Professional Documents
Culture Documents
DivalproexDRtoERClinicalTrialAPA Poster CITROME APA2004
DivalproexDRtoERClinicalTrialAPA Poster CITROME APA2004
Uploaded by
Leslie CitromeOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
DivalproexDRtoERClinicalTrialAPA Poster CITROME APA2004
DivalproexDRtoERClinicalTrialAPA Poster CITROME APA2004
Uploaded by
Leslie CitromeCopyright:
Available Formats
Switching from Standard Divalproex to Extended Release in Schizophrenia
351 Leslie Citrome, MD, MPH; Fabien Tremeau, MD; Pe Shein Wynn, MD, MPH; Biman Roy, MD; Hassan Dinakar, MD
Nathan S. Kline Institute for Psychiatric Research, Orangeburg, NY; New York University School of Medicine, Department of Psychiatry, New York, NY, and Rockland Psychiatric Center, Orangeburg, NY
Abstract Methods Results Discussion/Conclusions
Objective: To assess the safety, efficacy, and tolerability of switching from a multiple dose preparation The study was conducted with inpatients and outpatients at Rockland Psychiatric Center (RPC), A total of 30 subjects with schizophrenia were recruited, with 27 completing Although the two formulations of valproate, divalproex sodium extended release (ER) and the traditional
of divalproex sodium delayed release (DR) to once daily dosing with divalproex sodium extended a 400-bed hospital operated by New York State Office of Mental Health. The outpatients were housed the entire 28-day treatment period. There were 25 (83%) males and Figure 2. Valproate Plasma Levels: divalproex sodium delayed release (DR) formulations, are not bioequivalent, switching was successful
release (ER) in patients with schizophrenia already receiving the standard DR formulation. in residential facilities maintained on the hospital campus. Regardless of location, medications were 5 (17%) females. Most of the subjects (N=20) were inpatients, the remaining Trough-Trough and Peak-Trough Differences (mcg/mL) (i.e., no deterioration in psychopathology) even when conversion was done on a 1.0 mg per 1.0 mg
administered under the supervision of facility staff. ten were outpatients. The average age (with standard deviation) was basis. This was also seen in prior studies of patients with seizure disorder8 and in a group of 55 patients
Method: Thirty subjects with schizophrenia were switched from divalproex DR to a four-week 38.5±10.5 years and the average number of psychiatric hospitalizations was with a wide range of psychiatric disorders4. For our subjects whose baseline plasma levels were less
open-label treatment trial of the ER formulation. Baseline plasma levels of valproate were obtained In this open-label study, 30 subjects were switched from the standard formulation of divalproex 60
15±13. Ethnicity of the study participants were 17 (57%) African-American, than 85 mcg/mL, the switch was also well-tolerated, even though the daily dose was increased using
12 hours post dose. Patients were converted from divalproex DR to ER on a mg per mg basis (rounded sodium to a four-week treatment trial of the extended release formulation. No medication-free period a conversion rate of 1 mg to 1.2 mg, rounded up. The statistically significant improvement in total
6 (20%) White, 6 (20%) Hispanic, and 1 (3%) Other. 40
up to the nearest 500 mg increment) if baseline valproate plasma levels were greater than or equal to was involved. All subjects were between the ages of 18 to 65 years, with a chart diagnosis of DSM-IV BPRS scores, observed in the total sample and marginally among the subjects converted on a 1.0 mg
85 mcg/mL, otherwise the conversion rate was 1.0 mg to 1.2 mg, rounded up. Measured at baseline schizophrenia (supplemented by a SCID diagnosis of schizophrenia for those treated on the specialized Mean baseline BPRS total score was 37.9±9.2 (N=30). Endpoint average 20 per 1.0 mg basis, is small. It may represent the effect of additional time that the subjects are receiving
and end-point were the Brief Psychiatric Rating Scale (BPRS) and the UKU Side Effect Scale. Endpoint inpatient research unit) who have received the same daily dose of divalproex sodium within the range was 35.7±11.2 (N=29). Mean change and standard deviation for the total treatment. It is doubtful that these small changes in the BPRS (approximately a 6% improvement from
plasma levels were obtained at both 12 hours and 24 hours post dose. of 1000 to 3000 mg/day for at least 4 weeks. Patients with a diagnosis of schizoaffective or bipolar BPRS score was thus -2.3±5.4 (N=29). Figure 1 illustrates the variability 0 baseline) are clinically significant.
disorder were not eligible to participate in this study. Patients who were receiving divalproex for the of the changes in total BPRS scores.
