Professional Documents
Culture Documents
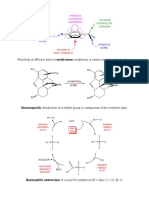
MO diagram for MO diagram for: σ-donor ligand system, octahedral complex π-acceptor ligand system, octahedral complex
MO diagram for MO diagram for: σ-donor ligand system, octahedral complex π-acceptor ligand system, octahedral complex
Uploaded by
Lurthu PushparajOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
MO diagram for MO diagram for: σ-donor ligand system, octahedral complex π-acceptor ligand system, octahedral complex
MO diagram for MO diagram for: σ-donor ligand system, octahedral complex π-acceptor ligand system, octahedral complex
Uploaded by
Lurthu PushparajCopyright:
Available Formats
MO diagram for σ-donor ligand system, octahedral complex MO diagram for π-acceptor ligand system, octahedral complex
a1g* a1g*
4p t1u* 4p t1u*
4s 4s
ligand
t2g* π*-orbitals
eg* eg*
3d 3d
t2g t2g
ligand ligand
σ-orbitals σ-orbitals
t2g
empty eg empty eg
partially filled partially filled
filled filled
t1u t1u
a1g a1g
Notes: – each ligand contributes an electron pair whether it is neutral or negatively charged.
– overlap between metal orbitals and ligand s-orbitals is in the order s > p > d.
– the six electron pairs derived from the ligands can be considered to fill the a1g, t1u and eg levels whilst the remaining
electrons derived from the metal are placed in the non-bonding t2g and antibonding eg* levels (of course, we cannot
distinguish between electrons derived from the ligand and those from the metal).
You might also like
- Melodie Heifetz PDFDocument3 pagesMelodie Heifetz PDFmpcgdNo ratings yet
- ML6 ComplexesDocument3 pagesML6 ComplexesCarlos PiñeiroNo ratings yet
- MOT ComplexDocument9 pagesMOT ComplexMyshaM099No ratings yet
- Sarah Tucker College TirunelveliDocument50 pagesSarah Tucker College TirunelveliTapas GhatakNo ratings yet
- Molecular Orbital Theory-2011Document25 pagesMolecular Orbital Theory-2011Nov IndaNo ratings yet
- Coordination Chemistry:: An OverviewDocument38 pagesCoordination Chemistry:: An OverviewAnmol KalantriNo ratings yet
- DalitzDocument15 pagesDalitzjvsdummyNo ratings yet
- Hybridization Part 1Document10 pagesHybridization Part 1ehap negm إيهاب نجمNo ratings yet
- rfx75 Schematic Rev4Document1 pagerfx75 Schematic Rev4Geraldrum Zyzcom HdzNo ratings yet
- Benefits of Operating Doubly Fed Induction Generators by ModularDocument8 pagesBenefits of Operating Doubly Fed Induction Generators by ModularDeepak GehlotNo ratings yet
- Magnetostatics Part1Document11 pagesMagnetostatics Part1SureshNo ratings yet
- Revised G0544, G5850Z & G5851Z Wiring Diagram: Rab-A35 Rab - A18Document1 pageRevised G0544, G5850Z & G5851Z Wiring Diagram: Rab-A35 Rab - A18JUAN RIVERA DUEÑASNo ratings yet
- SLD VSD Kontrol 1,5 KWDocument1 pageSLD VSD Kontrol 1,5 KWTeguh Puji HidayatNo ratings yet
- Stm32wb55ccu6 EschematicDocument1 pageStm32wb55ccu6 EschematicErnesto CorporanNo ratings yet
- Molecular Orbital Theory Approach To Bonding in Transition Metal ComplexesDocument19 pagesMolecular Orbital Theory Approach To Bonding in Transition Metal ComplexesMONIRUZZAMAN MONIRNo ratings yet
- Equations and ConstantsDocument1 pageEquations and ConstantsAbdalla ShaoebNo ratings yet
- Q1-Solution 1: 25 Mar 2022 14:44:39 - Svar-Exam180322.smDocument5 pagesQ1-Solution 1: 25 Mar 2022 14:44:39 - Svar-Exam180322.smmahmoudmousaviNo ratings yet
