Professional Documents
Culture Documents
18Mec101T Thermodynamics Week 1 Assignment Questions
18Mec101T Thermodynamics Week 1 Assignment Questions
Uploaded by
Saurav Das0 ratings0% found this document useful (0 votes)
20 views1 pageThis document contains 7 thermodynamics assignment questions about different topics:
1) Types of systems and examples of each
2) Definition of a quasi-static process
3) Derivation of an expression for work in a non-flow reversible adiabatic process
4) Calculation of various properties for a gas expanded adiabatically including mass, temperature, work, internal energy change, and enthalpy change
Original Description:
Original Title
ACFrOgB6DGRrunqlnKtJGrDwUrZJHyWlPLXjf263p2ZkoQcBDysHtvwmvfQpnByhzOSJ0vGJ6c8LM2Q3-F3KqTLV0p468V9-wU8Dpz9AsjmTe8d3rxiuGdUypJWC4OQc7aVoeHZ9ENu1Y5ydyNqx.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document contains 7 thermodynamics assignment questions about different topics:
1) Types of systems and examples of each
2) Definition of a quasi-static process
3) Derivation of an expression for work in a non-flow reversible adiabatic process
4) Calculation of various properties for a gas expanded adiabatically including mass, temperature, work, internal energy change, and enthalpy change
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
20 views1 page18Mec101T Thermodynamics Week 1 Assignment Questions
18Mec101T Thermodynamics Week 1 Assignment Questions
Uploaded by
Saurav DasThis document contains 7 thermodynamics assignment questions about different topics:
1) Types of systems and examples of each
2) Definition of a quasi-static process
3) Derivation of an expression for work in a non-flow reversible adiabatic process
4) Calculation of various properties for a gas expanded adiabatically including mass, temperature, work, internal energy change, and enthalpy change
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
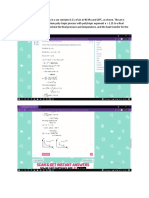
18MEC101T THERMODYNAMICS
Week 1 Assignment Questions
1. What are the three types of systems? Give example for each.
2. Define quasi-static process.
3. Derive an expression for the work done in a non flow reversible
adiabatic process.
4. A quantity of gas occupying 0.14 m3 at a pressure of 1400 kPa and
temperature of 3000 C is expanded adiabatically to 280 kPa. If for this
gas Cp = 1.005 kJ/kgK , Cv = 0.74 kJ/kgK, determine mass of the gas,
temperature after expansion, work done during expansion, change in
internal energy and change in enthalpy.
5. A mass of gas is compressed in a quasi-static process from 80 kPa,
0.2 m3 to 4 bar, 0.03 m3. Assuming that the pressure and volume are
related by pvn = C, find the work done by the gas system.
6. A mass of 5 kg of air is compressed in a quasi-static process from
1 MPa to 5 MPa for which PV = C. The initial density of air is
1.15 kg/m3. Find the work done and heat transfer for the process.
7. A gas is contained in a piston cylinder arrangement. Initial volume of the
gas is 0.5 m3. It is compressed from 1 bar to 10 bar such that the
temperature remains constant. Find the final volume and work done?
You might also like
- Course Work Tft-1Document4 pagesCourse Work Tft-1Ahmad HashemNo ratings yet
- Thermo ProblemsDocument12 pagesThermo ProblemsElaineNo ratings yet
- Question Bank (Numericals)Document12 pagesQuestion Bank (Numericals)Omid Karimi SadaghianiNo ratings yet
- 1 FormatsDocument1 page1 FormatsmsloveindiaNo ratings yet
- 1.1 Prob Sheet Energy Analysis of Closed SystemsDocument3 pages1.1 Prob Sheet Energy Analysis of Closed SystemsAnonymous mXicTi8hBNo ratings yet
- Eme Question Bank 08Document16 pagesEme Question Bank 08عبدالله عمرNo ratings yet
- Sheet 3 Work CalculationDocument3 pagesSheet 3 Work CalculationHassan El SayedNo ratings yet
- Problem Set - Thermodynamics & ICEDocument2 pagesProblem Set - Thermodynamics & ICEBea Therese RadubanNo ratings yet
- Our Official Android App - REJINPAUL NETWORK FromDocument2 pagesOur Official Android App - REJINPAUL NETWORK FromPradeep KumarNo ratings yet
- QB Unit 1Document6 pagesQB Unit 1Gaurav GadhesariaNo ratings yet
- Work Sheet 3 of ThermodynamicsDocument3 pagesWork Sheet 3 of ThermodynamicsTsedey bereketNo ratings yet
- Engineering Thermodynamics ProblemsDocument3 pagesEngineering Thermodynamics Problemsdhruv0010% (1)