Results: Patients switched from divalproex DR to ER had a small improvement noted on the total purposes of seizure or migraine headache prophylaxis were also excluded from participation. -20 As expected, plasma levels of valproate were lower with the ER preparation when converting on
BPRS at endpoint (mean change -2.3±5.4; t=-2.2538; df=28; p=0.0322) and on the UKU (mean a 1.0 mg per 1.0 mg basis. Converting on 1.2 mg per 1.0 mg basis resulted in equivalent trough levels
change -2.2±4.1; t=-2.7361; df=26; p=0.0111). Baseline and endpoint trough plasma levels were The principal dependent variable was the total Brief Psychiatric Rating Scale (BPRS) score6 Figure 1. Change in BPRS and UKU Scores -40
for the two preparations. These differences in plasma levels may not be clinically meaningful and may
80.1±20.4 mcg/mL and 73.1±24.2 mcg/mL respectively. Patients converted on a 1.0 mg per 1.0 mg administered at baseline and endpoint by the same rater for each subject. The BPRS is used routinely from Baseline to Endpoint -60 not result in any perceived improvement or worsening of psychopathology.
basis had lower end-point valproate trough plasma levels than at baseline, but did not experience by RPC clinicians in the regular assessment of their patients. Facility-wide training in the use of the
deterioration on their psychopathology. For all patients, endpoint valproate peak and trough plasma BPRS, including the scoring of interviews observed on videotape, was provided by one of the authors 15 Tolerability and side effects may be related to fluctuations in plasma levels. We have demonstrated
-80
levels were statistically significantly different (t=-3.8706; df=27; p=0.0006), but these differences (LC), however inter-rater reliability measures were not calculated. Trough-Trough Trough-Trough Endpoint peak-trough differences (mean ± standard deviation 14.6±19.6) that were statistically significant,
were small in magnitude (mean 14.6±19.6 mcg/mL). 10 1:1.2 Conversion 1:1 Conversion Peak-Trough but substantially less than comparable work done with the standard formulation of divalproex. This
The secondary outcome measure was the UKU Side Effect Rating Scale7, which allows for the Mean=3.3±34.9 Mean=-21.3±25.0 Mean=14.6±19.6
may explain our observation that the ER formulation was associated with statistically significant
Conclusions: Switching to a once daily formulation of extended release divalproex can be systematic assessment of side effects in several categories: psychic, neurologic, autonomic, (N=17) (N=10)* (N=28)
5 improvements in the UKU Side Effect Scale total score. Pharmacokinetic studies8 have established that
accomplished without a deterioration in psychopathology. The extended release formulation and other. Spontaneously occurring adverse events were also recorded when they occurred. * Excluding subject with unusually low endpoint plasma levels (trough 10 mcg/mL) ER achieves a lower Cmax central value and a higher Cmin mean than the corresponding values for the
of divalproex sodium appears well-tolerated. 0 and who may have been covertly non-compliant with medication treatment.
DR formulation, even after the daily dose of divalproex was increased by 14 – 20% when converting to
Baseline valproate plasma levels were measured 12 hours post dose. Endpoint valproate plasma levels
were measured at both 12 hours and 24 hours post dose. Valproate plasma levels were not obtained the ER preparation. In that study mean peak-to-trough fluctuations of valproate plasma concentrations
-5 Figure 2 illustrates the changes in plasma levels of valproate. Trough levels were 42 – 48% lower for the ER formulation compared with the DR (i.e., for 1500 mg ER, the peak-
in between baseline and endpoint. Additional safety measures included baseline and endpoint blood
chemistry and CBC, including platelet count. were obtained 12 hours post dose for the standard formulation and 24 hours trough difference was 33.8 mcg/mL, but for 1250 mg DR the difference was 60.6 mcg/ml). Reducing
Introduction Patients were converted on a mg per mg basis to the ER formulation if baseline trough (12 hour)
-10 post dose for the ER formulation. Endpoint peak levels were obtained
12 hours post dose for the ER formulation.
fluctuations may reduce side effects associated with peak plasma values. On the other hand,
fluctuations may not be relevant with regard to side effects such as local gastrointestinal irritation.
plasma levels of valproate were greater than or equal to 85 mcg/mL. If baseline trough plasma level -15
Although there is no FDA-approved indication for the use of valproate in patients with schizophrenia,
of valproate was less than 85 mcg/mL, the conversion rate was 1 mg standard formulation to 1.2 mg Mean and standard deviation of divalproex daily dose at study entry was The effect of once daily dosing on medication adherence could not be assessed in our study because
it is extensively prescribed in this population (see adjacent poster #350). Until recently, there have
ER, rounded up. All conversions were rounded up to the nearest 500 mg increment (the 250 mg ER -20 1592±498 mg. This resulted in an average and standard deviation baseline all subjects were administered medications under supervised conditions. However, we speculate that
been two commonly prescribed preparations of valproate, valproic acid and divalproex sodium delayed Total BPRS Score UKU Side Effect Scale
tablet was not available at study initiation) (e.g., all possible ER doses were 1000, 1500, 2000, 2500, Mean Change = -2.3±5.4 Mean Change = -2.2±4.1 trough plasma level of 80.1±20.4 mcg/mL (N=30). Endpoint mean and the convenience of once daily dosing, combined with a favorable tolerability profile, may enhance
release. Equivalent oral doses of either preparation deliver equivalent quantities of the valproate (N=29) (N=27)
3000, 3500, and 4000 mg/day – the latter two dosages could be used only if baseline plasma levels of standard deviation of divalproex dose was 1950±592 mg per day. This compliance with valproate in this challenging treatment population.
