Professional Documents
Culture Documents
HW 10
HW 10
Uploaded by
pCopyright:
Available Formats
You might also like
- Wjec A2 Chemistry Study and Revision Guide PDFDocument125 pagesWjec A2 Chemistry Study and Revision Guide PDFPakorn WinayanuwattikunNo ratings yet
- Woodward Fieser RuleDocument28 pagesWoodward Fieser RuleRakesh Kotta100% (3)
- HW 10 AnsDocument4 pagesHW 10 AnspNo ratings yet
- Exam 4 Carbohydrate Sample Problems KeyDocument2 pagesExam 4 Carbohydrate Sample Problems KeyTJ SmithNo ratings yet
- FLASH CARDS 2-) GlucidesDocument8 pagesFLASH CARDS 2-) Glucidestytwf6kmghNo ratings yet
- No Experiment Tittle/ Subtittle Reagent Used Function of Reagent ReactionDocument9 pagesNo Experiment Tittle/ Subtittle Reagent Used Function of Reagent ReactionLailatul BadriyahNo ratings yet
- 7.014 Handout: Biochemistry I-IIIDocument8 pages7.014 Handout: Biochemistry I-IIIRiriiiNo ratings yet
- 11 Chapter 2Document20 pages11 Chapter 2NeelutpalGogoiNo ratings yet
- Chemical Composition and ConversionDocument25 pagesChemical Composition and ConversionrubyNo ratings yet
- Reducing Sugar Structure PDFDocument7 pagesReducing Sugar Structure PDFKrutikNo ratings yet
- Drawing Haworth ProjectionsDocument6 pagesDrawing Haworth ProjectionschoconoodlesNo ratings yet
- Exam 4 Carbohydrate Sample ProblemsDocument2 pagesExam 4 Carbohydrate Sample ProblemsTJ SmithNo ratings yet
- Magnetic Nanoparticles Derivatives From Jack Bean Starch: Swelling and Solubility Studies and Ftir PatternsDocument14 pagesMagnetic Nanoparticles Derivatives From Jack Bean Starch: Swelling and Solubility Studies and Ftir PatternsMojirade EngracedNo ratings yet
- 去Boc保护Document1 page去Boc保护angle6321No ratings yet
- Kraft Reactin-Dye SynthesisDocument7 pagesKraft Reactin-Dye Synthesisgulft1777No ratings yet
- Conjugated Bile Salts Liaflet 2020Document2 pagesConjugated Bile Salts Liaflet 2020Balachandar BNo ratings yet
- Chemical Constituents of Fraction E5, Ethyl Acetate Extract of The Bark ofDocument46 pagesChemical Constituents of Fraction E5, Ethyl Acetate Extract of The Bark ofTâmNo ratings yet
- Fisiologi TumbuhanDocument63 pagesFisiologi TumbuhanMasnawati WatiNo ratings yet
- Kraft Pulping and Recovery Process BasicsDocument50 pagesKraft Pulping and Recovery Process BasicsTri PutriNo ratings yet
- Tetrodotoxin: TTX: BackgroundDocument12 pagesTetrodotoxin: TTX: BackgroundMarrauNo ratings yet
- Week 13 WorkshopDocument3 pagesWeek 13 Workshoplayla_loveNo ratings yet
- Organic Chemistry ReactionsDocument5 pagesOrganic Chemistry ReactionsTiffany LiuNo ratings yet
- Analysis of Sugars PDFDocument29 pagesAnalysis of Sugars PDFKhalidNo ratings yet
- Analysis of Sugars PDFDocument29 pagesAnalysis of Sugars PDFVasco BrancoNo ratings yet
- Analysis of Sugars PDFDocument29 pagesAnalysis of Sugars PDFAbu RaihanNo ratings yet
- Analysis of SugarsDocument29 pagesAnalysis of SugarsgirishtyagiNo ratings yet
- E Content 18C6 Isophthalic Acid Derivative Cocrystals NLO ApplicationsDocument48 pagesE Content 18C6 Isophthalic Acid Derivative Cocrystals NLO ApplicationsbalaNo ratings yet
- Biochem Self AssessmentDocument3 pagesBiochem Self AssessmentAmabella ShaeNo ratings yet
