Professional Documents
Culture Documents
ZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME CINP2006
ZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME CINP2006
Uploaded by
Leslie CitromeCopyright:
Available Formats
You might also like
- Hesi Psych Study GuideDocument16 pagesHesi Psych Study GuideR100% (17)
- Second and Third Generation Antipsychotics: A Comprehensive HandbookFrom EverandSecond and Third Generation Antipsychotics: A Comprehensive HandbookRating: 5 out of 5 stars5/5 (1)
- Apathy in Currently Nondepressed Patients Treated With A SSRI For A Major Depressive Episode - Outcomes Following RandomDocument8 pagesApathy in Currently Nondepressed Patients Treated With A SSRI For A Major Depressive Episode - Outcomes Following RandomMooyongNo ratings yet
- 174 Full PDFDocument7 pages174 Full PDFAsyha KantifaNo ratings yet
- Yoa70030 1115 1122Document8 pagesYoa70030 1115 1122Dewi NofiantiNo ratings yet
- Iosifescu (2011) - Electroencephalography-Derived Biomarkers of Antidepressant Response.Document11 pagesIosifescu (2011) - Electroencephalography-Derived Biomarkers of Antidepressant Response.Julieht RodriguezNo ratings yet
- S169726002300008XDocument7 pagesS169726002300008XAna Carolina SandovalNo ratings yet
- CGJ01Document8 pagesCGJ01Dewi NofiantiNo ratings yet
- Anhedonia and Reward Circuit Connectivity Distinguish Nonresponders From Responders To Dorsomedial Prefrontal Repetitive Transcranial Magnetic Stimulation in Major DepressionDocument10 pagesAnhedonia and Reward Circuit Connectivity Distinguish Nonresponders From Responders To Dorsomedial Prefrontal Repetitive Transcranial Magnetic Stimulation in Major DepressionKhory Aurora BertyNo ratings yet
- Ketamine NNTDocument8 pagesKetamine NNTBrent AllieNo ratings yet
- Revisión AntipsicóticosDocument6 pagesRevisión AntipsicóticosManel EMNo ratings yet
- Appi Ajp 2014 13121625Document9 pagesAppi Ajp 2014 13121625Orion OriNo ratings yet
- ESTUDIO CLINICO - Effects of Cannabidiol in The Treatment of Patients With ParkinsonDocument5 pagesESTUDIO CLINICO - Effects of Cannabidiol in The Treatment of Patients With ParkinsonBRIGITH NATALIA GUIO VARGASNo ratings yet
- Appi Ajp 2017 16091037 ds001Document6 pagesAppi Ajp 2017 16091037 ds001goldfishxNo ratings yet
- Exercise DepressionDocument17 pagesExercise Depressionlakshminivas PingaliNo ratings yet
- Artículo2 Grupo 5Document7 pagesArtículo2 Grupo 5LISBETH PEÑANo ratings yet
- Arbabi2013 PDFDocument4 pagesArbabi2013 PDFnur afniNo ratings yet
- Ejercicio y DepresiónDocument17 pagesEjercicio y DepresiónjosedegibesNo ratings yet
- BPD e DopaminaDocument11 pagesBPD e Dopaminaaire.puraNo ratings yet
- Predicting Treatment Response in Social Anxiety Disorder From Functional Magnetic Resonance ImagingDocument11 pagesPredicting Treatment Response in Social Anxiety Disorder From Functional Magnetic Resonance ImagingJave GajellomaNo ratings yet
- Wei Zheng, 2018Document8 pagesWei Zheng, 2018Hananya ManroeNo ratings yet
- OlanzapineDocument10 pagesOlanzapineWaoNo ratings yet
- Critical Review of Psilocybin DepressionDocument16 pagesCritical Review of Psilocybin DepressionAshok Kumar KrishnamoorthyNo ratings yet
- Altered Dynamic Regional Homogeneity in Patients With Conduct DisorderDocument7 pagesAltered Dynamic Regional Homogeneity in Patients With Conduct DisorderjojdoNo ratings yet
- Neuroimage: Clinical: SciencedirectDocument9 pagesNeuroimage: Clinical: SciencedirectMoumi PanditNo ratings yet
- PCN 12335Document8 pagesPCN 12335Ariel GaonNo ratings yet
- Brain Stimulation and Other Biological Non-Pharmacological Interventions in Mental Disorders: An Umbrella ReviewDocument18 pagesBrain Stimulation and Other Biological Non-Pharmacological Interventions in Mental Disorders: An Umbrella Reviewgermanos.dutraNo ratings yet
- Depression and Anxiety in Parkinson 'S Disease: Anette Schrag, Raquel N. TaddeiDocument33 pagesDepression and Anxiety in Parkinson 'S Disease: Anette Schrag, Raquel N. TaddeiMerari Lugo OcañaNo ratings yet
