Professional Documents
Culture Documents
R Q Q K F: Coulomb's Law
R Q Q K F: Coulomb's Law
Uploaded by
Kathy ChuaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
R Q Q K F: Coulomb's Law
R Q Q K F: Coulomb's Law
Uploaded by
Kathy ChuaCopyright:
Available Formats
Coulomb’s Law
Coulomb’s Law is the magnitude of the interactive force or electrical force between two charged
particles that separated by a fixed distance which states that the electrical force between two
charged objects is directly proportional to the product of the quantity of charge on the objects
and inversely proportional to the square of the separation distance between the two objects.
Electrical force has a magnitude similar to most types of forces. The force is attractive if the
charge are of opposite sign while the force is repulsive if the charges are of like sign and it is a
conservation force. There are a variety of factors that influence the magnitude of the electrical
force such as the quantity of charge on one of the particle because the more charged of the
particle, the greater the repulsive force. Second, the quantity of charge on the second particle will
also affect the strength of the repulsive force. Gently rub two balloons with animal fur and they
repel a little. Rub the two particles vigorously to impart more charge to both of them, and they
repel a lot. Finally, the distance between the two particles will have a significant and noticeable
effect upon the repulsive force. The last factor is decreasing of separation distance because the
electrical force is strongest when the particles are closest together. The magnitude of the force
and the distance between the two particles is said to be inversely related.
q1 q2
Fe k
r2
Coulomb’s Law, Equation
How a particle gain charge
Objects become electrically charged by gaining or losing electrons, so that they have unequal
numbers of protons and electrons. Gaining excess electrons causes a negative charge, while
losing electrons causes a positive charge. A charged object behaves differently than an object
with neutral charge, attracting objects with an opposite charge and repelling objects with a
similar charge
Charge occurs in discreet units, with each positive or negative charge being equal to some
multiple of the charge of individual protons or electrons. Individual charged particles are known
as ions. There are many processes that cause an object to become electrically charged such as
mechanical processes. For example, rubbing a balloon on hair because objects can be charged by
friction. This is because different elements have different electron affinities. When a substance
with a greater electron affinity rubs against one with a lesser affinity, it steals some of the
electrons, causing both to gain a charge.
You might also like
- Discover EasytronicDocument77 pagesDiscover EasytronicMohamed Mohamed100% (3)
- Physics Lab Report 5 (Electrostatic Force)Document6 pagesPhysics Lab Report 5 (Electrostatic Force)YugendranNairNo ratings yet
- Understanding of How Electrostatic Works Using PhET Colorado Experimental Laboratory SimulatorDocument12 pagesUnderstanding of How Electrostatic Works Using PhET Colorado Experimental Laboratory Simulator48MUHAMMAD ARIEF MULYANANo ratings yet
- General Physics 2 ElectrostaticsDocument32 pagesGeneral Physics 2 ElectrostaticsAriah KaliNo ratings yet