- N:WRN: Ulgfrqqhfwhg39V/Vwhp/Dujh Exloglqjdqgidup 7Kuhhskdvhlqyhuwhuzlwkpruhwkdqwzr$Uud/Er (HVDocument1 pageN:WRN: Ulgfrqqhfwhg39V/Vwhp/Dujh Exloglqjdqgidup 7Kuhhskdvhlqyhuwhuzlwkpruhwkdqwzr$Uud/Er (HVmahmoud12122012No ratings yet
- Lecture 9Document10 pagesLecture 9ishikashri165No ratings yet
- Caraca Node PDFDocument2 pagesCaraca Node PDFCadena CesarNo ratings yet
- Circuito Del Sistema Motor - Arranque Del Sistema Híbrido (W - o Entrada - Sistema de Arranque)Document7 pagesCircuito Del Sistema Motor - Arranque Del Sistema Híbrido (W - o Entrada - Sistema de Arranque)JaviersNo ratings yet
- Czerny Op 299 Heft 1 PianoDocument25 pagesCzerny Op 299 Heft 1 PianoRoger BurbanoNo ratings yet
- 6-Graficos Correccion DTML IntercambiadoresDocument13 pages6-Graficos Correccion DTML IntercambiadoresLeandroNo ratings yet
- rfx75 SchematicDocument1 pagerfx75 SchematicGeraldrum Zyzcom HdzNo ratings yet
- 2Sgh G H G: Service KW-HR Meter (Main Meter) Communication Service DropDocument1 page2Sgh G H G: Service KW-HR Meter (Main Meter) Communication Service DropMarc Kenneth ConchaNo ratings yet
- Tanabe-Sugano Diagrams: K.Sridharan Dean School of Chemical & Biotechnology SASTRA University Thanjavur - 613 401Document10 pagesTanabe-Sugano Diagrams: K.Sridharan Dean School of Chemical & Biotechnology SASTRA University Thanjavur - 613 401SonaliNo ratings yet
- Ciclo Brayton Cerrado: Jairo R. Barrera V Departamento de Ing. Química y Ambiental Universidad Nacional de ColombiaDocument8 pagesCiclo Brayton Cerrado: Jairo R. Barrera V Departamento de Ing. Química y Ambiental Universidad Nacional de ColombiaDaniel GonzálezNo ratings yet
- SS4H-SD Schematic v. 1.1Document1 pageSS4H-SD Schematic v. 1.1gigioNo ratings yet
- ECO - POWER - REV4C - SCHEMATICSDocument9 pagesECO - POWER - REV4C - SCHEMATICSkarl kraNo ratings yet
- A3 1st Floor ElecDocument1 pageA3 1st Floor ElecAJothamChristianNo ratings yet
- K 800 K Kmole M KN 314 - 8 M KN 808 Kmole KG 01 - 28Document4 pagesK 800 K Kmole M KN 314 - 8 M KN 808 Kmole KG 01 - 28MH MerhiNo ratings yet
- 30 Hilux: Power Source Theft Deterrent Wireless Door Lock Control (W/ Theft Deterrent System)Document1 page30 Hilux: Power Source Theft Deterrent Wireless Door Lock Control (W/ Theft Deterrent System)wilderNo ratings yet
- A B C D: Ground Floor Lighting LayoutDocument1 pageA B C D: Ground Floor Lighting LayoutAJothamChristianNo ratings yet
- Circuit Descriptions: Section E - HydraulicsDocument1 pageCircuit Descriptions: Section E - HydraulicsNikNo ratings yet
- EE316 ReceiverDocument1 pageEE316 ReceiverŞamil ŞirinNo ratings yet
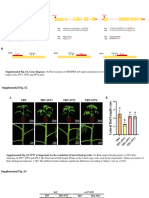
- Erad303 Suppl Supplementary Figures s1-s16 Tables s1-s2Document20 pagesErad303 Suppl Supplementary Figures s1-s16 Tables s1-s2MAnugrahRizkyPNo ratings yet
- ss4h-sd SchematicDocument1 pagess4h-sd Schematic003454No ratings yet
- ME 411-Lecture 22Document8 pagesME 411-Lecture 22Harun SarıçamNo ratings yet
- 750W Smps v.0.3Document1 page750W Smps v.0.3Sohail AhmedNo ratings yet
- Carel Co2Document15 pagesCarel Co2CaioNo ratings yet
- TSHWL FDocument4 pagesTSHWL FFix Gps GarminNo ratings yet
- Advanced Inorganic Chemistry - ROBERT L. CARTERDocument19 pagesAdvanced Inorganic Chemistry - ROBERT L. CARTERBRUNO RAMOS DE LIMANo ratings yet
- C T Ser Phe: GPD GPD GPD His Ile Ser Ser Leu GPD His Ile Phe Ser Leu GPD GPDDocument3 pagesC T Ser Phe: GPD GPD GPD His Ile Ser Ser Leu GPD His Ile Phe Ser Leu GPD GPDamzioujdaNo ratings yet