- Pharmacotherapy For Dissociative Disorders - A Systematic ReviewDocument9 pagesPharmacotherapy For Dissociative Disorders - A Systematic ReviewRita Saravia TasaycoNo ratings yet
- Management of Psychiatric and CognitiveDocument21 pagesManagement of Psychiatric and CognitiveloloasbNo ratings yet
- Estres Agudo Bryant Et Al (2008)Document9 pagesEstres Agudo Bryant Et Al (2008)Eleoner RamirezNo ratings yet
- What Does The PANSS Mean?Document8 pagesWhat Does The PANSS Mean?Farin MauliaNo ratings yet
- Asian Journal of PsychiatryDocument8 pagesAsian Journal of PsychiatryalbdapplimNo ratings yet
- Original PaperDocument9 pagesOriginal PaperSharvari ShahNo ratings yet
- Programme 1615Document29 pagesProgramme 1615Matias RizzoneNo ratings yet
- Treatment-Resistant Schizophrenia: Genetic and Neuroimaging CorrelatesDocument16 pagesTreatment-Resistant Schizophrenia: Genetic and Neuroimaging CorrelatesBianca CaterinalisendraNo ratings yet
- Pregabalina GADDocument7 pagesPregabalina GADGabriel LemosNo ratings yet
- Davidson 2004Document9 pagesDavidson 2004rf6yssdry2No ratings yet
- Pharmacological Treatment of Schizophrenia: Japanese Expert ConsensusDocument8 pagesPharmacological Treatment of Schizophrenia: Japanese Expert ConsensusArif IrpanNo ratings yet
- AripiprazolDocument15 pagesAripiprazolFabrício de Souza XavierNo ratings yet
- 7.tardive Dyskinesia Prevalence in The Period of Second-Generation Antipsychotic UseDocument15 pages7.tardive Dyskinesia Prevalence in The Period of Second-Generation Antipsychotic UseDebit SophasingNo ratings yet
- Whitton Et Al., 2021Document10 pagesWhitton Et Al., 2021Jonathan PitreNo ratings yet
- Predictors of Long-Term Improvement Following Cognitive Remediation in A Sample With Elevated Depressive SymptomsDocument11 pagesPredictors of Long-Term Improvement Following Cognitive Remediation in A Sample With Elevated Depressive SymptomsNerea Montero GarcíaNo ratings yet
- Kato 2005Document3 pagesKato 2005Huyền MiNo ratings yet
- Treatment of Acute Stress DisorderDocument9 pagesTreatment of Acute Stress DisorderAlessandra LacerdaNo ratings yet
- Metaanalysis of Efficacy and Safety of SGADocument12 pagesMetaanalysis of Efficacy and Safety of SGAdr.stojiljkovicNo ratings yet
- Fnhum 16 1029256Document22 pagesFnhum 16 1029256Krikunov KaNo ratings yet
- Translational Psychodelic Science RdocDocument39 pagesTranslational Psychodelic Science RdocRodrigo SosaNo ratings yet
- Ji-Hua Xu 2018Document3 pagesJi-Hua Xu 2018Mary FallNo ratings yet
- Pitman 2002 Biological-PsychiatryDocument4 pagesPitman 2002 Biological-PsychiatryQwerty QwertyNo ratings yet
- Add-On Pharmacotherapy For PatientsDocument2 pagesAdd-On Pharmacotherapy For PatientsmarcoNo ratings yet
- Cognitive Discernible Factors Between Schizophrenia and Schizoavective DisorderDocument4 pagesCognitive Discernible Factors Between Schizophrenia and Schizoavective DisorderdarkarotNo ratings yet
- NIH Public Access: Author ManuscriptDocument18 pagesNIH Public Access: Author ManuscriptAnonymous hvOuCjNo ratings yet
- Cranial Electrotherapy Stimulation For The Treatment of Chronically Symptomatic Bipolar PatientsDocument2 pagesCranial Electrotherapy Stimulation For The Treatment of Chronically Symptomatic Bipolar PatientsAna Maria AmanaturalisNo ratings yet
- Barret, 2006Document7 pagesBarret, 2006Elisabet GobelliNo ratings yet
- A Comparison of The Effectiveness of Three Drug Regimens On Cognitive Performance of Patients With Parkinson's DiseaseDocument12 pagesA Comparison of The Effectiveness of Three Drug Regimens On Cognitive Performance of Patients With Parkinson's DiseaseGolita emsakiNo ratings yet
- Tea Com LaserDocument20 pagesTea Com LaserPaloma ChagasNo ratings yet
- Physical Activity Reduces Clinical Symptoms and Restores Neuroplasticity in Major DepressionDocument15 pagesPhysical Activity Reduces Clinical Symptoms and Restores Neuroplasticity in Major DepressionVerônica DuquinnaNo ratings yet