- Bonding Forces and Energies: Intermolecular or Interatomic ForcesDocument3 pagesBonding Forces and Energies: Intermolecular or Interatomic ForcesManish SinghNo ratings yet
- PPT-Course 1-2-Charge Force Field Gaus PotentialDocument63 pagesPPT-Course 1-2-Charge Force Field Gaus PotentialRaul AtofaneiNo ratings yet
- Electricity: Introductio NDocument17 pagesElectricity: Introductio NPavelNo ratings yet
- AP Lec 1Document14 pagesAP Lec 1Muhammad HaziqNo ratings yet
- Unit 3Document12 pagesUnit 3re2phukanNo ratings yet
- What Is An Electrostatic Force?Document3 pagesWhat Is An Electrostatic Force?Carlton GrantNo ratings yet
- Q3 M1 Electric ChargeDocument6 pagesQ3 M1 Electric ChargeShielami SarapuddinNo ratings yet
- Electric Force and ColoumbDocument1 pageElectric Force and ColoumbEdmil Jhon AriquezNo ratings yet
- GP2 Q3W1Document24 pagesGP2 Q3W1DonggadongNo ratings yet
- What Is ElectricityDocument25 pagesWhat Is ElectricityJohn PetrovicNo ratings yet
- Unit 5 Electricity and MagnetismDocument7 pagesUnit 5 Electricity and MagnetismareejNo ratings yet
- Electric Charge and Electric FieldDocument3 pagesElectric Charge and Electric FieldAhmet UlusoyNo ratings yet
- Electr Ic Forces Charge Interactions: Opposites (+/-) Attract and Likes (+/+ or - / - ) RepelDocument8 pagesElectr Ic Forces Charge Interactions: Opposites (+/-) Attract and Likes (+/+ or - / - ) Repelapi-27085921No ratings yet
- Electrical ChargeDocument18 pagesElectrical ChargeSamrat100% (1)
- ElectrostaticsDocument15 pagesElectrostaticsDhruv TalwareNo ratings yet
- Electric ChargeDocument25 pagesElectric ChargeDragon Kits100% (1)
- Electric Charges and FieldDocument61 pagesElectric Charges and FieldGayu27No ratings yet
- MODULE 1 Electric Charge and Coulombs LawDocument12 pagesMODULE 1 Electric Charge and Coulombs LawVenus CaringalNo ratings yet
- L1 Electricity and MagnetismDocument20 pagesL1 Electricity and Magnetismbussanmugwati2004No ratings yet
- PHY102 Lecture Note 2023 - 2024 Session - 085214 - 102031Document7 pagesPHY102 Lecture Note 2023 - 2024 Session - 085214 - 102031Muhammad AbdulazizNo ratings yet
- Medical PhysicsDocument371 pagesMedical PhysicsAhmed khanNo ratings yet
- EED SCI 2 ElectricityDocument9 pagesEED SCI 2 ElectricityMaria Josette Baldomero CelebrarNo ratings yet
- Thiele LE18Document18 pagesThiele LE18amayaruiz55No ratings yet
- Electrostatics: Conservation of Charge and Coulomb'S LawDocument4 pagesElectrostatics: Conservation of Charge and Coulomb'S Lawash2560% (1)
- The Gravitational ForceDocument5 pagesThe Gravitational ForceGeorge RajnaNo ratings yet
- ElectrostaticDocument3 pagesElectrostaticPrincess Roan RobertoNo ratings yet
- AP CH 15-TeacherDocument36 pagesAP CH 15-TeacherOnur YavuzcetinNo ratings yet
- Artikel Electric ChargeDocument3 pagesArtikel Electric ChargeYeni SurantiNo ratings yet
- Electric ChargeDocument14 pagesElectric Chargefrancis solivenNo ratings yet
- Chapter 2 - ELECTROSTATICSDocument7 pagesChapter 2 - ELECTROSTATICSMohamed HanizamNo ratings yet
- Static Electricity, Electric Charge and Its ConservationDocument5 pagesStatic Electricity, Electric Charge and Its ConservationMarc Vincent CastilloNo ratings yet
- Charging by FrictionDocument33 pagesCharging by FrictionlostgirlNo ratings yet