molecule systemically1. Introduced in 2000 is an extended release (ER) preparation of divalproex
sodium. The FDA-approved indications are for prophylaxis of migraine headaches and for seizure valproate were less than 85 mcg/mL). The rationale for the 1:1 conversion came from a report (initially resulted in an average and standard deviation endpoint trough plasma level
disorder. It is intended for once-a-day oral administration. However, this formulation is not bioequivalent a poster when this study was designed) where a mg per mg switch was successful in a randomized Using paired t-tests, a statistically significant difference (improvement) of 73.1±24.2 mcg/mL (N=27). The average and standard deviation of the
to the standard delayed release tablets (DR); the ER tablet produces an average bioavailability of crossover study of extended-release vs. enteric-coated formulations of divalproex sodium in epileptic
patients8. The concern about the bioavailablity of the ER formulation being less than that of standard
was found between baseline and endpoint on the total BPRS score for
the entire sample (t=-2.2538; df=28; p=0.0322), and marginally for the
plasma valproate trough-trough differences between baseline and endpoint
for the 1:1.2 conversion group was 3.3±34.9 mcg/mL. That for the 1:1
References
81 – 89% relative to DR tablets given BID2,3.
divalproex sodium was mitigated in our study by the provision of a baseline plasma level “floor” group that was switched on a 1.0 mg per 1.0 mg basis (N=12) (mean conversion group was -21.3±25.0 mcg/mL. The plasma valproate trough- 1. Abbott Laboratories. Depacon – valproate sodium injection, Depakene – valproic acid capsules and syrup, Depakote Sprinkle
Information on switching from DR to ER is limited. Published reports include an open-label 7-day trough differences between baseline and endpoint for the 1 mg per 1 mg Capsules – divalproex sodium coated particles in capsules, Depakote Tablets – divalproex sodium delayed release tablets,
that minimizes the risk of decompensation should bioavailability issues reduce the plasma levels to change=-2.8±4.1; t=-2.1596; df=10; p=0.0561). For the group that Depakote ER – divalproex sodium extended release tablets product information. In Physicians’ Desk Reference, 57th Edition.
study of 55 patients with bipolar disorder, major depression, schizophrenia, schizoaffective disorder, a subtherapeutic amount. The study medication was given daily at 8 AM. There were no changes to was switched on a 1.2 mg per 1.0 mg basis (N=18), this difference was switch group were statistically significant (t=-2.5560; df=9; p=0.0309), Montvale, NJ: Thomson PDR, 2003:416-441.
Alzheimer’s disease, dementia, or intermittent explosive disorder4. Patients were successfully switched divalproex dosing during the course of the study. Inasmuch as possible, other standing medications not statistically significant (mean change=-2.0±6.3; t=-1.2698; df=16; but this was not the case for the subjects whose dose was increased 2. Abbott Laboratories. Depakote ER Divalproex Sodium Extended Release Tablets, Formulary Information. Abbott Park, IL:
on a mg per mg basis. In a report of 12 patients with bipolar disorder or schizoaffective disorder on a 1.2 mg per mg basis (t=0.3782; df=16; p=0.7102). The average and Abbott Laboratories, 2000.
were also held constant. p=0.2223). 3. Dutta S, Zhang Y, Selness DS, et al. Comparison of the bioavailability of unequal doses of divalproex sodium extended release
switched to ER in an open-label 6-week trial, doses were adjusted to maintain steady serum valproate standard deviation of the plasma valproate peak-trough differences at formulation relative to the delayed release formulation in healthy volunteers. Epilepsy Res, 2002;49(1):1-10.
concentrations5. Although the switch was well-tolerated, 21% higher doses of the ER preparation were The primary analysis tested the hypothesis that conversion to ER would result in no deterioration in the Mean baseline UKU total score was 8.8±6.7 (N=29). Endpoint average endpoint was 14.6±19.6 mcg/mL, and was statistically significant 4. Horne RL, Cunanan C. Safety and efficacy of switching psychiatric patients from a delayed-release to an extended release
required. Both of the above reports4, 5 are limited by their lack of a parallel group design and diagnostic total BPRS score. The sample size was selected on the basis of logistical constraints; a power analysis was 7.5±5.8 (N=28). Usable baseline and endpoint UKU ratings were (t=-3.8706; df=27; p=0.0006). formulation of divalproex sodium. J Clin Psychopharmacol, 2003;23(2):176-181.