- Fisher Projection Ball-&-Stick Structure Haworth Projection Chair/Envelope Conformation Complete NameDocument3 pagesFisher Projection Ball-&-Stick Structure Haworth Projection Chair/Envelope Conformation Complete NameAmabella ShaeNo ratings yet
- Fisher Projection Ball-&-Stick Structure Haworth Projection Chair/Envelope Conformation Complete NameDocument3 pagesFisher Projection Ball-&-Stick Structure Haworth Projection Chair/Envelope Conformation Complete NameAmabella ShaeNo ratings yet
- Learning ActivityDocument3 pagesLearning ActivityAmabella ShaeNo ratings yet
- Carbohydrates: Answers To QuestionsDocument3 pagesCarbohydrates: Answers To QuestionsGaby de GuzmanNo ratings yet
- Maillard Reaction of Milk Proteins-Wageningen University and Research 583115Document150 pagesMaillard Reaction of Milk Proteins-Wageningen University and Research 583115VanMarvasNo ratings yet
- Experiment 5 Properties of Carbohydrates: Solubility, Reactivity, and Specific RotationDocument9 pagesExperiment 5 Properties of Carbohydrates: Solubility, Reactivity, and Specific RotationCiara marie BernardoNo ratings yet
- B1.2 ProteinsDocument8 pagesB1.2 ProteinslittleianlauNo ratings yet
- Furans Biopolymer Materials-TfcDocument10 pagesFurans Biopolymer Materials-TfcGeorge Van BommelNo ratings yet
- Alcohols-Structure and Synthesis 2Document82 pagesAlcohols-Structure and Synthesis 2Ali Issa OthmanNo ratings yet
- Monosaccharides - Mind Maps - (JEE Ultimate CC 2.0 2023)Document1 pageMonosaccharides - Mind Maps - (JEE Ultimate CC 2.0 2023)ShahzadNo ratings yet
- P1-Hidrolisis de CarbohidratosDocument7 pagesP1-Hidrolisis de CarbohidratosgOnZo_86100% (1)
- Copia de Aldehyde ReactionsDocument5 pagesCopia de Aldehyde Reactionsileanajaiseh26No ratings yet
- 3 Activity Carbohyrdates IDocument10 pages3 Activity Carbohyrdates INur SetsuNo ratings yet
- OzonolysisDocument2 pagesOzonolysisKamaraj NaiduNo ratings yet
- C Ch-26 BiomoleculesDocument7 pagesC Ch-26 Biomoleculesmysoftinfo.incNo ratings yet
- Graphical Abstract-: BacosidesDocument5 pagesGraphical Abstract-: BacosidesDileep Kumar SharmaNo ratings yet
- Homework Problems: Structure, Bonding & Hybridization 1. The Molecule Shown Below Is Griseofulvin, An Antifungal CompoundDocument8 pagesHomework Problems: Structure, Bonding & Hybridization 1. The Molecule Shown Below Is Griseofulvin, An Antifungal CompoundPrachi KaushikNo ratings yet
- 315 Acid Cat MechsDocument13 pages315 Acid Cat MechsDeepak N SNo ratings yet
- 3-Introduction To Physiology Part II 2024Document36 pages3-Introduction To Physiology Part II 2024trieupg.22bi13431No ratings yet
- Exm N X11 Chem Biomol ADocument28 pagesExm N X11 Chem Biomol Asumair hejibNo ratings yet
- Steroid-4697 VFGGHDDGBJDocument15 pagesSteroid-4697 VFGGHDDGBJEdy PurnomoNo ratings yet
- Addition ReactionsDocument1 pageAddition ReactionsthirumalkothaNo ratings yet
- Guide To Fruit AcidsDocument1 pageGuide To Fruit AcidsJay McKearnNo ratings yet
- BIOMOLECULESDocument7 pagesBIOMOLECULESkanika guptaNo ratings yet
- Pertemuan 5 Reguler Pagi & Sore 2021-2022 - 2Document53 pagesPertemuan 5 Reguler Pagi & Sore 2021-2022 - 2Amelia Dwi Ramadhani AjitiaNo ratings yet
- Tutorial 7Document2 pagesTutorial 7Nicholas OwNo ratings yet
