriquettesROSELLE SIRUENo ratings yet
- Practice Book AnswersDocument6 pagesPractice Book Answersmohd razif ibrahimNo ratings yet
- New Magic Grammar 2BDocument34 pagesNew Magic Grammar 2BYume ZhuNo ratings yet
- GoutDocument16 pagesGoutfzee13No ratings yet
- Fish Nutrition PPT Title Defense 1Document17 pagesFish Nutrition PPT Title Defense 1Cristel Mae De GuzmanNo ratings yet
- The Typical Dishes From TarijaDocument4 pagesThe Typical Dishes From TarijaAlejandro RomeroNo ratings yet
- Consumer Affairs and Customer Care-1Document47 pagesConsumer Affairs and Customer Care-1Aditya PrakashNo ratings yet
- Spring Creek Sun December 3 2021Document24 pagesSpring Creek Sun December 3 2021amoses88No ratings yet
- Design and Development of Fresh Soybean Grinding and Filtration MachineDocument8 pagesDesign and Development of Fresh Soybean Grinding and Filtration Machinejenixson tamondongNo ratings yet
- Vanstiphout 1992 The Banquet Scene in The Mesopotamian Debate Poems ResOr 4Document8 pagesVanstiphout 1992 The Banquet Scene in The Mesopotamian Debate Poems ResOr 4Deniz ılgazNo ratings yet
- Borkar Super StoreDocument7 pagesBorkar Super StoreAshutosh SharmaNo ratings yet
- DW7 FoodDocument8 pagesDW7 FoodAlexander PeraltaNo ratings yet
- List Bolde Februari 2022Document6 pagesList Bolde Februari 2022Richard LiongNo ratings yet
- Ratios & ProportionsDocument11 pagesRatios & Proportionsmahesh.aptitudeNo ratings yet
- PEEEEDocument4 pagesPEEEEHaziel Joy GallegoNo ratings yet
- Đề 1Document5 pagesĐề 1PlumCatNo ratings yet
- Customer Behavior and Customer TrendDocument2 pagesCustomer Behavior and Customer TrendÁnh NgọcNo ratings yet
- Module 1 - CH 7 Gluten & MillingDocument25 pagesModule 1 - CH 7 Gluten & Millingaadityachandra1987No ratings yet