- Fuga Jędrzej StoczewskiDocument2 pagesFuga Jędrzej StoczewskiJędrzej StoczewskiNo ratings yet
- LM - TR: (With Acs Option) ,:,Rfliffi:XgiseDocument3 pagesLM - TR: (With Acs Option) ,:,Rfliffi:XgiseJuan LoNo ratings yet
- DrvpowersupplyschematicDocument1 pageDrvpowersupplyschematicbluesurviverNo ratings yet
- Schematic FinalDocument1 pageSchematic Finalvictor OliveiraNo ratings yet
- AssemblageDocument1 pageAssemblagehamzaNo ratings yet
- Hardware 3300Document1 pageHardware 3300Raphael LopesNo ratings yet
- Schedule of Test ResultsDocument2 pagesSchedule of Test ResultsHamza ButtNo ratings yet
- Coordination ChemistryDocument9 pagesCoordination ChemistryNITISH KUMARNo ratings yet
- Coordination ChemistryDocument9 pagesCoordination ChemistryChaudary Zain Ul AbideenNo ratings yet
- Design and Experiment of A Permanent Magnet Tubular Linear Generator For Wave Energy Conversion SystemDocument9 pagesDesign and Experiment of A Permanent Magnet Tubular Linear Generator For Wave Energy Conversion SystemSushant ChhotrayNo ratings yet
- Arima R ProgramasDocument27 pagesArima R ProgramasVladimiro Ibañez QuispeNo ratings yet
- 15 - JEE - Physics - Motion in Two Dimension - Circular Motion - Motion in Vertical CircleDocument4 pages15 - JEE - Physics - Motion in Two Dimension - Circular Motion - Motion in Vertical CirclePrasanth KumarNo ratings yet
- TL494 Psu PDFDocument1 pageTL494 Psu PDFDragan StojkovicNo ratings yet
- Scalable Non Ucd Class D Single With Protect Custom Sink SchematicDocument1 pageScalable Non Ucd Class D Single With Protect Custom Sink SchematicZamfir Vangu100% (1)
- InmoDocument36 pagesInmoGAMA INFORMACIÓNNo ratings yet
- Nano SynDocument4 pagesNano SynLurthu PushparajNo ratings yet
- Nano MaterialDocument5 pagesNano MaterialLurthu PushparajNo ratings yet
- NanoDocument6 pagesNanoLurthu PushparajNo ratings yet
- 12.ashnil KumaDocument9 pages12.ashnil KumaLurthu PushparajNo ratings yet
- Reactivity at Different Sites On Metal-Arene Complexes A Classic Example of UmpolungDocument1 pageReactivity at Different Sites On Metal-Arene Complexes A Classic Example of UmpolungLurthu PushparajNo ratings yet
- 16.yong LuoDocument14 pages16.yong LuoLurthu PushparajNo ratings yet
- Metallostar Assemblies Based On Dithiocarbamates For Use As MRI Contrast AgentsDocument11 pagesMetallostar Assemblies Based On Dithiocarbamates For Use As MRI Contrast AgentsLurthu PushparajNo ratings yet
- Organic Chemistry II / CHEM 252 Chapter 14 - : Aromatic CompoundsDocument28 pagesOrganic Chemistry II / CHEM 252 Chapter 14 - : Aromatic CompoundsLurthu PushparajNo ratings yet
- Catcycle PDFDocument1 pageCatcycle PDFLurthu PushparajNo ratings yet
- OAREDocument2 pagesOARELurthu PushparajNo ratings yet
- of Mo (η -C H) (η -CH Chchch) (η -C H) .: StructureDocument1 pageof Mo (η -C H) (η -CH Chchch) (η -C H) .: StructureLurthu PushparajNo ratings yet
- CNHN PDFDocument1 pageCNHN PDFLurthu PushparajNo ratings yet
- D3o - WikipediaDocument3 pagesD3o - WikipediaLurthu PushparajNo ratings yet
- ERRB-09-DRDO-Conference FormDocument2 pagesERRB-09-DRDO-Conference FormLurthu PushparajNo ratings yet
- 8 M. Sc. II Inorganic ChemistryDocument16 pages8 M. Sc. II Inorganic ChemistryLurthu PushparajNo ratings yet
- Fluorescein ArchvdateiDocument4 pagesFluorescein ArchvdateiLurthu PushparajNo ratings yet
- Proforma - V Department of BiotechnologyDocument5 pagesProforma - V Department of BiotechnologyLurthu PushparajNo ratings yet
