Professional Documents
Culture Documents
Properties of Pure Substances: Chapter IV
Properties of Pure Substances: Chapter IV
Uploaded by
Liz Arfin0 ratings0% found this document useful (0 votes)
69 views1 pageThe document summarizes a thermodynamics problem involving the isothermal expansion of refrigerant 12. Key details include:
- Refrigerant 12 is expanded at a constant temperature from a wet saturated vapor state at 80% quality to 70°C and 200 kPa.
- The flow rate is 13.6 kg/min, the change in kinetic energy is 3.5 kJ/kg, and heat is added at a rate of 21.81 kW.
- The problem asks to determine the system power based on the given states and properties of refrigerant 12 during the process.
Original Description:
Original Title
Thermo Solutions_Part74.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe document summarizes a thermodynamics problem involving the isothermal expansion of refrigerant 12. Key details include:
- Refrigerant 12 is expanded at a constant temperature from a wet saturated vapor state at 80% quality to 70°C and 200 kPa.
- The flow rate is 13.6 kg/min, the change in kinetic energy is 3.5 kJ/kg, and heat is added at a rate of 21.81 kW.
- The problem asks to determine the system power based on the given states and properties of refrigerant 12 during the process.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
69 views1 pageProperties of Pure Substances: Chapter IV
Properties of Pure Substances: Chapter IV
Uploaded by
Liz ArfinThe document summarizes a thermodynamics problem involving the isothermal expansion of refrigerant 12. Key details include:
- Refrigerant 12 is expanded at a constant temperature from a wet saturated vapor state at 80% quality to 70°C and 200 kPa.
- The flow rate is 13.6 kg/min, the change in kinetic energy is 3.5 kJ/kg, and heat is added at a rate of 21.81 kW.
- The problem asks to determine the system power based on the given states and properties of refrigerant 12 during the process.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
Chapter IV - PROPERTIES OF PURE SUBSTANCES
Problem 4.29
Refrigerant 12 is expanded steadily in an isothermal process. The flow rate is 13.6
kg/min with an inlet state of wet saturated vapor with an 80% quality to a final state
of 70°C and 200 kPa. The change of kinetic energy across the device is 3.5 kJ/kg and
the heat added is 21.81 kW. Determine the system power.
Given: R 12 being expanded isothermally with heat addition and change in kinetic
energy.
Find: Power.
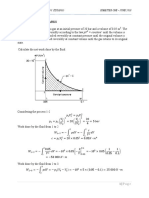
Sketch & Given Data:
T=C. 1a., ks/"'
I
Akt. • 3.S" kJ/"5
Q
Assumptions: 1) R 12 is in equilibrium.
2) Change in potential energy is negligible.
Analysis: Using Appendix All to find initial enthalpy.
he = 107.067 kJ/kg heg = 104.255 kJ/kg
h1 = hr + X hrg = 107.067 kJ/kg + (0.8)(104.255 kJ/kg)
= 190.471 kJ/kg
Using Appendix A12 to find exit enthalpy.
h2 = 234.291 kJ/kg
Writing first law equation for the open system.