- Clinical Implications of Brief Psychiatric Rating Scale ScoresDocument6 pagesClinical Implications of Brief Psychiatric Rating Scale Scores주병욱No ratings yet
- Fourth International Conference On Alzheimer'S Disease s137Document2 pagesFourth International Conference On Alzheimer'S Disease s137Sergiy Prokofiy MontanoNo ratings yet
- Variance in Neurocognitive Performance Is Associated With Dysbindin-1 in Schizophrenia: A Preliminary StudyDocument5 pagesVariance in Neurocognitive Performance Is Associated With Dysbindin-1 in Schizophrenia: A Preliminary Studyfajar tampubolonNo ratings yet
- ZolpidemProductLabel 0819 PDFDocument7 pagesZolpidemProductLabel 0819 PDFLeslie CitromeNo ratings yet
- Restoril™ (Temazepam) Capsules USP RX Only Warning: Risks From Concomitant Use With OpioidsDocument14 pagesRestoril™ (Temazepam) Capsules USP RX Only Warning: Risks From Concomitant Use With OpioidsLeslie CitromeNo ratings yet
- See Full Prescribing Information For Complete Boxed WarningDocument14 pagesSee Full Prescribing Information For Complete Boxed WarningLeslie CitromeNo ratings yet
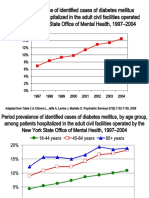
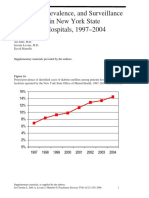
- DiabetesEpidemiologySlidesForDistribution CITROME AdaptedPsychServ2006Document8 pagesDiabetesEpidemiologySlidesForDistribution CITROME AdaptedPsychServ2006Leslie CitromeNo ratings yet
- WhatIsTranscranialMagneticStimulation CITROME KlineLine1999Document1 pageWhatIsTranscranialMagneticStimulation CITROME KlineLine1999Leslie CitromeNo ratings yet
- CATIENNTEditorialRegardingCITROME KERWIN IntJClinPract2006Document2 pagesCATIENNTEditorialRegardingCITROME KERWIN IntJClinPract2006Leslie CitromeNo ratings yet
- IncidencePrevalenceSurveillanceDiabetesMellitusInpatientsPoster CITROME NCDEU2006Document1 pageIncidencePrevalenceSurveillanceDiabetesMellitusInpatientsPoster CITROME NCDEU2006Leslie CitromeNo ratings yet
- Leslie Citrome, MD, MPH, Richard Josiassen, PHD, Nigel Bark, MD, Karen S Brown, MS, Suresh Mallikaarjun, PHD, Daniel E Salazar, PHDDocument1 pageLeslie Citrome, MD, MPH, Richard Josiassen, PHD, Nigel Bark, MD, Karen S Brown, MS, Suresh Mallikaarjun, PHD, Daniel E Salazar, PHDLeslie CitromeNo ratings yet
- DiabetesEpidemiologyFiguresSupplement CITROME PsychServ2006onlineDocument5 pagesDiabetesEpidemiologyFiguresSupplement CITROME PsychServ2006onlineLeslie CitromeNo ratings yet
- OlanzapineEarlyPredictorsWeightGainBipolarDisorder LIPKOVICH JClinPsychopharm2006Document5 pagesOlanzapineEarlyPredictorsWeightGainBipolarDisorder LIPKOVICH JClinPsychopharm2006Leslie CitromeNo ratings yet
- DiabetesSchizophreniaInterview CITROME BehavHealthCare2006Document8 pagesDiabetesSchizophreniaInterview CITROME BehavHealthCare2006Leslie CitromeNo ratings yet
- OlanzapineHighDoseRCTHGLFPoster KINON CINP2006Document19 pagesOlanzapineHighDoseRCTHGLFPoster KINON CINP2006Leslie CitromeNo ratings yet
- Sscchhiizzoopphhrreenniiaa: Ccuurrrreenntt Ttrreeaattm Meenntt CcoonnssiiddeerraattiioonnssDocument4 pagesSscchhiizzoopphhrreenniiaa: Ccuurrrreenntt Ttrreeaattm Meenntt CcoonnssiiddeerraattiioonnssLeslie CitromeNo ratings yet
- MedicalTrainingUnitedStatesAddendum CITROME CMAJ1992Document2 pagesMedicalTrainingUnitedStatesAddendum CITROME CMAJ1992Leslie CitromeNo ratings yet
- ZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME APA2006Document1 pageZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME APA2006Leslie CitromeNo ratings yet
- Benefits of A Second Dose of Intramuscular (IM) Aripiprazole To Control Agitation in Patients With Schizophrenia or Bipolar I DisorderDocument1 pageBenefits of A Second Dose of Intramuscular (IM) Aripiprazole To Control Agitation in Patients With Schizophrenia or Bipolar I DisorderLeslie CitromeNo ratings yet
- ZiprasidoneHaloperidolAgitationlPostHocPoster CITROME ACNP2004Document1 pageZiprasidoneHaloperidolAgitationlPostHocPoster CITROME ACNP2004Leslie CitromeNo ratings yet
- UpdateBiologicalTreatmentAggression VOLAVKA ActaEspPsiquiatr2006Document13 pagesUpdateBiologicalTreatmentAggression VOLAVKA ActaEspPsiquiatr2006Leslie CitromeNo ratings yet
