Professional Documents
Culture Documents
New Catalytic Process For Production of Olefins: 1. Theme Description
New Catalytic Process For Production of Olefins: 1. Theme Description
Uploaded by
Sandra Mayta SilloOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
New Catalytic Process For Production of Olefins: 1. Theme Description
New Catalytic Process For Production of Olefins: 1. Theme Description
Uploaded by
Sandra Mayta SilloCopyright:
Available Formats
New Catalytic Process for
Production of Olefins
Author: Marcello De Falco, Associate Professor, University
UCBM – Rome (Italy)
1. Theme description
Olefins, mainly ethylene (C2H4) and propylene (C3H6), are key
intermediate and feedstock for the production of a wide number
of chemical products, as the polyolefins (polyethylene – PE,
polypropylene – PP), Mono-ethylene glycol (MEG), Ethylene
Oxide (EO) and derivatives, Propylene Oxide (PO) and
derivatives, Polyvinyl chloride (PVC), ethylene dichloride
(EDC), Styrene, Acrylonitrile, Cumene, Acetic Acid, etc.
At the present, the worldwide demand of ethylene/propylene is
more than 200 million tons per year but the conventional
processes suffer for a series of problems as the high cost and
low conversion efficiency.
In the following, the traditional technologies, i.e. the
Thermal Steam Cracking and the Fluid Catalytic Cracking, are
firstly presented. Then the innovation in the olefins
production are described and assessed.
2. Olefins production conventional
processes
The most used olefins industrial production processes are:
Thermal Steam Cracking (TSC);
Fluid Catalytic Cracking (FCC).
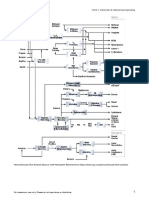
TSC is a thermal process by which a feedstock, typically
composed by naphtha, ethane or propane, is heated up in a
furnace composed by both a convection and radiant section, and
mixed with steam to reduce the coke formation. The steam
addition depends on the TSC feedstock (from 0.2 kg steam to kg
of hydrocarbon for ethane to 0.8 kg steam to kg of hydrocarbon
for naphtha).
Then the products (ethylene, propylene, butadiene, hydrogen)
are quickly cooled down to avoid subsequent reactions
(quenching) and then are separated by means of a series of
operations (refer to Figure 1).
The reactions structure involved in thermal cracking is
complex and, generally, is based on a free radical mechanism.
Basically, two types of reactions are supported in a thermal
cracking process:
primary cracking, with the initial formation of paraffin
and olefins;
secondary cracking, with the formation of light products
rich in olefins are formed.
TSC is an energy intensive process: the specific energy
consumption per kg of produced olefin is 3.050 kcal/kg.
FCC is a multi-component catalytic system, where the catalyst
pellets are “fluidized” thanks to the inlet steam flow-rate
and the cracking process is supported at lower temperature
than TSC. A typical block diagram is shown in Figure 2, while
a FCC reactor drawing is reported in Figure 3.
Olefins production traditional technologies suffer from
inefficiency due to high temperature/high energy costs,
complex and expensive separation units and significant CO 2
emissions.
As a consequence, a strong interest towards the development of
more flexible, more efficient with a lower environmental
impact and less expensive catalytic olefin production
technologies is growing.
In the following, some of the most interesting technologies
developed during the last years are presented and described.
Fig. 1 – Thermal Steam Cracking plant layout [1]
Fig. 2 – Fluid Catalytic Cracking block diagram [2]
Fig. 3 – FCC reactor drawing [2]
3. Innovative Technologies
Advanced Catalytic Olefins (ACO)
The Advanced Catalytic Olefins (ACO TM ) technology has been
developed by Kellogg Brown & Root LLC (KBR) and SK Innovation
Global Technology. The process is an FCC-type with an improved
catalyst able to convert the feedstock in larger quantities of
ethylene and propylene, with a higher share of propylene than
conventional processes (the ratio of produced propylene to
produced ethylene is 1 versus 0.7 of the commercial
processes). The ACO process produces 10-25% more olefins than
the traditional FCC processes, with a reduction of consumed
energy per unit of olefins by 7-10% [3].