- Mechanisms 1-10: CHEM 725: Davey 1Document7 pagesMechanisms 1-10: CHEM 725: Davey 1Bradley DaveyNo ratings yet
- 6ALDOSASDocument1 page6ALDOSASapi-3700689No ratings yet
- Grafic Sea Cucumber AntelminticDocument1 pageGrafic Sea Cucumber AntelminticFeraCygCatzNo ratings yet
- Exam 4 Sample ProblemsDocument2 pagesExam 4 Sample ProblemsTJ SmithNo ratings yet
- Reaksi AlkenaDocument1 pageReaksi Alkenajoel13No ratings yet
- APIChem Featured Products PDFDocument5 pagesAPIChem Featured Products PDFTezozómocNo ratings yet
- Chemical Constituents of The Mexican Mistletoe (Psittacanthus Calyculatus)Document7 pagesChemical Constituents of The Mexican Mistletoe (Psittacanthus Calyculatus)Educación UniversidadNo ratings yet
- Course SummaryDocument152 pagesCourse SummarypNo ratings yet
- Organic Chemistry Lab Report: CHEM 203 - Fall 2017 Supervisor - Dr. Jay WickendenDocument1 pageOrganic Chemistry Lab Report: CHEM 203 - Fall 2017 Supervisor - Dr. Jay WickendenpNo ratings yet
- Nanh (1 Equiv.) 2. CH - CH - I H, Lindlar Catalyst Mcpba CH Li Cubr TSCL Pyridine NacnDocument12 pagesNanh (1 Equiv.) 2. CH - CH - I H, Lindlar Catalyst Mcpba CH Li Cubr TSCL Pyridine NacnpNo ratings yet
- Hcno C H H H C H H H C H H H: Remains +1 ThroughoutDocument4 pagesHcno C H H H C H H H C H H H: Remains +1 ThroughoutpNo ratings yet
- hw03 2017Document3 pageshw03 2017pNo ratings yet
- Chem 213 Practice Final Exam V3Document11 pagesChem 213 Practice Final Exam V3pNo ratings yet
- B, Best Depicts Pyridine N-Oxide? Why?Document2 pagesB, Best Depicts Pyridine N-Oxide? Why?pNo ratings yet
- CHEM 203 Midterm Exam 2Document7 pagesCHEM 203 Midterm Exam 2pNo ratings yet
- CHEM 203 Midterm Exam 2Document7 pagesCHEM 203 Midterm Exam 2pNo ratings yet
- HW 10 AnsDocument4 pagesHW 10 AnspNo ratings yet
- CHEM 203 Midterm Exam 2Document7 pagesCHEM 203 Midterm Exam 2pNo ratings yet
- IFOS NUA Biological Innovations NuaDHA 1000 Lot 3309-5-16 G1Document2 pagesIFOS NUA Biological Innovations NuaDHA 1000 Lot 3309-5-16 G1MayoNo ratings yet
- Mind Map of Organic Complete - 1Document1 pageMind Map of Organic Complete - 1Shalini 沙鹿 ShaluNo ratings yet
- CEE 370 Environmental Engineering PrinciplesDocument29 pagesCEE 370 Environmental Engineering PrinciplesARYAN_FATHONI_AMRINo ratings yet
- Marine Hazardous Substances Data System - Volume 2Document98 pagesMarine Hazardous Substances Data System - Volume 2Andrzej SzymańskiNo ratings yet
- Class 10 Chemistry Chapter 2 Revision NotesDocument2 pagesClass 10 Chemistry Chapter 2 Revision NotesMd TaaseenNo ratings yet
- Raw Mix DesignDocument53 pagesRaw Mix DesignDilnesa Ejigu100% (1)
- Chapter 5 Gastrointestinal Agents Reviewer PDFDocument6 pagesChapter 5 Gastrointestinal Agents Reviewer PDFdavenNo ratings yet
- Carbonyl Compounds - XII PDFDocument39 pagesCarbonyl Compounds - XII PDFMathoholic OjaNo ratings yet
- Synthsis PDFDocument30 pagesSynthsis PDF2967449CEENo ratings yet
- Elimination ReactionDocument89 pagesElimination ReactionManahil FatimaNo ratings yet
- Sulfite: Iodate-Iodide Method Method 8071 0-500 MG/L As SO (Or 0 To More Than 500 MG/L) Buret TitrationDocument6 pagesSulfite: Iodate-Iodide Method Method 8071 0-500 MG/L As SO (Or 0 To More Than 500 MG/L) Buret Titrationahmad sutejaNo ratings yet