was not done. We compared baseline and endpoint total BPRS scores using the paired t-test. Secondary available for 27 subjects, resulting in mean change and standard deviation 5. Centorrino F, Kelleher JP, Berry JM, et al. Pilot comparison of extended release and standard preparations of divalproex sodium
heterogeneity. in patients with bipolar and schizoaffective disorders. Am J Psychiatry, 2003;160(7):1348-1350.
analyses tested the hypotheses that conversion to ER will result in lower UKU Side Effect Scores. This for the total UKU score to be -2.2±4.1. Using paired t-tests, a statistically 6. Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep, 1962;10:799-812.
Switching to a once daily preparation of valproate may result in improved compliance and lower risk of was examined by using paired t-tests (two-tailed). significant difference was found between baseline and endpoint on the total 7. Lingjaerde O, Ahlfors UG, Bech P, et al. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic
relapse for those patients with schizophrenia who derive benefit from adjunctive valproate, and perhaps UKU score for the entire sample (t=-2.7361; df=26; p=0.0111). drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl, 1987;334:1-100.
greater patient satisfaction. To our knowledge, this is the first report of a systematic study examining Baseline and endpoint plasma valproate levels were compared using paired t-tests (two-tailed) to test 8. Thibault M, Blume WT, Saint-Hilaire JM, et al. Divalproex extended release versus the original divalproex tablet: results
the hypothesis that conversion to the ER formulation will result in lower plasma levels if the switch is of a randomized, crossover study of well-controlled epileptic patients with primary generalized seizures. Epilepsy Res, 2002;50(3):
the feasibility of switching to ER from DR in patients with schizophrenia (and excluding patients with 243-249.
schizoaffective or bipolar disorder). done on a mg per mg basis.
Presented at the 157th Annual Meeting of the American Psychiatric Association, New York City, NY • May 4, 2004.
This poster contains information that has not been
Supported by funding from Abbott Laboratories In Press in the Journal of Clinical Psychopharmacology. approved by the U.S. Food and Drug Administration.
0418929_02A
You might also like
- Second and Third Generation Antipsychotics: A Comprehensive HandbookFrom EverandSecond and Third Generation Antipsychotics: A Comprehensive HandbookRating: 5 out of 5 stars5/5 (1)
- Distributor ObatDocument3 pagesDistributor Obatkimiafarma antang75% (4)
- Tohen 2003Document9 pagesTohen 2003Francisco VillalonNo ratings yet
- Jurnal ReadingDocument10 pagesJurnal ReadingAndreas NatanNo ratings yet
- Clotiapine - Another Forgotten Treassure in PsychiatryDocument1 pageClotiapine - Another Forgotten Treassure in PsychiatryJuan IgnacioNo ratings yet
- Clozapine Alone Versus Clozapine and Risperidone With Refractory SchizophreniaDocument11 pagesClozapine Alone Versus Clozapine and Risperidone With Refractory SchizophreniawardahNo ratings yet
- Longterm Safety and Effectiveness of Lurasidone in Schizophrenia A 22month Openlabel Extension StudyDocument10 pagesLongterm Safety and Effectiveness of Lurasidone in Schizophrenia A 22month Openlabel Extension Studyromany hosnyNo ratings yet
- Green 2009Document8 pagesGreen 2009Nadia SaiNo ratings yet
- Treatment of Delirium With Quetiapine: One Personal Copy May Be PrintedDocument3 pagesTreatment of Delirium With Quetiapine: One Personal Copy May Be PrintedMahmoud WardNo ratings yet
- Potkin2002 QTP+ Hal Risp Thiridazine RCTDocument10 pagesPotkin2002 QTP+ Hal Risp Thiridazine RCTIulia CiocotisanNo ratings yet
- Rituximab in Hephrotic SyndromeDocument8 pagesRituximab in Hephrotic Syndromejennylau0809No ratings yet