The plant configuration is composed of 4 sections:
riser/reactor, disengager, stripper and regenerator. Figure 4
shows a simplified process scheme, while Figure 5 illustrates
a picture of the first ACO commercial demonstration unit,
installed in South Korea and with a production capacity of 40
kta of olefins.
Fig. 4 – ACO plant process scheme [3]
Fig. 5 – ACO Commercial Demonstration unit installed in Ulsan
(South Korea)
PCC Process
The Propylene Catalytic Cracking is a fluid solids naphtha
cracking process patented by Exxon Mobil and based on an
optimization of catalyst, reactor design and operating
conditions set able to modulate the reactions selectivity,
leading to crucial economic benefits in comparison with the
conventional processes.
The PCC process is able to produce directly the propylene at
the chemical grade concentrations, thus avoiding the expensive
fractionation units. Moreover, the specific operating
conditions allows the minimization of aromatics
production [4].
Exxon is testing the innovative solutions on tailored pilot
facilities.
Indmax FCC Process
The Indmax process, developed by the Indian Oil Corporation,
is able to convert heavy feedstock to light olefins. It is a
FCC-type process where the reactions are supported by a
patented catalyst, able to reduce the contact time and thus
leading to higher selectivity to light olefins (ethylene and
propylene).
Another crucial characteristic of I-FCC process is the high
production flexibility: the process can be easily adjusted to
modulate the output, maximizing propylene, gasoline or
producing combinations (propylene and ethylene or propylene
and gasoline) [5].
Aither Chemicals’ catalytic process
Aither Chemicals, a company located in the U.S., developed an
innovative catalytic cracking process for the production of
ethylene, acetic acid, ethylene derivatives as ethylene oxide
(EO) and ethylene glycol (EG), polyethylene (PE, LLDPE, HDPE),
acetic acid derivatives as acetic anhydride, ethylene-acetic-
acid derivatives such as vinyl acetate monomer (VAM), ethyl
vinyl acetate (EVA) and other chemicals and plastics [6]. The
process uses oxygen instead of water steam and, globally,
needs much lower energy (-80%) and produces 90% less carbon
dioxide, being more environmentally sustainable.
Moreover, the CO2 and CO streams are captured at the outlet of
the catalytic process and utilized for producing chemicals and
polymer, thus nullifying the GreenHouse Gases emissions.
The production volumes foreseen for the innovative process are
224 ktons of ethylene, 112 ktons of acetic acid, 30 ktons of
CO2 and 15 ktons of CO.
Methane-to-olefins processes
Many research efforts are devoted to find new routes and
process configurations to convert directly natural gas to
olefins by low temperature reactors.
There are two possible methane-to-olefins (MTO) processes:
Indirect process, by which methane is converted into
syngas, methanol or ethane and then olefins are
produced;
Direct process, by which olefins are directly produced
from the methane in a single conversion step composed by
modified Fischer-Tropsch reaction.
Even if the direct route seems to be more interesting, at the
present not a good light olefins selectivity has been
obtained [7] and the MTO processes are more energy intensive
than the conventional cracking technologies. The only pre-
commercial scale application has been developed by UOP and
Total Petrochemicals in Feluy (Belgium): the plan is an
indirect process able to produce ethylene and propylene
through methanol and syngas.
Fig. 6 – MTO plant in Feluy, Belgium [8]
Some interesting patents have been produced on the MTO
topic [9], [10], [11], as well some accurate scientific
publications on important international journals [12], [13],
[14].
Propane Dehydrogenation
The company UOP developed an innovative Propane
Dehydrogenation (PDH) process able to produce ethylene and
propylene at lower cost thanks to a lower energy usage and a
more stable platinum-based catalyst [15]. The process, called
Oleflex, is divided in three sections: the reaction,
consisting of four radial-flow reactors, the product
purification and the catalyst regeneration. Fig. 7 shows a
process layout. Currently, 6 Oleflex units are installed and
produce more than 1.250.000 MTA of propylene worldwide.
Fig. 7 – UOP’s Oleflex process layout [8]
Shell Higher Olefins Process
The Shell Higher Olefins Process (SHOP) is an innovative
olefins production technology, developed by Royal Dutch Shell,
based on a homogeneous catalyst and used for production of
linear α – olefins (from C4 to C40) and internal olefins from
ethene.