- Nutritional and Antinutritional Composition of Adenopus Breviflorus Benth Seed Protein IsolateDocument7 pagesNutritional and Antinutritional Composition of Adenopus Breviflorus Benth Seed Protein IsolateIOSRjournalNo ratings yet
- SAM Organic Chemistry Amines, AmidesDocument11 pagesSAM Organic Chemistry Amines, AmidesChangWeiTanNo ratings yet
- Problem Set 3 - Alkanes and StereochemDocument6 pagesProblem Set 3 - Alkanes and StereochemKatrina Louise GonzalesNo ratings yet
- Corrosion Behavior of Carbon Steel in The Monoethanolamine-H2O-CO2-O2Document11 pagesCorrosion Behavior of Carbon Steel in The Monoethanolamine-H2O-CO2-O2gabriel norbertNo ratings yet
- Anaemia TabletsDocument4 pagesAnaemia TabletsReginald AhorluNo ratings yet
- Methyl BenzoateDocument4 pagesMethyl Benzoateapi-318284296No ratings yet
- Logsheet Lab Print Februari 2022Document3 pagesLogsheet Lab Print Februari 2022zainudin simonNo ratings yet
- Uop303 97Document7 pagesUop303 97Anix DiazNo ratings yet
- Experiment 2:: Complexometric TitrationsDocument6 pagesExperiment 2:: Complexometric TitrationsGhali El SamadNo ratings yet
- Synthesis - of - Terpineol - From - Pinene - by Homogeneous Acid CatalystDocument5 pagesSynthesis - of - Terpineol - From - Pinene - by Homogeneous Acid CatalystPhạm NgânNo ratings yet
- SaponinDocument1 pageSaponinIbrahim AlmesallamyNo ratings yet
- Ledapol Basic Lingerie and Clothing Collection 23Document108 pagesLedapol Basic Lingerie and Clothing Collection 23Latex, Leather and LaceNo ratings yet
- Sulfoximines From A Medicinal Chemists PerspectiveDocument21 pagesSulfoximines From A Medicinal Chemists PerspectiveMara AlananNo ratings yet
- Acc Scope NAL 056 - 2022Document9 pagesAcc Scope NAL 056 - 2022mezghichebiovitalNo ratings yet
- Dyeing Lab Department Calculation and Others - TEXTILE TECHNOLOGY ( ) PDFDocument10 pagesDyeing Lab Department Calculation and Others - TEXTILE TECHNOLOGY ( ) PDFshakilsai100% (1)
- Microsoft PowerPoint - SOLUTIONS - Midterm CoverageDocument10 pagesMicrosoft PowerPoint - SOLUTIONS - Midterm CoverageAnthony Luj MirandoNo ratings yet
- Chloride Ion Content Determination Kit PART NO. 144-40: InstructionsDocument4 pagesChloride Ion Content Determination Kit PART NO. 144-40: InstructionsWindy MartdianzahNo ratings yet
HW 10
HW 10
Uploaded by
pOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
HW 10
HW 10
Uploaded by
pCopyright:
Available Formats
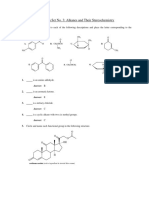
CHEM 203 HOMEWORK 10 Carbohydrates
1. Write the structure of:
a. A Fischer projection of D-galactose:
HO OH
H
O
H
HO
HO H OH
H
D-galactose
b. The more thermodynamically stable pyranose form of D -xylose:
CHO
H OH
HO H
H OH
OH
D-xylose
c. The α-methyl glycoside derivative of D-glucose:
CHO
H OH
HO H
H OH
H OH
OH
D-glucose
d. A Fischer projection of any D -aldopentose and one of any L-aldopentose
e. A Fischer projection of a D-ketohexose
CHEM 203 HOMEWORK 10 p. 2
2. Indicate whether the following carbohydrates are reducing or nonreducing
OH
HO O
HO OH HO OH
O H O OH
O O HO HO
HO H HO OH O O
HO HO O
O HO HO
HO H OH
HO OH HO OH
H OH
HO
3. Predict the product(s) of the following reactions:
H OH OH
OH Ag(NH3)2NO3 OH
O O
HO
a. HO HO
aq. NH3
H H OH HO
H O
d. O dil. aq.