- Childhood Idiopathic Nephrotic Syndrome in Turkey: Original ArticleDocument4 pagesChildhood Idiopathic Nephrotic Syndrome in Turkey: Original ArticleDrago BakovicNo ratings yet
- Clozapin RisperidonDocument7 pagesClozapin RisperidonAnonymous 2LcGdsRNo ratings yet
- Alpha-Dihydroergocryptine vs. Pramipexole As Adjunct Symptomatic Treatment of Idiopathic Parkinson'sDocument9 pagesAlpha-Dihydroergocryptine vs. Pramipexole As Adjunct Symptomatic Treatment of Idiopathic Parkinson'sRizka Leonita FahmyNo ratings yet
- Journal Presentation Dr. Jasmine RSSSDocument48 pagesJournal Presentation Dr. Jasmine RSSSAhuja SaurabhNo ratings yet
- Britishjournalofpsychiatry BRITISHJOURNALOFPSYCHIATRY (2001), 179,514 518 (2 0 0 1), 1 7 9, 5 1 4 5 1 8Document6 pagesBritishjournalofpsychiatry BRITISHJOURNALOFPSYCHIATRY (2001), 179,514 518 (2 0 0 1), 1 7 9, 5 1 4 5 1 8Imam FirdausNo ratings yet
- Valproic Acid and Risperidone: A Drug Interaction?: To The EditorDocument2 pagesValproic Acid and Risperidone: A Drug Interaction?: To The EditorFariz RifqiNo ratings yet
- Valproate For Agitation in Critically Ill Patients - A Retrospective Study-2017Document7 pagesValproate For Agitation in Critically Ill Patients - A Retrospective Study-2017Juan ParedesNo ratings yet
- A Randomized, Double-Blind, Placebo-Controlled Study of Citalopram in Adolescents With Major Depressive Disorder - Von Knorring 2006Document5 pagesA Randomized, Double-Blind, Placebo-Controlled Study of Citalopram in Adolescents With Major Depressive Disorder - Von Knorring 2006Julio JuarezNo ratings yet
- J Schres 2004 01 014Document17 pagesJ Schres 2004 01 014xhdrv7nvdrNo ratings yet
- 1 s2.0 S2772408522006408 MainDocument2 pages1 s2.0 S2772408522006408 MainloloasbNo ratings yet
- Ajp 156 5 702Document8 pagesAjp 156 5 7029 PsychologyNo ratings yet
- TX Nmda EncefDocument9 pagesTX Nmda EncefserftyNo ratings yet
- Alzheimer PicoDocument6 pagesAlzheimer PicoRaja Friska YulandaNo ratings yet
- Apathy in Currently Nondepressed Patients Treated With A SSRI For A Major Depressive Episode - Outcomes Following RandomDocument8 pagesApathy in Currently Nondepressed Patients Treated With A SSRI For A Major Depressive Episode - Outcomes Following RandomMooyongNo ratings yet
- Aac 37 2 178Document5 pagesAac 37 2 178Marcelo salvador silva MacotoraNo ratings yet
- Safety and Effectiveness of Lurasidone in Adolescents With Schizophrenia: Results of A 2-Year, Open-Label Extension StudyDocument11 pagesSafety and Effectiveness of Lurasidone in Adolescents With Schizophrenia: Results of A 2-Year, Open-Label Extension StudyNaiana PaulaNo ratings yet
- Dexmedetomidine Is Effective For Sedation For Outpatient ElectroencephalographyDocument6 pagesDexmedetomidine Is Effective For Sedation For Outpatient ElectroencephalographyRudi HerdiansyahNo ratings yet
- Clozapine and Haloperidol in ModeratelyDocument8 pagesClozapine and Haloperidol in Moderatelyrinaldiapt08No ratings yet
- The Efficacy of Propofol and Midazolam in Treatment of Refractory Status Epilepticus in ChildrenDocument6 pagesThe Efficacy of Propofol and Midazolam in Treatment of Refractory Status Epilepticus in ChildrenReci MaulitaNo ratings yet
- Ajp.161.10.1837 2Document11 pagesAjp.161.10.1837 2HKANo ratings yet
- Use of Very-High-Dose Olanzapine in Treatment-Resistant SchizophreniaDocument4 pagesUse of Very-High-Dose Olanzapine in Treatment-Resistant SchizophreniaPutu Agus GrantikaNo ratings yet
- Coping Therapy Dependence: Naltrexone and Skills For AlcoholDocument7 pagesCoping Therapy Dependence: Naltrexone and Skills For AlcoholOana BumbucNo ratings yet
- Lurasidone in Older Adults With Bipolar DepressionDocument8 pagesLurasidone in Older Adults With Bipolar DepressionSarah El HalabiNo ratings yet