- DivalproexDRtoERClinicalTrialAPA Poster CITROME APA2004Document1 pageDivalproexDRtoERClinicalTrialAPA Poster CITROME APA2004Leslie CitromeNo ratings yet
- AtypicalAntipsychoticsDiabetesMellitusCaseControlACNPPoster CITROME ACNP2003Document1 pageAtypicalAntipsychoticsDiabetesMellitusCaseControlACNPPoster CITROME ACNP2003Leslie CitromeNo ratings yet
- MoodStabilizerUtilizationAbbottAPAPoster CITROME 2004Document1 pageMoodStabilizerUtilizationAbbottAPAPoster CITROME 2004Leslie CitromeNo ratings yet
- AtypicalAntipsychoticsDiabetesMellitusCaseControlAPAPoster CITROME APA2004Document1 pageAtypicalAntipsychoticsDiabetesMellitusCaseControlAPAPoster CITROME APA2004Leslie CitromeNo ratings yet
- DosingSGAPosterWCBPHandout CITROME 2005Document4 pagesDosingSGAPosterWCBPHandout CITROME 2005Leslie CitromeNo ratings yet
- NewTreatmentsAgitationReview CITROME PsychQuarterly2004Document18 pagesNewTreatmentsAgitationReview CITROME PsychQuarterly2004Leslie CitromeNo ratings yet
- Mood Stabilizer and Antipsychotic Medication Coprescribing (Polypharmacy)Document1 pageMood Stabilizer and Antipsychotic Medication Coprescribing (Polypharmacy)Leslie CitromeNo ratings yet
- Antipsychotic Drugs in EpilepsyDocument5 pagesAntipsychotic Drugs in EpilepsyAttaufiq IrawanNo ratings yet
- Treatment in Schizophrenia: Factors For Adherence: Francisca Caiado de BragançaDocument39 pagesTreatment in Schizophrenia: Factors For Adherence: Francisca Caiado de BragançaArif IrpanNo ratings yet
- Schizophrenia - Wikipedia, The Free EncyclopediaDocument20 pagesSchizophrenia - Wikipedia, The Free EncyclopediaChitransh SaxenaNo ratings yet
- Agitation After TBIDocument66 pagesAgitation After TBIerika_mak23No ratings yet
- Chapter 18. Mood StabilizersDocument12 pagesChapter 18. Mood StabilizersAlfen PranataNo ratings yet
- Pharmacology (Psychosis and Mania)Document53 pagesPharmacology (Psychosis and Mania)lavanyakakarlaNo ratings yet
- SchizophreniaDocument46 pagesSchizophreniaEmilyne Joy Mendoza CabayaNo ratings yet
- Practical Points of Psychotropic Drugs - 19!7!2021Document51 pagesPractical Points of Psychotropic Drugs - 19!7!2021HayaaaNo ratings yet
- Drug Induced Parkinsonism: A Common Cause of Parkinsonism in Older PeopleDocument6 pagesDrug Induced Parkinsonism: A Common Cause of Parkinsonism in Older Peoplekung_pauNo ratings yet
- CH 16Document3 pagesCH 16Venice LopezNo ratings yet
- Review ArticleDocument9 pagesReview ArticleSamuel MillerNo ratings yet
- Prevalensi Tardive Dyskinesia Pada Pasien Skizofrenia Yang Mendapat Terapi Antipsikotik Di RSJ HB Saanin Padang Wenny Sagita Dita HasniDocument7 pagesPrevalensi Tardive Dyskinesia Pada Pasien Skizofrenia Yang Mendapat Terapi Antipsikotik Di RSJ HB Saanin Padang Wenny Sagita Dita HasniArif IrpanNo ratings yet
- MCQ II March 09 PDFDocument9 pagesMCQ II March 09 PDFSpacetoon DaysNo ratings yet
- 39207618Document6 pages39207618api-301074100% (1)
- Perceived Awareness of Clozapine Associated With Socio - Demographic Status, Clinical, and Side Effect Profile Among Patients From Mental Health Hospital, Taif, Saudi ArabiaDocument8 pagesPerceived Awareness of Clozapine Associated With Socio - Demographic Status, Clinical, and Side Effect Profile Among Patients From Mental Health Hospital, Taif, Saudi ArabiaJAVED ATHER SIDDIQUINo ratings yet
- Neurobiologic Theories and PsychopharmacologyDocument7 pagesNeurobiologic Theories and PsychopharmacologyhoneyNo ratings yet
- Dip 2Document129 pagesDip 2Raju Teach KapsNo ratings yet
- TG Chapter15Document12 pagesTG Chapter15ibrahim muashiNo ratings yet
- Passmedicine Mcqs PsychiatryDocument54 pagesPassmedicine Mcqs PsychiatryMayar Wael100% (3)
- Mood-Stabilizing AgentsDocument9 pagesMood-Stabilizing AgentsRoci ArceNo ratings yet
- Schizophrenia: Sattish Harbance-Singh 2013Document18 pagesSchizophrenia: Sattish Harbance-Singh 2013Aaron WallaceNo ratings yet
- Psychiatry Uworld A4Document133 pagesPsychiatry Uworld A4farheennnahmedNo ratings yet