- Me8391 Engineering Thermodynamics Tutorial - I Me8391 Engineering Thermodynamics Tutorial - IDocument1 pageMe8391 Engineering Thermodynamics Tutorial - I Me8391 Engineering Thermodynamics Tutorial - IBuckshu PhdNo ratings yet
- Work Sheet1Document4 pagesWork Sheet1Tesfa negaNo ratings yet
- ThermodynamicsDocument2 pagesThermodynamicsKathryn BellNo ratings yet
- I3611TT - Tutorial 2Document6 pagesI3611TT - Tutorial 2Rebekka Ndahafa100% (1)
- XXDocument1 pageXXGetachew TikueNo ratings yet
- XXDocument1 pageXXGetachew TikueNo ratings yet
- Assignment 1Document2 pagesAssignment 1Văn TrầnNo ratings yet
- Question Bank-Thermal EngineeringDocument4 pagesQuestion Bank-Thermal EngineeringIrfan ShaikhNo ratings yet
- ME 266 Tutorial Questions Set 1Document3 pagesME 266 Tutorial Questions Set 1Eric AnsahNo ratings yet
- Etd Ti, T2.T3Document5 pagesEtd Ti, T2.T3Kumaran RNo ratings yet
- Tutorial 7 (Lecture 7, Chapter 7) Engineering Mechanics DepartmentDocument3 pagesTutorial 7 (Lecture 7, Chapter 7) Engineering Mechanics DepartmentAhmedSeragNo ratings yet
- Assignment 1 - Work DoneDocument2 pagesAssignment 1 - Work DoneShubhenduGuptaNo ratings yet
- Thermodynamics Questions and AnswersDocument5 pagesThermodynamics Questions and AnswersMD SHOEBUDDIN0% (1)
- Assignment 2Document2 pagesAssignment 2api-3802845No ratings yet
- Processes and Carnot CycleDocument4 pagesProcesses and Carnot CycleRagh AhmedNo ratings yet
- Thermo QuestionsDocument3 pagesThermo QuestionsHimanshu VasisthaNo ratings yet
- EXAMDocument1 pageEXAMkelly evangelistaNo ratings yet
- Etd Question-2Document2 pagesEtd Question-2karthiyuvenNo ratings yet
- TD Tutorial 2018 EditedDocument34 pagesTD Tutorial 2018 EditedRochakNo ratings yet
- In A Gas TurbineDocument7 pagesIn A Gas TurbineANBU RAJ ANo ratings yet
- Me3391-Engineering Thermodynamics-805217166-Important Question For Engineering ThermodynamicsDocument10 pagesMe3391-Engineering Thermodynamics-805217166-Important Question For Engineering ThermodynamicsRamakrishnan NNo ratings yet
- Assignment 1 ThermodynamicsDocument1 pageAssignment 1 ThermodynamicsMarvin BayanayNo ratings yet
- MEG 212 Practise QuestionsdocxDocument11 pagesMEG 212 Practise Questionsdocxoyetunde ridwanNo ratings yet
- Day 22 - Thermodynamics 2 L Lecture ProblemsDocument2 pagesDay 22 - Thermodynamics 2 L Lecture Problemsj4240300No ratings yet
- Question Bank MechDocument102 pagesQuestion Bank MechKaradam PatelNo ratings yet
- Thermo 2Document2 pagesThermo 2kj gandaNo ratings yet
- 11me201 Thermodynamics QuestionsDocument12 pages11me201 Thermodynamics QuestionsramsastryNo ratings yet
- Unit4 - NotesDocument38 pagesUnit4 - NotesKrishna AgarwalNo ratings yet
- ThermoDocument4 pagesThermowong zhi chengNo ratings yet
- Engineering ThermodynamicsDocument21 pagesEngineering Thermodynamicsrkrajesh86No ratings yet
- QUESTION BANK ThermodynamicsDocument6 pagesQUESTION BANK Thermodynamicsvikas_1989No ratings yet
- Contoh Soal Termo - Tk.Document17 pagesContoh Soal Termo - Tk.dit doankNo ratings yet
- Supplementary Problems For Practice: 1. A Mass of 0.15 KG of Air Is Initially Exists at 2 Mpa and 350Document1 pageSupplementary Problems For Practice: 1. A Mass of 0.15 KG of Air Is Initially Exists at 2 Mpa and 350physics a2No ratings yet
- Assignment No. 4Document2 pagesAssignment No. 4Charie EralinoNo ratings yet
- Basics of ThermodynamicsDocument36 pagesBasics of ThermodynamicsYeditha Satyanarayana MurthyNo ratings yet
- TDCE Question Bank - 2018 Unit IDocument11 pagesTDCE Question Bank - 2018 Unit IvinodNo ratings yet
- KF 1Document19 pagesKF 1Diana Fitriani SurtikaNo ratings yet
- Thermodynamics QuestionsDocument4 pagesThermodynamics Questionsprateek vyasNo ratings yet
- Adamson UniversityDocument3 pagesAdamson UniversityVanessa Elaine CaoNo ratings yet
- Plate5 SpecialDocument1 pagePlate5 SpecialDG Deku YTNo ratings yet
- ThermoDocument5 pagesThermoTerry Clarice DecatoriaNo ratings yet
- Pset 1 CombustionDocument13 pagesPset 1 CombustionMicaella Jaime De LeonNo ratings yet
- MMAN2700ThermoProblemSheet6 - 1st Law Steady FlowDocument3 pagesMMAN2700ThermoProblemSheet6 - 1st Law Steady Flowgrandw9524No ratings yet
- Thermodynamics 1Document5 pagesThermodynamics 1ArgielJohn LlagasNo ratings yet
- Unit IDocument5 pagesUnit INallappan Rajj ANo ratings yet