13 6
= 21.81kw + ( · kg/m) (190.471kJ/kg-234.291kg/kg)
60s/m
+ ( 13.6kg/m) ( _3 _SkJ/kg)
60s/m
=11.08kw
4-46
You might also like
- TD WorksheetDocument4 pagesTD WorksheetrtyiookNo ratings yet
- Chapter 9 Examples&SolutionDocument42 pagesChapter 9 Examples&SolutionSami ullahNo ratings yet
- HW 9 Solutions - Chapter 7Document4 pagesHW 9 Solutions - Chapter 7goodfellas121No ratings yet
- Mer 231 Steady Flow ExamplesDocument9 pagesMer 231 Steady Flow ExamplesOmar CorralNo ratings yet
- Thermodynamics 3Document23 pagesThermodynamics 3Jo Ces27% (11)
- Thermochemistry: QuestionsDocument32 pagesThermochemistry: QuestionsBe like BruhNo ratings yet
- Solutions 4 F14Document5 pagesSolutions 4 F14nageshNo ratings yet
- A7 - 20-SEP-2016 - RM001 - POCE5 - Module-4-Energy Balance-NumericalsDocument17 pagesA7 - 20-SEP-2016 - RM001 - POCE5 - Module-4-Energy Balance-NumericalssantoshNo ratings yet
- ch14 PDFDocument8 pagesch14 PDFAkash ThummarNo ratings yet
- Solution Manual For Chemistry 10th by ZumdahlDocument33 pagesSolution Manual For Chemistry 10th by ZumdahlAmandaHarrissftia100% (97)
- HW 13Document5 pagesHW 13Maria Mikaela PelagioNo ratings yet
- Week 7Document6 pagesWeek 7shmyeNo ratings yet
- MME 3334b Assignment #1: P 160 Bar, H H VP P 190.01 KJ / KGDocument5 pagesMME 3334b Assignment #1: P 160 Bar, H H VP P 190.01 KJ / KGTimNo ratings yet
- Solution Manual For Chemistry An Atoms First Approach 2nd EditionDocument31 pagesSolution Manual For Chemistry An Atoms First Approach 2nd EditionChristianGonzalezsrybm100% (93)
- TD 1 Properties EnonceDocument2 pagesTD 1 Properties EnonceLuc AusterNo ratings yet
- c08 - Pending 8.36Document262 pagesc08 - Pending 8.36SeungMin LeeNo ratings yet
- Thermochemistry: QuestionsDocument32 pagesThermochemistry: QuestionsChala1989No ratings yet
- Cycles Revision SolutionsDocument11 pagesCycles Revision SolutionsLayla JhNo ratings yet
- Solution Manual For Chemistry An Atoms First Approach 2nd Edition Steven S Zumdahl Susan A ZumdahlDocument31 pagesSolution Manual For Chemistry An Atoms First Approach 2nd Edition Steven S Zumdahl Susan A ZumdahlJenniferLarsonpsdc100% (50)
- Chapter IV - : Problem 4.53Document1 pageChapter IV - : Problem 4.53Liz ArfinNo ratings yet
- Applied Thermodynamics Exam 2018 Wirh SolutionsDocument9 pagesApplied Thermodynamics Exam 2018 Wirh SolutionsFarouk BassaNo ratings yet
- Lec Exercises 4 Energy and ChemistryDocument4 pagesLec Exercises 4 Energy and ChemistryILEENVIRUSNo ratings yet
- Chapter 6 Thermodynamics: The First Law: Systems, States, and Energy (Sections 6.1-6.8)Document12 pagesChapter 6 Thermodynamics: The First Law: Systems, States, and Energy (Sections 6.1-6.8)MostafaRock100% (2)
- Vapour Power Cycles - 275Document53 pagesVapour Power Cycles - 275aditya yadavNo ratings yet
- Formulas - PipeDocument34 pagesFormulas - PipeReuben Madera DabaNo ratings yet
- Amanda Harris (1037441) - CHM3107 Worksheet 1Document7 pagesAmanda Harris (1037441) - CHM3107 Worksheet 1Amanda harrisNo ratings yet
- Ejercicio para CompararDocument4 pagesEjercicio para CompararCamila SarabiaNo ratings yet
- HW02 SolnDocument34 pagesHW02 Solnsenen.selenNo ratings yet
- Thermo LQ3Document2 pagesThermo LQ3Su-ho HanNo ratings yet