- Canadian GPC AnsiedadDocument83 pagesCanadian GPC AnsiedadFabiana FloresNo ratings yet
- What Is SchizophreniaDocument18 pagesWhat Is SchizophreniachristnavNo ratings yet
- B2B Psychopharmacology 2015Document128 pagesB2B Psychopharmacology 2015Soleil DaddouNo ratings yet
- Lec 11-Psychotic Disorders p1Document6 pagesLec 11-Psychotic Disorders p1AnmarNo ratings yet
- History of Community PsychiatryDocument19 pagesHistory of Community PsychiatryNoremozachhom JahidNo ratings yet
- Physical and Chemical Restraint (An Update)Document15 pagesPhysical and Chemical Restraint (An Update)Alifvia IntanNo ratings yet
- Determination of Packaging Efficiency of Norflu (Fluphenazine-Nortriptyline) in Case of Normal PackagingDocument74 pagesDetermination of Packaging Efficiency of Norflu (Fluphenazine-Nortriptyline) in Case of Normal PackagingRinta MoonNo ratings yet
ZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME CINP2006
ZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME CINP2006
Uploaded by
Leslie CitromeOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
ZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME CINP2006
ZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME CINP2006
Uploaded by
Leslie CitromeCopyright:
Available Formats
T654 Citrome Poster sv4 6/23/06 12:26 PM Page 1
Efficacy of Ziprasidone Against Hostility
in Schizophrenia
Leslie Citrome, MD, MPH1; Jan Volavka, MD, PhD1; Pal Czobor, PhD1; Shlomo Brook, MD2; Antony D. Loebel, MD 3;
Francine S. Mandel, PhD 3; Hilda Templeton, MD4
1
Nathan S. Kline Institute for Psychiatric Research and the New York University School of Medicine, Orangeburg, NY,
USA; 2Sterkfontein Hospital, Krugersdorp, South Africa; 3Pfizer Inc, New York, NY, USA; 4Pfizer Inc, Pittsburgh, PA, USA
Table 1. Baseline Demographic and Treatment Characteristics
ABSTRACT
Ziprasidone Haloperidol
Objective: The objective was to determine the effects of sequential intramuscular/oral ziprasidone on hostility. Characteristic (N=429) (N=138)
Methods: A total of 572 patients diagnosed with schizophrenia or schizoaffective disorder were the subjects Men, N (%) 286 (66.7) 91 (65.9)
in a randomized, rater-blinded, 6-week open-label study comparing sequential intramuscular and oral
Race, N (%)
ziprasidone with haloperidol. The Brief Psychiatric Rating Scale (BPRS) was the principal outcome measure. To
determine the effect of ziprasidone on hostility, post-hoc analyses of the “hostility” item from the BPRS were White 338 (78.8) 110 (79.7)
conducted. Introducing positive symptoms and akathisia as covariates tested specific anti-hostility effect. Black 64 (14.9) 19 (13.8)
Results: Ziprasidone demonstrated specific anti-hostility effects over time throughout the 42-day study Asian 8 (1.9) 3 (2.2)
period, and statistically significant superiority to haloperidol on this measure in the first week of treatment.
Other 19 (4.4) 6 (4.3)
Conclusion: Ziprasidone is an effective treatment for hostility in patients with schizophrenia or
schizoaffective disorder. Age, mean (SD)/range, yr 34.0 (10.5)/18–67 34.6 (10.5)/17–65
Schizophrenia, N (%) 384 (89.5) 121 (87.7)
BPRS total, mean (SD) 57 (10.5) 57 (9.6)
Abbreviations: BAS=Barnes Akathisia Scale; BPRS=Brief Psychiatric Rating Scale; CI=confidence interval; GEE=generalized BPRS positive symptoms, mean (SD)* 19.44 (4.85) 19.24 (4.46)
estimating equations; IM=intramuscular; OR=odds ratio; SD=standard deviation
BPRS hostility item, mean (SD) 2.82 (1.45) 2.44 (1.45)
BAS global score, mean (SD) 0.35 (0.72) 0.51 (0.86)
IM medication on day 2, N (%) 280 (65) 86 (62)
BACKGROUND
IM medication on day 3, N (%) 166 (39) 48 (35)
■ Assessing the effects of antipsychotic agents in treating aggression is important for their clinical use 1
* Sum of the BPRS items of suspiciousness, grandiosity, unusual thought content, conceptual disorganization, and hallucinatory
– Agitated or hostile behavior is a frequent reason for admission to a psychiatric inpatient facility behavior.