H HO
H OH
CH3CH2OH HO H2SO4
O
HO HO
b. HO HO
cat. H2SO4 OH O
HO H OH – H2O O
H
OH
HO OH HO
H
O aq. NH3 excess NaH
H e. product of
c. HO question b.
HO H OH excess CH3I
H
4. The world demand for aminoacids is so large that many such compounds have to be produced by synthesis,
rather than by extraction from naturally occurring proteinaceous material. A key reaction in the industrial
preparation of aminoacids is the so-called Strecker synthesis (diagram below). Thus, an aldehyde, e.g.,
phenylacetaldehye, A, ammonia, ammonium chloride (pKa ≈ 9), and hydrogen cyanide (pKa ≈ 10) are
combined to give an aminonitrile, B, which is then transformed into an aminoacid, e.g., phenylalanine, C,
by a reaction that you will study in CHEM 213. Propose a mechanism for the formation of B.
NH3, NH4Cl CN COOH
CHO
HCN NH2 NH2
A B C
note: meterorites contain several simple aminoacids, which are believed to form by a Strecker reaction
in outer space, where simple aldehydes, ammonia, and HCN are common.
5. Propose a method to achieve the following transformations:
CN
a. b.
CH3O O OCH3 OH
You might also like
- Wjec A2 Chemistry Study and Revision Guide PDFDocument125 pagesWjec A2 Chemistry Study and Revision Guide PDFPakorn WinayanuwattikunNo ratings yet
- Woodward Fieser RuleDocument28 pagesWoodward Fieser RuleRakesh Kotta100% (3)
- HW 10 AnsDocument4 pagesHW 10 AnspNo ratings yet
- Exam 4 Carbohydrate Sample Problems KeyDocument2 pagesExam 4 Carbohydrate Sample Problems KeyTJ SmithNo ratings yet
- FLASH CARDS 2-) GlucidesDocument8 pagesFLASH CARDS 2-) Glucidestytwf6kmghNo ratings yet
- No Experiment Tittle/ Subtittle Reagent Used Function of Reagent ReactionDocument9 pagesNo Experiment Tittle/ Subtittle Reagent Used Function of Reagent ReactionLailatul BadriyahNo ratings yet
- 7.014 Handout: Biochemistry I-IIIDocument8 pages7.014 Handout: Biochemistry I-IIIRiriiiNo ratings yet
- 11 Chapter 2Document20 pages11 Chapter 2NeelutpalGogoiNo ratings yet
- Chemical Composition and ConversionDocument25 pagesChemical Composition and ConversionrubyNo ratings yet
- Reducing Sugar Structure PDFDocument7 pagesReducing Sugar Structure PDFKrutikNo ratings yet
- Drawing Haworth ProjectionsDocument6 pagesDrawing Haworth ProjectionschoconoodlesNo ratings yet
- Exam 4 Carbohydrate Sample ProblemsDocument2 pagesExam 4 Carbohydrate Sample ProblemsTJ SmithNo ratings yet
- Magnetic Nanoparticles Derivatives From Jack Bean Starch: Swelling and Solubility Studies and Ftir PatternsDocument14 pagesMagnetic Nanoparticles Derivatives From Jack Bean Starch: Swelling and Solubility Studies and Ftir PatternsMojirade EngracedNo ratings yet
- 去Boc保护Document1 page去Boc保护angle6321No ratings yet
- Kraft Reactin-Dye SynthesisDocument7 pagesKraft Reactin-Dye Synthesisgulft1777No ratings yet
- Conjugated Bile Salts Liaflet 2020Document2 pagesConjugated Bile Salts Liaflet 2020Balachandar BNo ratings yet
- Chemical Constituents of Fraction E5, Ethyl Acetate Extract of The Bark ofDocument46 pagesChemical Constituents of Fraction E5, Ethyl Acetate Extract of The Bark ofTâmNo ratings yet
- Fisiologi TumbuhanDocument63 pagesFisiologi TumbuhanMasnawati WatiNo ratings yet
- Kraft Pulping and Recovery Process BasicsDocument50 pagesKraft Pulping and Recovery Process BasicsTri PutriNo ratings yet