The process architecture consists of three steps:
Oligomerization (conversion of a monomer or a mixture of
monomers into an oligomer, temperature = 90–100°C,
pressure = 100–110 bar, polar solvent);
Isomerization (molecules rearrangement reaction by a
metal catalyst, 100–125° C and 10 bar);
Methathesis (alkenes are converted into new products by
breaking – up and reformation of C-C double bonds by an
alumina-based catalyst, 100–125° C and 10 bar) [17].
At the present, SHOP is widely applied and the worldwide
production capacity is 1.190.000 t of linear alpha and
internal olefins per year.
Catalytic Partial Oxidation of ethane
ENI and the Italian research centre CNR developed an ethylene
production process through Short Contact Time – Catalytic
Partial Oxidation (CPO) of ethane.
The process is supported by a patented monolithic catalyst
able to improve the ethylene yield up to 55 wt.% [18].
At the present, the technology has been validated trough a
bench-scale unit, by which the optimal operating conditions
have been identified. However, the industrial scale
application is not ready yet, since an optimization of the CPO
reactor design and the improvement of the catalyst reliability
are needed.
_______________
[1]
http://nptel.ac.in/courses/103107082/module7/lecture2/lecture2
.pdf
[2]
http://nptel.ac.in/courses/103107082/module6/lecture5/lecture5
.pdf
[3]
http://www.kbr.com/Newsroom/Publications/Articles/Advanced-Cat
alytic-Olefins-ACO-First-Commercial-Demonstration-Unit-Begins-
Operations.pdf
[4] M.W. Bedell, P.A. Ruziska, T.R. Steffens. On-Purpose
Propylene from Olefinic Streams. Davison Catalagram, 94 (2004)
– Special Edition: Propylene –
[5] http://www.cbi.com/images/uploads/tech_sheets/I-FCC-12.pdf
[6]
http://www.aitherchemicals.com/2012/06/21/aither-announces-ope
n-season/
[7] https://www.tut.fi/ms/muo/polyko/materiaalit/aa/apdf/polyk
o_technology_for_the_production_of_olefins.pdf
[8]
http://plasticsengineeringblog.com/2013/02/20/how-shale-gas-is
-changing-propylene/
[9] http://www.google.co.in/patents/US4450310
[10] http://www.google.com/patents/WO2014031524A1?cl=en
[11] http://www.freepatentsonline.com/7091391.html
[12]
http://www.sciencedirect.com/science/article/pii/S036054420800
0042
[13] http://www.hindawi.com/journals/jchem/2013/676901/
[14] http://www.netl.doe.gov/KMD/Cds/disk28/NG7-4.PDF
[15]
http://www.uop.com/?document=uop-olefin-production-solutions-b
rochure&download=1
[16] epg.science.cmu.ac.th/…/article-download.php?id=
[17] http://www.kataliza.chemia.polsl.pl/makro-ic-wyk4a.pdf
[18] L. Basini, S. Cimino, A. Guarinoni “Short Contact Time
Catalytic Partial Oxidation (SCT-CPO) for Synthesis Gas
Processes and Olefins Production”. Ind. Eng. Chem. Res., 2013,
52 (48), 17023–17037.