– Continuation of such behaviors after admission can prolong the hospitalization
■ The standard of care for agitated or aggressive behavior in patients with psychotic disorders has been the
Figure 2. Decreases in Hostility With Ziprasidone and Haloperidol
short-term use of intramuscular haloperidol (at times combined with lorazepam), followed by oral (no adjustment for covariates)
antipsychotics2
■ Choice of acute intramuscular agent has expanded recently to include rapid-acting intramuscular formulations
of second-generation antipsychotics3-6
■ A randomized, open-label study found sequential administration of intramuscular and oral ziprasidone to be
superior to haloperidol in the treatment of acute exacerbation of schizophrenia or schizoaffective disorder7
■ We conducted a post-hoc analysis of data from this study pertaining specifically to hostility.8 We used the
hostility item of the BPRS as the primary efficacy measure because it has been used extensively as a proxy
measure to estimate potential antiaggressive effects of antipsychotic medications. Clinical experience as well
as empirical evidence indicates that increased hostility may precede overt aggression1
METHODS
Study Design
■ 42-day, international (20 countries), multicenter (76 sites) study
■ Randomized, parallel-group, open-label, flexible-dose design
■ All assessments were conducted by evaluators blinded to drug allocation
■ Subjects were randomly assigned in a 3:1 ratio to receive either ziprasidone or haloperidol
Patients
■ Hospitalized men and women, age 18 to 70 years Figure 3. Decreases in Hostility With Ziprasidone and Haloperidol
(after adjustment for covariates — specific antihostility effect)
■ DSM-IV diagnosis of acute exacerbation of schizophrenia or schizoaffective disorder
■ BPRS total score of ≥40
■ Exclusions
– Treatment with an investigational agent within the past 6 months, clozapine within the past 3 months, an
antipsychotic within the past 12 hours, a depot injection of an antipsychotic within the past 2 weeks
– History of resistance to conventional drugs on at least 2 occasions within the past 2 years
– Substance abuse within the past 3 months (or a positive urine screen for amphetamines, cocaine, or opioids)
– Previous diagnosis of organic mental disease including mental retardation
– Immediate risk of harm to self or others
■ Concomitant medications were permitted, including anticholinergic medications as needed for extrapyramidal
symptoms, propranolol for akathisia, benzodiazepines for additional sedation, and temazepam (up to 20 mg per
night) for insomnia
Treatments
■ Transition from intramuscular to oral administration was done when clinically appropriate. Oral ziprasidone was
started at 80 mg/day, and oral haloperidol at 10 mg/day
Assessments
■ BPRS at baseline; upon transition to oral medication (day 1, 2, or 3); day 5; week 1; week 2; week 4; and week 6
or at early termination
■ BAS at baseline; upon transition to oral medication (day 1, 2, or 3 ); week 1; week 4; and week 6 or at early Safety and Tolerability
termination
Akathisia
Statistical Analysis
■ Primary outcome measure for this post-hoc analysis was the BPRS hostility item. This item is defined as Table 2. BAS Global Scores
“animosity, contempt, belligerence, and disdain for other people outside the interview situation” and is scored
Mean (SD)
on a scale ranging from 1 (indicating not present) to 7 (very severe)
Ziprasidone Haloperidol
■ Safety measures included the BAS global score
Baseline 0.35 (0.72) 0.51 (0.86)
■ P =0.05 (2-sided) was adopted for all analyses for statistical significance
Endpoint change –0.03* (0.82) 0.41† (1.09)
■ Principal statistical approach for this analysis: GEE
– The technique of generalized estimating equations (GEE) is a method of analyzing categorical data (binary * Indicates nonstatistically significant improvement (t=0.72; P =0.474)
† Indicates worsening of akathisia (paired t test, t=4.32; P <0.0001)
or polychotomous). This method is an extension of traditional linear repeated-measures models and is used
to handle nonnormally distributed categorical data
– Since the hostility item is essentially a polychotomous categorical variable, GEE permitted appropriate
analysis of change in the presence of a very skewed distribution of the hostility variable. Treatment group ■ This difference in effect on akathisia was evident from the first time period (Days 1–3) (mean change for
was used as the between-subject variable. Time served as the within-subject (repeated-measures) factor. ziprasidone [SD]= – 0.02 [0.55]; paired t test, t=0.63, P = 0.532 vs mean change for haloperidol [SD]= 0.30
The time (overall change over time) and the interaction effect between group and time (group difference in [0.81]; paired t test, t=4.24; P < 0.0001)
change over time) constituted the main effects of interest in the analysis ■ The incidence of new cases of treatment-emergent akathisia over the 6 weeks of the study in the haloperidol
■ Effect size for change in hostility status over time group was 32.6% (of which 17.5% resolved), and in the ziprasidone group it was 13.2% (of which 46.8%