- Tetrodotoxin: TTX: BackgroundDocument12 pagesTetrodotoxin: TTX: BackgroundMarrauNo ratings yet
- Week 13 WorkshopDocument3 pagesWeek 13 Workshoplayla_loveNo ratings yet
- Organic Chemistry ReactionsDocument5 pagesOrganic Chemistry ReactionsTiffany LiuNo ratings yet
- Analysis of Sugars PDFDocument29 pagesAnalysis of Sugars PDFKhalidNo ratings yet
- Analysis of Sugars PDFDocument29 pagesAnalysis of Sugars PDFVasco BrancoNo ratings yet
- Analysis of Sugars PDFDocument29 pagesAnalysis of Sugars PDFAbu RaihanNo ratings yet
- Analysis of SugarsDocument29 pagesAnalysis of SugarsgirishtyagiNo ratings yet
- E Content 18C6 Isophthalic Acid Derivative Cocrystals NLO ApplicationsDocument48 pagesE Content 18C6 Isophthalic Acid Derivative Cocrystals NLO ApplicationsbalaNo ratings yet
- Biochem Self AssessmentDocument3 pagesBiochem Self AssessmentAmabella ShaeNo ratings yet
- Fisher Projection Ball-&-Stick Structure Haworth Projection Chair/Envelope Conformation Complete NameDocument3 pagesFisher Projection Ball-&-Stick Structure Haworth Projection Chair/Envelope Conformation Complete NameAmabella ShaeNo ratings yet
- Fisher Projection Ball-&-Stick Structure Haworth Projection Chair/Envelope Conformation Complete NameDocument3 pagesFisher Projection Ball-&-Stick Structure Haworth Projection Chair/Envelope Conformation Complete NameAmabella ShaeNo ratings yet
- Learning ActivityDocument3 pagesLearning ActivityAmabella ShaeNo ratings yet
- Carbohydrates: Answers To QuestionsDocument3 pagesCarbohydrates: Answers To QuestionsGaby de GuzmanNo ratings yet
- Maillard Reaction of Milk Proteins-Wageningen University and Research 583115Document150 pagesMaillard Reaction of Milk Proteins-Wageningen University and Research 583115VanMarvasNo ratings yet
- Experiment 5 Properties of Carbohydrates: Solubility, Reactivity, and Specific RotationDocument9 pagesExperiment 5 Properties of Carbohydrates: Solubility, Reactivity, and Specific RotationCiara marie BernardoNo ratings yet
- B1.2 ProteinsDocument8 pagesB1.2 ProteinslittleianlauNo ratings yet
- Furans Biopolymer Materials-TfcDocument10 pagesFurans Biopolymer Materials-TfcGeorge Van BommelNo ratings yet
- Alcohols-Structure and Synthesis 2Document82 pagesAlcohols-Structure and Synthesis 2Ali Issa OthmanNo ratings yet
- Monosaccharides - Mind Maps - (JEE Ultimate CC 2.0 2023)Document1 pageMonosaccharides - Mind Maps - (JEE Ultimate CC 2.0 2023)ShahzadNo ratings yet
- P1-Hidrolisis de CarbohidratosDocument7 pagesP1-Hidrolisis de CarbohidratosgOnZo_86100% (1)
- Copia de Aldehyde ReactionsDocument5 pagesCopia de Aldehyde Reactionsileanajaiseh26No ratings yet
- 3 Activity Carbohyrdates IDocument10 pages3 Activity Carbohyrdates INur SetsuNo ratings yet
- OzonolysisDocument2 pagesOzonolysisKamaraj NaiduNo ratings yet
- C Ch-26 BiomoleculesDocument7 pagesC Ch-26 Biomoleculesmysoftinfo.incNo ratings yet
- Graphical Abstract-: BacosidesDocument5 pagesGraphical Abstract-: BacosidesDileep Kumar SharmaNo ratings yet
- Homework Problems: Structure, Bonding & Hybridization 1. The Molecule Shown Below Is Griseofulvin, An Antifungal CompoundDocument8 pagesHomework Problems: Structure, Bonding & Hybridization 1. The Molecule Shown Below Is Griseofulvin, An Antifungal CompoundPrachi KaushikNo ratings yet