You might also like
- New Olefin Production Technologies in SINOPECDocument10 pagesNew Olefin Production Technologies in SINOPECTrevor J. HutleyNo ratings yet
- UOP Oleflex ProcessDocument2 pagesUOP Oleflex ProcessssslayerNo ratings yet
- EO Technology OverviewDocument23 pagesEO Technology OverviewSoumitra DeshmukhNo ratings yet
- 01pa JB 2 6 PDFDocument9 pages01pa JB 2 6 PDFMarcelo Varejão CasarinNo ratings yet
- Ethylbenzene A4Document8 pagesEthylbenzene A4Sữa Chua VinamilkNo ratings yet
- Advanced Catalytic Olefins ACO First Commercial Demonstration Unit Begins OperationsDocument12 pagesAdvanced Catalytic Olefins ACO First Commercial Demonstration Unit Begins OperationsmakhadermfNo ratings yet
- Uop HF Alkylation Technology: Kurt A. Detrick, James F. Himes, Jill M. Meister, and Franz-Marcus NowakDocument24 pagesUop HF Alkylation Technology: Kurt A. Detrick, James F. Himes, Jill M. Meister, and Franz-Marcus NowakBharavi K SNo ratings yet
- Ethylene From EthanolDocument45 pagesEthylene From EthanolDannica Keyence MagnayeNo ratings yet
- Procesos de Obtencion de PolipropilenoDocument2 pagesProcesos de Obtencion de PolipropilenoInés Diaz GuevaraNo ratings yet
- Advances in The OCC Process For Propylene ProductionDocument6 pagesAdvances in The OCC Process For Propylene ProductionAngel Richard MamaniNo ratings yet
- The Production of Cumene Using Zeolite Catalyst Aspen Model DocumentationDocument16 pagesThe Production of Cumene Using Zeolite Catalyst Aspen Model Documentationديانا محمدNo ratings yet
- Chemi Properties (Full Permission)Document9 pagesChemi Properties (Full Permission)Vinay BabuNo ratings yet
- Industrial Catalytic Processes-Phenol Production: Robert J. SchmidtDocument15 pagesIndustrial Catalytic Processes-Phenol Production: Robert J. SchmidtUzair WahidNo ratings yet
- Propylene, Propylene Oxide and Isopropanol: Course: Chemical Technology (Organic) Module VIIDocument12 pagesPropylene, Propylene Oxide and Isopropanol: Course: Chemical Technology (Organic) Module VIImaheshNo ratings yet
- 0910 8 AbsDocument9 pages0910 8 Absjlcheefei9258No ratings yet
- Process Design For The Production of Ethylene From EthanolDocument144 pagesProcess Design For The Production of Ethylene From EthanolJorge RicoNo ratings yet
- Process Design For The Production of Ethylene From EthanolDocument144 pagesProcess Design For The Production of Ethylene From EthanolWilmer Rios DiazNo ratings yet
- Gas: China: Volume One-Scienceand TechnologyDocument4 pagesGas: China: Volume One-Scienceand TechnologyAnurita GhoshNo ratings yet
- Extended AbstractDocument4 pagesExtended AbstractEdrian A. MañalongNo ratings yet
- Che MaturDocument4 pagesChe MaturTralalaNo ratings yet
- Ethylene Glycol ProductionDocument3 pagesEthylene Glycol ProductionQuang NguyễnNo ratings yet
- Ethylene Midterms PetrochemDocument8 pagesEthylene Midterms PetrochemEfraim AbuelNo ratings yet
- Methanol-to-Propylene Technology: by Intratec SolutionsDocument4 pagesMethanol-to-Propylene Technology: by Intratec SolutionsYuliaDwiRahmawatiNo ratings yet
- Development of Highly Selective Process For Mono-Ethylene Glycol Production From Ethylene Oxide Via Ethylene Carbonate Using Phosphonium Salt CatalystDocument5 pagesDevelopment of Highly Selective Process For Mono-Ethylene Glycol Production From Ethylene Oxide Via Ethylene Carbonate Using Phosphonium Salt CatalystirfanNo ratings yet
- 1 .1-S2.0-S2213343721006503-MainDocument14 pages1 .1-S2.0-S2213343721006503-Mainmohamed magedNo ratings yet
- Processes Review of Propylene Production by Catalytic Cracking of C4-C8 OlefinsDocument12 pagesProcesses Review of Propylene Production by Catalytic Cracking of C4-C8 OlefinsAndres Carmona OsorioNo ratings yet