- Clozapine: SchizophrenicDocument8 pagesClozapine: SchizophrenicVictoria FellowsNo ratings yet
- Update On Therapeutics: Shehan Williams and Thilini RajapakseDocument3 pagesUpdate On Therapeutics: Shehan Williams and Thilini RajapakseAnjula KumarasingheNo ratings yet
- ZiprasidoneHaloperidolAgitationlPostHocAbstractICOSR CITROME SchizBull2005Document2 pagesZiprasidoneHaloperidolAgitationlPostHocAbstractICOSR CITROME SchizBull2005Leslie CitromeNo ratings yet
- Propofol Kelly 1999Document11 pagesPropofol Kelly 1999dai leNo ratings yet
- Deberdt 2008Document8 pagesDeberdt 2008Lidia BorleanNo ratings yet
- A Comparison of Risperidone and Haloperidol For The Prevention of Relapse in Patients With SchizophreniaDocument8 pagesA Comparison of Risperidone and Haloperidol For The Prevention of Relapse in Patients With SchizophreniaIlham WahyuNo ratings yet
- LurasidoneDocument1 pageLurasidoneRaju Teach KapsNo ratings yet
- IJP Volume 4 Issue 1 Pages 1233-1242Document10 pagesIJP Volume 4 Issue 1 Pages 1233-1242Anca AdamNo ratings yet
- HEAD NeurologieDocument13 pagesHEAD NeurologieMihaela MavrodinNo ratings yet
- Rathore SS. Digoxin in Treatment of Heart FailureDocument8 pagesRathore SS. Digoxin in Treatment of Heart FailureDinhLinhNo ratings yet
- Espironolactona en DiálisisDocument11 pagesEspironolactona en DiálisisMaria Juliana ValenzuelaNo ratings yet
- Weinblad 2001Document1 pageWeinblad 2001remiNo ratings yet
- Daytime Sleepiness Associated With LurasidoneDocument9 pagesDaytime Sleepiness Associated With Lurasidoneromany hosnyNo ratings yet
- Donepezil in Vascular Dementia: A Randomized, Placebo-Controlled StudyDocument9 pagesDonepezil in Vascular Dementia: A Randomized, Placebo-Controlled StudyDian ArdiansyahNo ratings yet
- Aripiprazole Monotherapy in Acute Mania: 12-Week Randomised Placebo-And Haloperidol - Controlled StudyDocument12 pagesAripiprazole Monotherapy in Acute Mania: 12-Week Randomised Placebo-And Haloperidol - Controlled StudySherly VeronicaNo ratings yet
- Modafinil and Armodafinil in Schizophrenia: Chittaranjan Andrade, MDDocument3 pagesModafinil and Armodafinil in Schizophrenia: Chittaranjan Andrade, MDbenedicte lewinNo ratings yet
- Riociguat - ReviewDocument4 pagesRiociguat - Reviewapi-302147754No ratings yet
- Kane1988 PDFDocument8 pagesKane1988 PDFRavi KumarNo ratings yet
- Singh. NMP Better Result VS DYDDocument2 pagesSingh. NMP Better Result VS DYDRuth RachmawatyNo ratings yet
- Wei Zheng, 2018Document8 pagesWei Zheng, 2018Hananya ManroeNo ratings yet
- Wilcock 2008Document11 pagesWilcock 2008Nadia SaiNo ratings yet
- Ann Pharmacother 2011 Owenby 95 100Document6 pagesAnn Pharmacother 2011 Owenby 95 100Mahmud AnshoriNo ratings yet
- Hyper Na CorrectionDocument11 pagesHyper Na CorrectionalizanNo ratings yet
- Research Paper: NeuropsychiatryDocument11 pagesResearch Paper: NeuropsychiatryveerrajuNo ratings yet
- Clinical Cardiology - May 1992 - Massie - 24 Hour Efficacy of Once Daily Diltiazem in Essential HypertensionDocument4 pagesClinical Cardiology - May 1992 - Massie - 24 Hour Efficacy of Once Daily Diltiazem in Essential Hypertensionarkarchitecture2023No ratings yet
- 5 PharmacologyDocument4 pages5 Pharmacologyshiv_prhNo ratings yet
- Treatment–Refractory Schizophrenia: A Clinical ConundrumFrom EverandTreatment–Refractory Schizophrenia: A Clinical ConundrumPeter F. BuckleyNo ratings yet
- ZolpidemProductLabel 0819 PDFDocument7 pagesZolpidemProductLabel 0819 PDFLeslie CitromeNo ratings yet
- Restoril™ (Temazepam) Capsules USP RX Only Warning: Risks From Concomitant Use With OpioidsDocument14 pagesRestoril™ (Temazepam) Capsules USP RX Only Warning: Risks From Concomitant Use With OpioidsLeslie CitromeNo ratings yet