- 315 Acid Cat MechsDocument13 pages315 Acid Cat MechsDeepak N SNo ratings yet
- 3-Introduction To Physiology Part II 2024Document36 pages3-Introduction To Physiology Part II 2024trieupg.22bi13431No ratings yet
- Exm N X11 Chem Biomol ADocument28 pagesExm N X11 Chem Biomol Asumair hejibNo ratings yet
- Steroid-4697 VFGGHDDGBJDocument15 pagesSteroid-4697 VFGGHDDGBJEdy PurnomoNo ratings yet
- Addition ReactionsDocument1 pageAddition ReactionsthirumalkothaNo ratings yet
- Guide To Fruit AcidsDocument1 pageGuide To Fruit AcidsJay McKearnNo ratings yet
- BIOMOLECULESDocument7 pagesBIOMOLECULESkanika guptaNo ratings yet
- Pertemuan 5 Reguler Pagi & Sore 2021-2022 - 2Document53 pagesPertemuan 5 Reguler Pagi & Sore 2021-2022 - 2Amelia Dwi Ramadhani AjitiaNo ratings yet
- Tutorial 7Document2 pagesTutorial 7Nicholas OwNo ratings yet
- Mechanisms 1-10: CHEM 725: Davey 1Document7 pagesMechanisms 1-10: CHEM 725: Davey 1Bradley DaveyNo ratings yet
- 6ALDOSASDocument1 page6ALDOSASapi-3700689No ratings yet
- Grafic Sea Cucumber AntelminticDocument1 pageGrafic Sea Cucumber AntelminticFeraCygCatzNo ratings yet
- Exam 4 Sample ProblemsDocument2 pagesExam 4 Sample ProblemsTJ SmithNo ratings yet
- Reaksi AlkenaDocument1 pageReaksi Alkenajoel13No ratings yet
- APIChem Featured Products PDFDocument5 pagesAPIChem Featured Products PDFTezozómocNo ratings yet
- Chemical Constituents of The Mexican Mistletoe (Psittacanthus Calyculatus)Document7 pagesChemical Constituents of The Mexican Mistletoe (Psittacanthus Calyculatus)Educación UniversidadNo ratings yet
- Course SummaryDocument152 pagesCourse SummarypNo ratings yet
- Organic Chemistry Lab Report: CHEM 203 - Fall 2017 Supervisor - Dr. Jay WickendenDocument1 pageOrganic Chemistry Lab Report: CHEM 203 - Fall 2017 Supervisor - Dr. Jay WickendenpNo ratings yet
- Nanh (1 Equiv.) 2. CH - CH - I H, Lindlar Catalyst Mcpba CH Li Cubr TSCL Pyridine NacnDocument12 pagesNanh (1 Equiv.) 2. CH - CH - I H, Lindlar Catalyst Mcpba CH Li Cubr TSCL Pyridine NacnpNo ratings yet
- Hcno C H H H C H H H C H H H: Remains +1 ThroughoutDocument4 pagesHcno C H H H C H H H C H H H: Remains +1 ThroughoutpNo ratings yet
- hw03 2017Document3 pageshw03 2017pNo ratings yet
- Chem 213 Practice Final Exam V3Document11 pagesChem 213 Practice Final Exam V3pNo ratings yet
- B, Best Depicts Pyridine N-Oxide? Why?Document2 pagesB, Best Depicts Pyridine N-Oxide? Why?pNo ratings yet
- CHEM 203 Midterm Exam 2Document7 pagesCHEM 203 Midterm Exam 2pNo ratings yet
- CHEM 203 Midterm Exam 2Document7 pagesCHEM 203 Midterm Exam 2pNo ratings yet
- HW 10 AnsDocument4 pagesHW 10 AnspNo ratings yet
- CHEM 203 Midterm Exam 2Document7 pagesCHEM 203 Midterm Exam 2pNo ratings yet
- IFOS NUA Biological Innovations NuaDHA 1000 Lot 3309-5-16 G1Document2 pagesIFOS NUA Biological Innovations NuaDHA 1000 Lot 3309-5-16 G1MayoNo ratings yet
- Mind Map of Organic Complete - 1Document1 pageMind Map of Organic Complete - 1Shalini 沙鹿 ShaluNo ratings yet
- CEE 370 Environmental Engineering PrinciplesDocument29 pagesCEE 370 Environmental Engineering PrinciplesARYAN_FATHONI_AMRINo ratings yet