- Soal PR TermodinamikaDocument10 pagesSoal PR TermodinamikaanjaniNo ratings yet
- Sample 35313Document16 pagesSample 35313Francis CometaNo ratings yet
- Gibbs Free Energy WorksheetDocument2 pagesGibbs Free Energy WorksheetMo NassifNo ratings yet
- Questions and Answers To Problems Number 11 & 29Document1 pageQuestions and Answers To Problems Number 11 & 29owl knightNo ratings yet
- Steam Turbinedocx PDF FreeDocument13 pagesSteam Turbinedocx PDF Freeben richNo ratings yet
- Final Exam Thermodynamics QDocument4 pagesFinal Exam Thermodynamics QEnrico BorjaNo ratings yet
- Thermodynamics 2 Quiz #3 - T01: Name: ID #: Problem:: 1 Mark 1 MarkDocument2 pagesThermodynamics 2 Quiz #3 - T01: Name: ID #: Problem:: 1 Mark 1 MarkPratulya KolheNo ratings yet
- EXERCISE 1 - SolDocument19 pagesEXERCISE 1 - SolkelvinNo ratings yet
- Spontaneous Change Entropy and Free EnergyDocument46 pagesSpontaneous Change Entropy and Free EnergyStephanie MejiaNo ratings yet
- Chemistry An Atoms First Approach 2nd Edition Zumdahl Solutions Manual DownloadDocument42 pagesChemistry An Atoms First Approach 2nd Edition Zumdahl Solutions Manual DownloadRita Schwartz100% (26)
- Solutions ProblemSet8 Sem22007Document7 pagesSolutions ProblemSet8 Sem22007clearcastingNo ratings yet
- Heat of Combustion Lab 2Document14 pagesHeat of Combustion Lab 2Sarah GoinsNo ratings yet
- Exercise 6.2a - EnergyDocument3 pagesExercise 6.2a - Energysamuel.bennettNo ratings yet
- Example 11 RefrigerationDocument3 pagesExample 11 RefrigerationSantosh RathodNo ratings yet
- Tarea No 7 ExergiaDocument3 pagesTarea No 7 ExergiaAndres RomeroNo ratings yet
- 05 - Thermodynamic - Cycles - (Rankine) PDFDocument6 pages05 - Thermodynamic - Cycles - (Rankine) PDFAntonio Di FioreNo ratings yet
- 12th PhysucsvipDocument3 pages12th Physucsvipphysics a2No ratings yet
- LESSON 3.3 Calculating Free EnergyDocument25 pagesLESSON 3.3 Calculating Free EnergyQueenie TalabocNo ratings yet
- BTDDocument20 pagesBTDYeditha Satyanarayana MurthyNo ratings yet
- Phychem 1 Review 1 Sept 2015Document2 pagesPhychem 1 Review 1 Sept 2015Jupert Jasser AbellanaNo ratings yet
- 5 Thermochemistry: ChangesDocument53 pages5 Thermochemistry: ChangesPrashant AchariNo ratings yet
- Tutorial Sheet 02 Answers 2014Document24 pagesTutorial Sheet 02 Answers 2014checkmeout803100% (1)
- Physical Chemistry 2nd Edition Ball Solutions Manual 1Document36 pagesPhysical Chemistry 2nd Edition Ball Solutions Manual 1hannahbridgesotdcrxnbae100% (30)
- Chapter 8 - Tut-1Document5 pagesChapter 8 - Tut-1Ram AroraNo ratings yet
- Sheet (1&2) ThermoDocument17 pagesSheet (1&2) ThermoAhmed A. TaimaNo ratings yet
- Adamson UniversityDocument3 pagesAdamson UniversityVanessa Elaine CaoNo ratings yet
- Ip Group 12 - ThermoDocument12 pagesIp Group 12 - ThermoLAU POEY YEE STUDENTNo ratings yet
- Chapter 7. Energy and Energy BalanceDocument40 pagesChapter 7. Energy and Energy BalancezuksmanNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Chapter V - Ideal and Actual Gases: Problem 5.5Document1 pageChapter V - Ideal and Actual Gases: Problem 5.5Liz ArfinNo ratings yet
- Conservation of Mass and Energy: Problem 3.33Document1 pageConservation of Mass and Energy: Problem 3.33Liz ArfinNo ratings yet