- See Full Prescribing Information For Complete Boxed WarningDocument14 pagesSee Full Prescribing Information For Complete Boxed WarningLeslie CitromeNo ratings yet
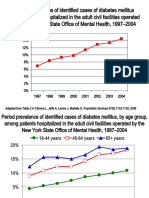
- DiabetesEpidemiologySlidesForDistribution CITROME AdaptedPsychServ2006Document8 pagesDiabetesEpidemiologySlidesForDistribution CITROME AdaptedPsychServ2006Leslie CitromeNo ratings yet
- WhatIsTranscranialMagneticStimulation CITROME KlineLine1999Document1 pageWhatIsTranscranialMagneticStimulation CITROME KlineLine1999Leslie CitromeNo ratings yet
- CATIENNTEditorialRegardingCITROME KERWIN IntJClinPract2006Document2 pagesCATIENNTEditorialRegardingCITROME KERWIN IntJClinPract2006Leslie CitromeNo ratings yet
- ZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME CINP2006Document1 pageZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME CINP2006Leslie CitromeNo ratings yet
- UpdateBiologicalTreatmentAggression VOLAVKA ActaEspPsiquiatr2006Document13 pagesUpdateBiologicalTreatmentAggression VOLAVKA ActaEspPsiquiatr2006Leslie CitromeNo ratings yet
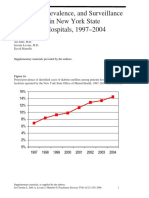
- DiabetesEpidemiologyFiguresSupplement CITROME PsychServ2006onlineDocument5 pagesDiabetesEpidemiologyFiguresSupplement CITROME PsychServ2006onlineLeslie CitromeNo ratings yet
- ZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME APA2006Document1 pageZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME APA2006Leslie CitromeNo ratings yet
- OlanzapineEarlyPredictorsWeightGainBipolarDisorder LIPKOVICH JClinPsychopharm2006Document5 pagesOlanzapineEarlyPredictorsWeightGainBipolarDisorder LIPKOVICH JClinPsychopharm2006Leslie CitromeNo ratings yet
- OlanzapineHighDoseRCTHGLFPoster KINON CINP2006Document19 pagesOlanzapineHighDoseRCTHGLFPoster KINON CINP2006Leslie CitromeNo ratings yet
- IncidencePrevalenceSurveillanceDiabetesMellitusInpatientsPoster CITROME NCDEU2006Document1 pageIncidencePrevalenceSurveillanceDiabetesMellitusInpatientsPoster CITROME NCDEU2006Leslie CitromeNo ratings yet
- DiabetesSchizophreniaInterview CITROME BehavHealthCare2006Document8 pagesDiabetesSchizophreniaInterview CITROME BehavHealthCare2006Leslie CitromeNo ratings yet
- Benefits of A Second Dose of Intramuscular (IM) Aripiprazole To Control Agitation in Patients With Schizophrenia or Bipolar I DisorderDocument1 pageBenefits of A Second Dose of Intramuscular (IM) Aripiprazole To Control Agitation in Patients With Schizophrenia or Bipolar I DisorderLeslie CitromeNo ratings yet
- Sscchhiizzoopphhrreenniiaa: Ccuurrrreenntt Ttrreeaattm Meenntt CcoonnssiiddeerraattiioonnssDocument4 pagesSscchhiizzoopphhrreenniiaa: Ccuurrrreenntt Ttrreeaattm Meenntt CcoonnssiiddeerraattiioonnssLeslie CitromeNo ratings yet
- ZiprasidoneHaloperidolAgitationlPostHocPoster CITROME ACNP2004Document1 pageZiprasidoneHaloperidolAgitationlPostHocPoster CITROME ACNP2004Leslie CitromeNo ratings yet
- MedicalTrainingUnitedStatesAddendum CITROME CMAJ1992Document2 pagesMedicalTrainingUnitedStatesAddendum CITROME CMAJ1992Leslie CitromeNo ratings yet
- Leslie Citrome, MD, MPH, Richard Josiassen, PHD, Nigel Bark, MD, Karen S Brown, MS, Suresh Mallikaarjun, PHD, Daniel E Salazar, PHDDocument1 pageLeslie Citrome, MD, MPH, Richard Josiassen, PHD, Nigel Bark, MD, Karen S Brown, MS, Suresh Mallikaarjun, PHD, Daniel E Salazar, PHDLeslie CitromeNo ratings yet
- AtypicalAntipsychoticsDiabetesMellitusCaseControlACNPPoster CITROME ACNP2003Document1 pageAtypicalAntipsychoticsDiabetesMellitusCaseControlACNPPoster CITROME ACNP2003Leslie CitromeNo ratings yet
- MoodStabilizerUtilizationAbbottAPAPoster CITROME 2004Document1 pageMoodStabilizerUtilizationAbbottAPAPoster CITROME 2004Leslie CitromeNo ratings yet