- TVK Ethylene Plant OverviewDocument32 pagesTVK Ethylene Plant Overviewstavros7100% (2)
- Converting Olefins To Propene - Ethene To Propene and Olefin CrackingDocument59 pagesConverting Olefins To Propene - Ethene To Propene and Olefin Crackingsaeed babaeiNo ratings yet
- ChemBioEng Reviews - 2017 - Mohsenzadeh - Bioethylene Production From Ethanol A Review and Techno Economical EvaluationDocument17 pagesChemBioEng Reviews - 2017 - Mohsenzadeh - Bioethylene Production From Ethanol A Review and Techno Economical EvaluationHossam M. Abd El RahmanNo ratings yet
- Process Analytics in Ethylene Production PlantsDocument11 pagesProcess Analytics in Ethylene Production PlantsIka SulistyaningtiyasNo ratings yet
- FILE 20220921 173401 Homogeneously Catalyzed Industrial ProcessesDocument31 pagesFILE 20220921 173401 Homogeneously Catalyzed Industrial ProcessesPham ThaoNo ratings yet
- Synthesis of Biomass-Derived Gasoline Fuel Oxygenates by Microwave IrradiationDocument18 pagesSynthesis of Biomass-Derived Gasoline Fuel Oxygenates by Microwave IrradiationDevesh Pratap ChandNo ratings yet
- Alkylation and Polymerization ProcessDocument61 pagesAlkylation and Polymerization ProcessWan Afiff100% (2)
- Pce-II Unit-I & II 1Document84 pagesPce-II Unit-I & II 1Pavan SatishNo ratings yet
- Industrial Catalytic Processes Phenol PRDocument15 pagesIndustrial Catalytic Processes Phenol PRJesús MorenoNo ratings yet
- Applications of The Olefin Metathesis Reaction To Industrial ProcessesDocument15 pagesApplications of The Olefin Metathesis Reaction To Industrial ProcessesPetr Vaněk100% (1)
- The Industrial Applications of Fluidized Bed Reactors Is As FollowsDocument3 pagesThe Industrial Applications of Fluidized Bed Reactors Is As FollowsAyodeji Arowolo MustaphaNo ratings yet
- Design of EthylbenzeneDocument5 pagesDesign of Ethylbenzenesahar vahdatifarNo ratings yet
- Ethylene ProductionDocument23 pagesEthylene ProductionDavid ZamoraNo ratings yet
- SINOPEC Methanol-To-Olefins (S-MTO) Technology Process PDFDocument1 pageSINOPEC Methanol-To-Olefins (S-MTO) Technology Process PDFHendriyana StNo ratings yet
- Glycol PlantDocument8 pagesGlycol PlantDian Risti PurwantiNo ratings yet
- Executive Summary: Ethylene OxideDocument7 pagesExecutive Summary: Ethylene OxideBlueblurrr100% (1)
- Assignment 1Document8 pagesAssignment 1Sulman KhalidNo ratings yet
- UOP Olefin Production Solutions BrochureDocument2 pagesUOP Olefin Production Solutions BrochureAbhik Banerjee100% (1)
- Butenediol PDFDocument6 pagesButenediol PDFJaamac Dhiil100% (1)
- Ethylene Glycol ProductionDocument1 pageEthylene Glycol ProductionAlexander50% (2)
- Report Control SystemDocument17 pagesReport Control SystemHussein Al HabebNo ratings yet
- Phenol Evaluation - FinalDocument23 pagesPhenol Evaluation - FinalCristian TorrezNo ratings yet
- Production of Ethylene and Propylene From Methanol: I. Over ViewDocument10 pagesProduction of Ethylene and Propylene From Methanol: I. Over ViewPhạm Ngọc ThạchNo ratings yet
- 7 - OligomerizationDocument16 pages7 - OligomerizationAn Lê TrườngNo ratings yet
- Industrial Applications of Olefin MetathesisDocument7 pagesIndustrial Applications of Olefin Metathesisdogmanstar100% (1)
- Advances in Biofeedstocks and Biofuels, Volume 2: Production Technologies for BiofuelsFrom EverandAdvances in Biofeedstocks and Biofuels, Volume 2: Production Technologies for BiofuelsLalit Kumar SinghNo ratings yet
- Clean Ironmaking and Steelmaking Processes: Efficient Technologies for Greenhouse Emissions AbatementFrom EverandClean Ironmaking and Steelmaking Processes: Efficient Technologies for Greenhouse Emissions AbatementNo ratings yet
- Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and PowerFrom EverandThermochemical Processing of Biomass: Conversion into Fuels, Chemicals and PowerNo ratings yet
- Carbon Capture and Storage: The Legal Landscape of Climate Change Mitigation TechnologyFrom EverandCarbon Capture and Storage: The Legal Landscape of Climate Change Mitigation TechnologyNo ratings yet
- Solid Waste Incinerator ManufacturersDocument2 pagesSolid Waste Incinerator Manufacturersbl engineeringNo ratings yet
- Structure Determination of Organic Compounds - ChemvoiceDocument37 pagesStructure Determination of Organic Compounds - ChemvoiceJubin Kumar0% (1)
- 750 Mapefibrest42 GBDocument2 pages750 Mapefibrest42 GBJulianNo ratings yet
- Determination of COD From Supplied Sample.Document3 pagesDetermination of COD From Supplied Sample.Anonymous G304U86No ratings yet
- Heat Transfer Oil SheetDocument1 pageHeat Transfer Oil SheetShamim SazzadNo ratings yet
- Biochemistry Papers MSUDocument20 pagesBiochemistry Papers MSUShiv BhattNo ratings yet
- Prime CoatDocument2 pagesPrime CoatBilal AhmadNo ratings yet
- Materials, Equipments and Fabrication TechniqueDocument17 pagesMaterials, Equipments and Fabrication TechniqueNagarjun CherukuriNo ratings yet
- Chem NotesDocument148 pagesChem Noteskiruba devi .kNo ratings yet
- 978 1 62100 769 2 - ch1Document45 pages978 1 62100 769 2 - ch1João RodriguesNo ratings yet
- TP135 Full ContentDocument65 pagesTP135 Full ContentedgardNo ratings yet
- Paste ThickenerDocument18 pagesPaste Thickenerraymondt153905No ratings yet
- Carbohydrates and Amino Acids Polymers-01-TheoryDocument36 pagesCarbohydrates and Amino Acids Polymers-01-TheoryRaju SinghNo ratings yet
- Chapter.5.ENV HYDRAULICS Students HandoutDocument53 pagesChapter.5.ENV HYDRAULICS Students HandoutTimothy KiryaNo ratings yet
- Topic: Metals and Non-Metals Worksheet KeyDocument6 pagesTopic: Metals and Non-Metals Worksheet KeyPranav SaiNo ratings yet
- MSDS - Loctite Silver Grade Anti-SiezeDocument8 pagesMSDS - Loctite Silver Grade Anti-SiezeToddy SamuelNo ratings yet
- Reactions of Synthetic ImportanceDocument28 pagesReactions of Synthetic ImportanceRx Nadeem ChhipaNo ratings yet
- Chemistry H3 Syllabus 2012Document29 pagesChemistry H3 Syllabus 2012Simon KohNo ratings yet
- Material Equivalent GradeDocument3 pagesMaterial Equivalent GradeRevanNo ratings yet
- MD1 Intro Block Sample ExamDocument13 pagesMD1 Intro Block Sample ExamFrank Mbuli EmorolaNo ratings yet
- GEA Spray Drying: Small-Scale Solutions For R&D and ProductionDocument20 pagesGEA Spray Drying: Small-Scale Solutions For R&D and ProductionPatricio ValenciaNo ratings yet
- Technical Bulletin: Pickling Methods For Duplex Stainless SteelDocument3 pagesTechnical Bulletin: Pickling Methods For Duplex Stainless SteelPhoenix KukuruyukNo ratings yet
- 22 - Analytical TechniquesDocument19 pages22 - Analytical TechniquesRaisa Binte HudaNo ratings yet
- Hafta 6Document22 pagesHafta 6n24zz5hvjwNo ratings yet
- Theoretical and Electrochemical AssessmeDocument10 pagesTheoretical and Electrochemical Assessmechérifa boulechfarNo ratings yet
- Analisa Fizik SPMDocument1 pageAnalisa Fizik SPMkamalharmozaNo ratings yet
- Chapter 5 Refrigerant Systems Chemistry.Document12 pagesChapter 5 Refrigerant Systems Chemistry.Arib RahhimNo ratings yet
- Kids Toothpaste: Phase Ingredients % W/WDocument2 pagesKids Toothpaste: Phase Ingredients % W/WrekhilaNo ratings yet
- The Solubility of Gases in Liquids: Introductory InformationDocument7 pagesThe Solubility of Gases in Liquids: Introductory InformationEwindNo ratings yet
- Ppe MCQ - 300Document36 pagesPpe MCQ - 300raveen_82mechNo ratings yet