- Chapter V - Ideal and Actual Gases: 350 KpcaDocument1 pageChapter V - Ideal and Actual Gases: 350 KpcaLiz ArfinNo ratings yet
- Thermo Solutions - Part66 PDFDocument1 pageThermo Solutions - Part66 PDFLiz ArfinNo ratings yet
- Thermo Solutions - Part88 PDFDocument1 pageThermo Solutions - Part88 PDFLiz ArfinNo ratings yet
- Properties of Pure Substances: Chapter IVDocument1 pageProperties of Pure Substances: Chapter IVLiz ArfinNo ratings yet
- Problem 4.5: Chapter Iv - Properties of Pure SubstancesDocument1 pageProblem 4.5: Chapter Iv - Properties of Pure SubstancesLiz ArfinNo ratings yet
- Thermo Solutions - Part75 PDFDocument1 pageThermo Solutions - Part75 PDFLiz ArfinNo ratings yet
- Problem 3.13: Chapter Iii - Conservation of Mass and EnergyDocument1 pageProblem 3.13: Chapter Iii - Conservation of Mass and EnergyLiz ArfinNo ratings yet
- Chapter Id - Conservation of Mass and EnergyDocument1 pageChapter Id - Conservation of Mass and EnergyLiz ArfinNo ratings yet
- Conservation of Mass and Energy: Problem 3.37Document1 pageConservation of Mass and Energy: Problem 3.37Liz ArfinNo ratings yet
- Thermo Solutions - Part55 PDFDocument1 pageThermo Solutions - Part55 PDFLiz ArfinNo ratings yet
- M (H M (H M (H M (H: Chapter Ill - Conservation of Mass and EnergyDocument1 pageM (H M (H M (H M (H: Chapter Ill - Conservation of Mass and EnergyLiz ArfinNo ratings yet
- Thermo Solutions - Part94 PDFDocument1 pageThermo Solutions - Part94 PDFLiz ArfinNo ratings yet
- Thermo Solutions - Part81 PDFDocument1 pageThermo Solutions - Part81 PDFLiz ArfinNo ratings yet
- Thermo Solutions - Part79 PDFDocument1 pageThermo Solutions - Part79 PDFLiz ArfinNo ratings yet
- Thermo Solutions - Part51 PDFDocument1 pageThermo Solutions - Part51 PDFLiz ArfinNo ratings yet
- Iii - Conservation of Mass and Energy: Fl. FLDocument1 pageIii - Conservation of Mass and Energy: Fl. FLLiz ArfinNo ratings yet
- Thermo Solutions - Part67 PDFDocument1 pageThermo Solutions - Part67 PDFLiz ArfinNo ratings yet
- Thermo Solutions - Part87 PDFDocument1 pageThermo Solutions - Part87 PDFLiz ArfinNo ratings yet
- Thermo Solutions - Part91 PDFDocument1 pageThermo Solutions - Part91 PDFLiz ArfinNo ratings yet
- Thermo Solutions - Part73 PDFDocument1 pageThermo Solutions - Part73 PDFLiz ArfinNo ratings yet
- Problem 4.1: Chapter Iv - Properties of Pure SubstancesDocument1 pageProblem 4.1: Chapter Iv - Properties of Pure SubstancesLiz ArfinNo ratings yet
- Problem 3.41: Chapter Ill - Conservation of Mass and EnergyDocument1 pageProblem 3.41: Chapter Ill - Conservation of Mass and EnergyLiz ArfinNo ratings yet
- Thermo Solutions - Part93 PDFDocument1 pageThermo Solutions - Part93 PDFLiz ArfinNo ratings yet
- Conservation of Mass and Energy: Isc.MDocument1 pageConservation of Mass and Energy: Isc.MLiz ArfinNo ratings yet
- Thermo Solutions - Part92 PDFDocument1 pageThermo Solutions - Part92 PDFLiz ArfinNo ratings yet
- Chapter IV - : Problem 4.53Document1 pageChapter IV - : Problem 4.53Liz ArfinNo ratings yet
- Thermo Solutions - Part86 PDFDocument1 pageThermo Solutions - Part86 PDFLiz ArfinNo ratings yet
- Problem 3.55: Chapter Iii - Conservation of Mass and EnergyDocument1 pageProblem 3.55: Chapter Iii - Conservation of Mass and EnergyLiz ArfinNo ratings yet