- AtypicalAntipsychoticsDiabetesMellitusCaseControlAPAPoster CITROME APA2004Document1 pageAtypicalAntipsychoticsDiabetesMellitusCaseControlAPAPoster CITROME APA2004Leslie CitromeNo ratings yet
- DosingSGAPosterWCBPHandout CITROME 2005Document4 pagesDosingSGAPosterWCBPHandout CITROME 2005Leslie CitromeNo ratings yet
- NewTreatmentsAgitationReview CITROME PsychQuarterly2004Document18 pagesNewTreatmentsAgitationReview CITROME PsychQuarterly2004Leslie CitromeNo ratings yet
- Mood Stabilizer and Antipsychotic Medication Coprescribing (Polypharmacy)Document1 pageMood Stabilizer and Antipsychotic Medication Coprescribing (Polypharmacy)Leslie CitromeNo ratings yet
- Annex 1 - Health Facilities For The Police and PrisonsDocument4 pagesAnnex 1 - Health Facilities For The Police and PrisonsZuhura NassorNo ratings yet
- Activity 12: Parts of A Drug Guide: Generic NameDocument8 pagesActivity 12: Parts of A Drug Guide: Generic NameJerry LicayanNo ratings yet
- Regulatory AffairsDocument8 pagesRegulatory Affairsnsk79in@gmail.com100% (1)
- CostPlusDrugs - Price ListDocument66 pagesCostPlusDrugs - Price ListCBS 11 NewsNo ratings yet
- BiopharmaceuticsDocument21 pagesBiopharmaceuticsStefano PorzioNo ratings yet
- IP121lec - Topic 3 - Calculation of Doses PDFDocument47 pagesIP121lec - Topic 3 - Calculation of Doses PDFBj LarracasNo ratings yet
- Nada Lincomycyn and Espectynomycin Solubleucm061812Document4 pagesNada Lincomycyn and Espectynomycin Solubleucm061812laurz95No ratings yet
- A Simple Practice Guide For Dose Conversion Between Human and AnimalsDocument5 pagesA Simple Practice Guide For Dose Conversion Between Human and AnimalsKishor BajgainNo ratings yet
- 9 - DR Arun Bhatt - Schedule YDocument27 pages9 - DR Arun Bhatt - Schedule Yvivek100% (1)
- Drugs ActDocument44 pagesDrugs ActBhavithavNo ratings yet
- Unit 13-English for Medicine and Pharmacy 2: *Bắt buộcDocument8 pagesUnit 13-English for Medicine and Pharmacy 2: *Bắt buộcNguyễn Thành LongNo ratings yet
- Drug ResearchDocument33 pagesDrug ResearchJakobus Benny SalimNo ratings yet
- E PrescribingDocument2 pagesE PrescribingDakota SimbsNo ratings yet
- CONOL - BETA BLOCKERS (Selective and Non Selective)Document22 pagesCONOL - BETA BLOCKERS (Selective and Non Selective)Jewelyn ConolNo ratings yet
- Grade 6 Answer KeyDocument8 pagesGrade 6 Answer KeyAngelica BuquiranNo ratings yet
- HJM 25 Okt 2023Document16 pagesHJM 25 Okt 2023Mel WsNo ratings yet
- Study Guide-Pharmacology-PrelimDocument20 pagesStudy Guide-Pharmacology-Prelimcath payotNo ratings yet
- Buffer Stock RJDocument9 pagesBuffer Stock RJardinaNo ratings yet
- Pharmacy CareDocument9 pagesPharmacy CareJoslin RozNo ratings yet
- Medicine Prescriptions JavaDocument6 pagesMedicine Prescriptions Javaapi-636650239No ratings yet
- Pharmaceuticals Executive Summary PDFDocument10 pagesPharmaceuticals Executive Summary PDFPaes C. MarceloNo ratings yet
- Inactive Ingredient Search For Approved Dhtghrug ProductsDocument2 pagesInactive Ingredient Search For Approved Dhtghrug ProductsDang Chi CongNo ratings yet
- Ijpab 2015 3 1 224 235 PDFDocument12 pagesIjpab 2015 3 1 224 235 PDFDrAmit VermaNo ratings yet
- Pharmacology Daily Practice PaperDocument7 pagesPharmacology Daily Practice PaperSunil Kumar MahapatraNo ratings yet
- Julfar Ksa - Google SearchDocument1 pageJulfar Ksa - Google Searchptq9cpxpf9No ratings yet
- Annex A1 - KonSulTa Meds PDFDocument4 pagesAnnex A1 - KonSulTa Meds PDFEric Monte de RamosNo ratings yet
- Covid Resource Bhopal v1.0.KTDocument14 pagesCovid Resource Bhopal v1.0.KTKapil TilwaniNo ratings yet
- Polypharmacy: Gina Desevo Bellotti and Emily R. HajjarDocument8 pagesPolypharmacy: Gina Desevo Bellotti and Emily R. Hajjarwidodo adi prasetyoNo ratings yet
- Laiman'S Pharmacy: Pre-Test ExaminationDocument3 pagesLaiman'S Pharmacy: Pre-Test ExaminationChristine PaulineNo ratings yet