– Estimated using the OR computed from the GEE resolved)
– The analysis comparing ziprasidone with haloperidol was set up so that the OR indicates the likelihood (odds) ■ Because of the baseline differences in the BAS global scores between the ziprasidone and haloperidol groups,
of shifting 1 point down on the hostility item in the ziprasidone group compared to the haloperidol group additional tests were conducted for confirmation of the above findings
(thus, an OR >1 would indicate superiority for ziprasidone) – Analysis of covariance, using the baseline BAS score in order to adjust for the baseline difference, did not
■ Testing for specific antihostility effect substantially affect the results we have reported
– Controlling for general antipsychotic effect, as well as akathisia, permitted the testing for specific ■ Stratification of the sample based on baseline presence or absence of akathisia showed that ziprasidone’s
antihostility effect9,10 superiority to haloperidol for akathisia was heightened in the group with baseline akathisia
– Controlling for general antipsychotic effect was done by introducing into the model the change in the sum ■ Haloperidol-treated patients also exhibited significantly greater increases in Extrapyramidal Symptom Rating
of the BPRS items of suspiciousness, grandiosity, unusual thought content, conceptual disorganization, and Scale at end of IM treatment and at endpoint than ziprasidone-treated patients (P<0.0001). (Data not shown but
hallucinatory behavior as a single covariate shown in Brook S, et al.7)
– Controlling for akathisia was done by introducing the BAS global score as a covariate
CONCLUSIONS
RESULTS
■ Ziprasidone effectively treated hostility in patients with schizophrenia or schizoaffective disorder. Sequential
Figure 1. Disposition of Patients intramuscular-to-oral ziprasidone was superior to haloperidol in reducing hostility, showing evidence of a
specific antihostility effect in the first week of treatment.
■ Ziprasidone’s specific effect on hostility was superior to that of haloperidol, even after correcting for the
akathisia observed in the haloperidol group. This difference was statistically significant for the IM
treatment period ( P =0.002) and was a trend at study end point (P =0.15)
■ Ziprasidone demonstrated superior tolerability to haloperidol with respect to akathisia and EPS
REFERENCES
01. Citrome L, Nolan KA, Volavka J. Science-based treatment of aggression and agitation. In: Fishbein D, ed. The Science,
Treatment, and Prevention of Antisocial Behaviors: Evidence-Based Practice. 1st ed. Kingston, NJ: Civic Research Institute, Inc;
2004;11.1–11.31.
02. Hughes DH. Acute pharmacological management of the aggressive psychotic patient. Psychiatr Serv. 1999;50:1135–1137.
03. Winans EA, Janicak PG. IM olanzapine in the treatment of agitation and aggression. Expert Rev Neurother. 2001;1:28–32.
04. Krakowski MI, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent schizophrenic and
schizoaffective patients. Arch Gen Psychiatry. In press.
05. Wright P, Meehan K, Birkett M, et al. A comparison of the efficacy and safety of olanzapine versus haloperidol during transition
from intramuscular to oral therapy. Clin Ther. 2003;25:1420–1428.
■ Without accounting for any covariates, both the ziprasidone group and the haloperidol group improved with 06. Volavka J, Czobor P, Citrome L, et al. Efficacy of aripiprazole against hostility in schizophrenia and schizoaffective disorder. J
respect to hostility over time. However, ziprasidone was superior to haloperidol in the likelihood of reduction of Clin Psychiatry. 2005;66:1362–1366.
hostility, as noted by OR >1 for the effect of treatment and time (Figure 2) 07. Brook S, Walden J, Benattia I, et al. Ziprasidone and haloperidol in the treatment of acute exacerbation of schizophrenia and
schizoaffective disorder: comparison of intramuscular and oral formulations in a 6-week, randomized, blinded-assessment
■ Statistically significant differences were maintained until day 42, at which point the differences reached trend study. Psychopharmacology (Berl). 2005;178:514–523.
levels (P =0.056) 08. Citrome L, Volavka J, Czobor P, et al. Efficacy of ziprasidone against hostility in schizophrenia: post hoc analysis of randomized
open-label study data. J Clin Psychiatry. 2006;67:638–642.
■ When the analysis controlled for general antipsychotic effect and akathisia, the ziprasidone group
09. Keckich WA. Neuroleptics: violence as a manifestation of akathisia. JAMA. 1978;240:2185.
demonstrated a statistically significant improvement in hostility at each time point, whereas the haloperidol
10. Crowner ML, Douyon R, Convit A, et al. Akathisia and violence. Psychopharmacol Bull. 1990;26:115–117.
group showed no significant improvement in hostility over baseline (i.e., the lower limit of the 95% CI was
always >1 for the ziprasidone group and always <1 for the placebo group)
■ The between-group comparison also significantly favored ziprasidone over haloperidol (effect of treatment and
time) until day 14 (Figure 3)
Supported by funding from Pfizer Inc.
Presented at CINP 2006 Chicago Congress, July 9-13, 2006, Chicago, Illinois.