- Static Electricity: Lesson 1 - Basic Terminology & ConceptsDocument77 pagesStatic Electricity: Lesson 1 - Basic Terminology & ConceptsDERRICKKEANNo ratings yet
- Lightning in Bottle: Seoion 1Document34 pagesLightning in Bottle: Seoion 1afaflotfi_155696459No ratings yet
- Week 1 - Electric Charge ELECTRIC CHARGE - We Can Trace All Electrical Effects To Electrons and Protons Inside Every Atom. This IsDocument61 pagesWeek 1 - Electric Charge ELECTRIC CHARGE - We Can Trace All Electrical Effects To Electrons and Protons Inside Every Atom. This IsCrizza Mae CuregNo ratings yet
- Muhammad AkhtarDocument25 pagesMuhammad AkhtarMuhammad akhtarNo ratings yet
- Concept Summary: Batesville High School PhysicsDocument29 pagesConcept Summary: Batesville High School PhysicsKirstie KJSNo ratings yet
- Malabanan-Matthew Gt2 Phyla1Document6 pagesMalabanan-Matthew Gt2 Phyla1Matt MalabananNo ratings yet
- Riri Physics 3.1Document1 pageRiri Physics 3.1joannapiezaNo ratings yet
- Electric ChargesDocument18 pagesElectric Chargesviswathkumaraa2005No ratings yet
- PTP 102Document20 pagesPTP 102memo110No ratings yet
- ElectronDocument2 pagesElectronJohn paul ServillonNo ratings yet
- Lecture - Electric Force and Electric FieldDocument58 pagesLecture - Electric Force and Electric FieldnafsNo ratings yet
- Physics ProjectDocument44 pagesPhysics ProjectAvnish TewariNo ratings yet
- Electric ChargesDocument31 pagesElectric ChargesJeorge QuiboyNo ratings yet
- Electrostatics Involves Electric Charges, The Forces Between Them, and Their Behavior in MaterialsDocument93 pagesElectrostatics Involves Electric Charges, The Forces Between Them, and Their Behavior in MaterialsMaryThereseCartagenasNo ratings yet
- Sourabh 1Document6 pagesSourabh 1vishudhakciyaNo ratings yet
- FACEL2Document27 pagesFACEL2deepak90rewaNo ratings yet
- Static ElectricityDocument10 pagesStatic ElectricityAditya NandaNo ratings yet
- Snell's Law: Force As A Vector QuantityDocument2 pagesSnell's Law: Force As A Vector QuantityMegan CabahugNo ratings yet
- Genphy2 Electrostatics 1Document60 pagesGenphy2 Electrostatics 1Pamela AzurinNo ratings yet
- Task 1Document5 pagesTask 1Marfe Salamat ManilaNo ratings yet
- States of Matter - Gas and Liquid: Sem-IIDocument22 pagesStates of Matter - Gas and Liquid: Sem-IIapi-233404189No ratings yet
- Phy2 Unit Test 1Document21 pagesPhy2 Unit Test 1JACOB SANTOSNo ratings yet
- Introductory Electricity and Magnetism Worksheets and SolutionsDocument34 pagesIntroductory Electricity and Magnetism Worksheets and SolutionsIbnu kusnoNo ratings yet
- Electrostatics Is The Branch of Physics That Deals With The Phenomena and Properties of Stationary or SlowDocument6 pagesElectrostatics Is The Branch of Physics That Deals With The Phenomena and Properties of Stationary or SlowfunandruinsNo ratings yet
- What is Charge? – The Redefinition of Atom - Energy to Matter ConversionFrom EverandWhat is Charge? – The Redefinition of Atom - Energy to Matter ConversionNo ratings yet
- Solution Problem Chapter 1 Electric Machinery FundamentalsDocument8 pagesSolution Problem Chapter 1 Electric Machinery FundamentalsAinaaSyahirahNo ratings yet
- Thesis 77Document128 pagesThesis 77AmanuelNo ratings yet
- Closed Conduit Measurement Techniques: Monroe L. Weber-Shirk S Civil Environmental EngineeringDocument19 pagesClosed Conduit Measurement Techniques: Monroe L. Weber-Shirk S Civil Environmental EngineeringMuaz MushtaqNo ratings yet