- Pulp & Paper-SystemGuide-0221Document8 pagesPulp & Paper-SystemGuide-0221Surung P. Kreison (CARBOLINE)No ratings yet
- Haloalkane Part I New Syllabus 1.Document16 pagesHaloalkane Part I New Syllabus 1.grgrohit1424No ratings yet
- F T. Topolimero. 24022020Document2 pagesF T. Topolimero. 24022020FedericoNo ratings yet
- Biodiesel Plant Lists PDFDocument1 pageBiodiesel Plant Lists PDFRaju GummaNo ratings yet
- 107th AgendaDocument123 pages107th Agendakinanantha100% (1)
- DermaDamp HDDocument2 pagesDermaDamp HDJacob Norman CruzNo ratings yet
- Wessely GmbH-product CatalougueDocument52 pagesWessely GmbH-product CatalougueFurqan ShabbirNo ratings yet
- Oil Fractional Distillation ProcessDocument3 pagesOil Fractional Distillation ProcessMica Dell MartinezNo ratings yet
- Organic Name Reactions/Revision List For Top Ranks in IIT/AIIMSDocument54 pagesOrganic Name Reactions/Revision List For Top Ranks in IIT/AIIMSraza anandNo ratings yet
- 05 Arcel EvacleanDocument3 pages05 Arcel EvacleanKemas RamaNo ratings yet
- Material Compatibility Chart-SpectraDocument4 pagesMaterial Compatibility Chart-SpectraMachineryengNo ratings yet
- To Polymers: Submitted by Chander Shekher First Sem Branch - :aee Roll No-: 38Document8 pagesTo Polymers: Submitted by Chander Shekher First Sem Branch - :aee Roll No-: 38NirmalKrishanPrasadNo ratings yet
- Chromatography of Aroma Compounds and FragrancesDocument47 pagesChromatography of Aroma Compounds and Fragrancesilab6638No ratings yet
- Laporan Biologi Molekuler Isolasi DNA MukosaDocument2 pagesLaporan Biologi Molekuler Isolasi DNA MukosaRosyadiAdnanNo ratings yet
- Unit Test 4 Alchemist Science Academy PDFDocument10 pagesUnit Test 4 Alchemist Science Academy PDFAanchal PathakNo ratings yet
- Lab Report SBT Sem 5 ProteinDocument11 pagesLab Report SBT Sem 5 ProteinIyana RazaliNo ratings yet
- Types of TabletDocument5 pagesTypes of TabletBasit AliNo ratings yet
- Xanthan Gum Its Biopharmaceutical Applications: An OverviewDocument14 pagesXanthan Gum Its Biopharmaceutical Applications: An OverviewmaizhafiraNo ratings yet
- Protein TestsDocument13 pagesProtein TestsMa. Loucel RodriguezNo ratings yet
- FRP Fish TanksDocument33 pagesFRP Fish TanksA.Subin DasNo ratings yet
- SukanyaDocument2 pagesSukanyaSrijaNo ratings yet
- PC3150 PDFDocument8 pagesPC3150 PDFMaffone NumerounoNo ratings yet
- Osmo TopOil EN71-3 and DIN53160Document1 pageOsmo TopOil EN71-3 and DIN53160krystobalNo ratings yet
- Consumables-Catalogue Lincoln ElectricDocument702 pagesConsumables-Catalogue Lincoln ElectrickoppelaarNo ratings yet
- Polymer Project For Class 12thDocument11 pagesPolymer Project For Class 12thVinod KaushikNo ratings yet
- Manufacture and Thermal Deformation Analysis of Semicrystalline Polymer Polyether Ether Ketone by 3D PrintingDocument6 pagesManufacture and Thermal Deformation Analysis of Semicrystalline Polymer Polyether Ether Ketone by 3D PrintingLucas VillalobosNo ratings yet
- (Doi 10.1002/9783527610426.bard050402) Bard, Allen J. - Encyclopedia of Electrochemistry (Online) - The Electrolytic Production of AluminumDocument43 pages(Doi 10.1002/9783527610426.bard050402) Bard, Allen J. - Encyclopedia of Electrochemistry (Online) - The Electrolytic Production of AluminummiladrahimianNo ratings yet
- Home Made ExplosivesDocument6 pagesHome Made ExplosivesJozy BizzyNo ratings yet
- AcetobacterDocument11 pagesAcetobacterdiantinurwindaNo ratings yet
- PkoDocument63 pagesPkoLeonel VillaltaNo ratings yet