- Marine Hazardous Substances Data System - Volume 2Document98 pagesMarine Hazardous Substances Data System - Volume 2Andrzej SzymańskiNo ratings yet
- Class 10 Chemistry Chapter 2 Revision NotesDocument2 pagesClass 10 Chemistry Chapter 2 Revision NotesMd TaaseenNo ratings yet
- Raw Mix DesignDocument53 pagesRaw Mix DesignDilnesa Ejigu100% (1)
- Chapter 5 Gastrointestinal Agents Reviewer PDFDocument6 pagesChapter 5 Gastrointestinal Agents Reviewer PDFdavenNo ratings yet
- Carbonyl Compounds - XII PDFDocument39 pagesCarbonyl Compounds - XII PDFMathoholic OjaNo ratings yet
- Synthsis PDFDocument30 pagesSynthsis PDF2967449CEENo ratings yet
- Elimination ReactionDocument89 pagesElimination ReactionManahil FatimaNo ratings yet
- Sulfite: Iodate-Iodide Method Method 8071 0-500 MG/L As SO (Or 0 To More Than 500 MG/L) Buret TitrationDocument6 pagesSulfite: Iodate-Iodide Method Method 8071 0-500 MG/L As SO (Or 0 To More Than 500 MG/L) Buret Titrationahmad sutejaNo ratings yet
- Nutritional and Antinutritional Composition of Adenopus Breviflorus Benth Seed Protein IsolateDocument7 pagesNutritional and Antinutritional Composition of Adenopus Breviflorus Benth Seed Protein IsolateIOSRjournalNo ratings yet
- SAM Organic Chemistry Amines, AmidesDocument11 pagesSAM Organic Chemistry Amines, AmidesChangWeiTanNo ratings yet
- Problem Set 3 - Alkanes and StereochemDocument6 pagesProblem Set 3 - Alkanes and StereochemKatrina Louise GonzalesNo ratings yet
- Corrosion Behavior of Carbon Steel in The Monoethanolamine-H2O-CO2-O2Document11 pagesCorrosion Behavior of Carbon Steel in The Monoethanolamine-H2O-CO2-O2gabriel norbertNo ratings yet
- Anaemia TabletsDocument4 pagesAnaemia TabletsReginald AhorluNo ratings yet
- Methyl BenzoateDocument4 pagesMethyl Benzoateapi-318284296No ratings yet
- Logsheet Lab Print Februari 2022Document3 pagesLogsheet Lab Print Februari 2022zainudin simonNo ratings yet
- Uop303 97Document7 pagesUop303 97Anix DiazNo ratings yet
- Experiment 2:: Complexometric TitrationsDocument6 pagesExperiment 2:: Complexometric TitrationsGhali El SamadNo ratings yet
- Synthesis - of - Terpineol - From - Pinene - by Homogeneous Acid CatalystDocument5 pagesSynthesis - of - Terpineol - From - Pinene - by Homogeneous Acid CatalystPhạm NgânNo ratings yet
- SaponinDocument1 pageSaponinIbrahim AlmesallamyNo ratings yet
- Ledapol Basic Lingerie and Clothing Collection 23Document108 pagesLedapol Basic Lingerie and Clothing Collection 23Latex, Leather and LaceNo ratings yet
- Sulfoximines From A Medicinal Chemists PerspectiveDocument21 pagesSulfoximines From A Medicinal Chemists PerspectiveMara AlananNo ratings yet
- Acc Scope NAL 056 - 2022Document9 pagesAcc Scope NAL 056 - 2022mezghichebiovitalNo ratings yet
- Dyeing Lab Department Calculation and Others - TEXTILE TECHNOLOGY ( ) PDFDocument10 pagesDyeing Lab Department Calculation and Others - TEXTILE TECHNOLOGY ( ) PDFshakilsai100% (1)
- Microsoft PowerPoint - SOLUTIONS - Midterm CoverageDocument10 pagesMicrosoft PowerPoint - SOLUTIONS - Midterm CoverageAnthony Luj MirandoNo ratings yet
- Chloride Ion Content Determination Kit PART NO. 144-40: InstructionsDocument4 pagesChloride Ion Content Determination Kit PART NO. 144-40: InstructionsWindy MartdianzahNo ratings yet