- CFD-Analysis of Free Surface Flow Inside A Twin Screw ExtruderDocument10 pagesCFD-Analysis of Free Surface Flow Inside A Twin Screw ExtruderAbkar AhmedNo ratings yet
- Review Questions: Written/Composed By: - SHAHZAD IFTIKHAR Contact # 0313-5665666 WebsiteDocument6 pagesReview Questions: Written/Composed By: - SHAHZAD IFTIKHAR Contact # 0313-5665666 WebsitesohailNo ratings yet
- CH2010 Chemical Engineering Thermodynamics Assignment - 2Document2 pagesCH2010 Chemical Engineering Thermodynamics Assignment - 2Aryan PandeyNo ratings yet
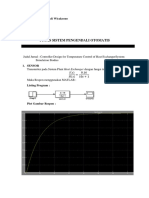
- Tugas Sistem Pengendali Otomatis: Nama: Bagus Pram Adi Wicaksono Kelas: MS - 4C/07 NIM: 4.21.18.8.07Document10 pagesTugas Sistem Pengendali Otomatis: Nama: Bagus Pram Adi Wicaksono Kelas: MS - 4C/07 NIM: 4.21.18.8.07baguspNo ratings yet
- A Meraghini Chemisky - PA66GF30 Composite - Effect of Relative HumidityDocument9 pagesA Meraghini Chemisky - PA66GF30 Composite - Effect of Relative Humiditydon-donnolaNo ratings yet
- C StructuralDesign FanellaDocument3 pagesC StructuralDesign FanellasadelgadoproinciNo ratings yet
- DLL (Motion in 2 Dimension)Document3 pagesDLL (Motion in 2 Dimension)JeanRachoPaynandosNo ratings yet
- Physics Lecture NotesDocument8 pagesPhysics Lecture NotesbluesNo ratings yet
- Aero9500 STK T2Document18 pagesAero9500 STK T2John SmithNo ratings yet
- Chapter 1.2 (Separable Eq)Document10 pagesChapter 1.2 (Separable Eq)Noi IvyNo ratings yet
- Atomic Structure PDFDocument75 pagesAtomic Structure PDFSudheerkhan MuhammedNo ratings yet
- Criticism of Current Seismic Design and Construction Practice in Venezuela: A Bleak PerspectiveDocument14 pagesCriticism of Current Seismic Design and Construction Practice in Venezuela: A Bleak PerspectiveLissette PérezNo ratings yet
- Recent Publications On Leaky-Wave AntennasDocument3 pagesRecent Publications On Leaky-Wave Antennassambala4444No ratings yet
- Engineering Science Handbook For Technicians - Level IDocument122 pagesEngineering Science Handbook For Technicians - Level IKennedy MulengaNo ratings yet
- Types of TorsionDocument4 pagesTypes of TorsionDECODE BDNo ratings yet
- Well Engineering Level 1Document4 pagesWell Engineering Level 1SHOBHIT KUMAR100% (1)
- Reaction MechanismDocument14 pagesReaction MechanismMasterJEE SPSNo ratings yet
- 01 U3 Ws 1 Force DiagramsDocument4 pages01 U3 Ws 1 Force Diagramsapi-197108354No ratings yet
- 5 Cross Section Plastic Resistance (Plastic Hinge)Document15 pages5 Cross Section Plastic Resistance (Plastic Hinge)Imran SaikatNo ratings yet
- CIVI 381 LAB 1 Mayank Guglani MainDocument8 pagesCIVI 381 LAB 1 Mayank Guglani MainAhmed ZarkhaizNo ratings yet
- YLAC-1808 应力分析报告(Stress Analysis-1808)Document19 pagesYLAC-1808 应力分析报告(Stress Analysis-1808)arisandiyusufNo ratings yet
- TRB Mechanical Engineering SyllabusDocument4 pagesTRB Mechanical Engineering SyllabusgvijaymNo ratings yet
- QB Topic 4 SHMDocument5 pagesQB Topic 4 SHMmarufinoNo ratings yet
- M 389 ContentDocument52 pagesM 389 ContentokahertaberNo ratings yet
- Falkner SkanDocument16 pagesFalkner SkanBenedict EgboiyiNo ratings yet
- Electrostatics Class 12 Physics Chapter 1Document151 pagesElectrostatics Class 12 Physics Chapter 1Mr. X OfficialNo ratings yet