You might also like
- Hesi Psych Study GuideDocument16 pagesHesi Psych Study GuideR100% (17)
- Second and Third Generation Antipsychotics: A Comprehensive HandbookFrom EverandSecond and Third Generation Antipsychotics: A Comprehensive HandbookRating: 5 out of 5 stars5/5 (1)
- Apathy in Currently Nondepressed Patients Treated With A SSRI For A Major Depressive Episode - Outcomes Following RandomDocument8 pagesApathy in Currently Nondepressed Patients Treated With A SSRI For A Major Depressive Episode - Outcomes Following RandomMooyongNo ratings yet
- 174 Full PDFDocument7 pages174 Full PDFAsyha KantifaNo ratings yet
- Yoa70030 1115 1122Document8 pagesYoa70030 1115 1122Dewi NofiantiNo ratings yet
- Iosifescu (2011) - Electroencephalography-Derived Biomarkers of Antidepressant Response.Document11 pagesIosifescu (2011) - Electroencephalography-Derived Biomarkers of Antidepressant Response.Julieht RodriguezNo ratings yet
- S169726002300008XDocument7 pagesS169726002300008XAna Carolina SandovalNo ratings yet
- CGJ01Document8 pagesCGJ01Dewi NofiantiNo ratings yet
- Anhedonia and Reward Circuit Connectivity Distinguish Nonresponders From Responders To Dorsomedial Prefrontal Repetitive Transcranial Magnetic Stimulation in Major DepressionDocument10 pagesAnhedonia and Reward Circuit Connectivity Distinguish Nonresponders From Responders To Dorsomedial Prefrontal Repetitive Transcranial Magnetic Stimulation in Major DepressionKhory Aurora BertyNo ratings yet
- Ketamine NNTDocument8 pagesKetamine NNTBrent AllieNo ratings yet
- Revisión AntipsicóticosDocument6 pagesRevisión AntipsicóticosManel EMNo ratings yet
- Appi Ajp 2014 13121625Document9 pagesAppi Ajp 2014 13121625Orion OriNo ratings yet
- ESTUDIO CLINICO - Effects of Cannabidiol in The Treatment of Patients With ParkinsonDocument5 pagesESTUDIO CLINICO - Effects of Cannabidiol in The Treatment of Patients With ParkinsonBRIGITH NATALIA GUIO VARGASNo ratings yet
- Appi Ajp 2017 16091037 ds001Document6 pagesAppi Ajp 2017 16091037 ds001goldfishxNo ratings yet
- Exercise DepressionDocument17 pagesExercise Depressionlakshminivas PingaliNo ratings yet
- Artículo2 Grupo 5Document7 pagesArtículo2 Grupo 5LISBETH PEÑANo ratings yet
- Arbabi2013 PDFDocument4 pagesArbabi2013 PDFnur afniNo ratings yet
- Ejercicio y DepresiónDocument17 pagesEjercicio y DepresiónjosedegibesNo ratings yet
- BPD e DopaminaDocument11 pagesBPD e Dopaminaaire.puraNo ratings yet
- Predicting Treatment Response in Social Anxiety Disorder From Functional Magnetic Resonance ImagingDocument11 pagesPredicting Treatment Response in Social Anxiety Disorder From Functional Magnetic Resonance ImagingJave GajellomaNo ratings yet
- Wei Zheng, 2018Document8 pagesWei Zheng, 2018Hananya ManroeNo ratings yet
- OlanzapineDocument10 pagesOlanzapineWaoNo ratings yet
- Critical Review of Psilocybin DepressionDocument16 pagesCritical Review of Psilocybin DepressionAshok Kumar KrishnamoorthyNo ratings yet
- Altered Dynamic Regional Homogeneity in Patients With Conduct DisorderDocument7 pagesAltered Dynamic Regional Homogeneity in Patients With Conduct DisorderjojdoNo ratings yet
- Neuroimage: Clinical: SciencedirectDocument9 pagesNeuroimage: Clinical: SciencedirectMoumi PanditNo ratings yet
- PCN 12335Document8 pagesPCN 12335Ariel GaonNo ratings yet
- Brain Stimulation and Other Biological Non-Pharmacological Interventions in Mental Disorders: An Umbrella ReviewDocument18 pagesBrain Stimulation and Other Biological Non-Pharmacological Interventions in Mental Disorders: An Umbrella Reviewgermanos.dutraNo ratings yet
- Depression and Anxiety in Parkinson 'S Disease: Anette Schrag, Raquel N. TaddeiDocument33 pagesDepression and Anxiety in Parkinson 'S Disease: Anette Schrag, Raquel N. TaddeiMerari Lugo OcañaNo ratings yet
- Pharmacotherapy For Dissociative Disorders - A Systematic ReviewDocument9 pagesPharmacotherapy For Dissociative Disorders - A Systematic ReviewRita Saravia TasaycoNo ratings yet
- Management of Psychiatric and CognitiveDocument21 pagesManagement of Psychiatric and CognitiveloloasbNo ratings yet
- Estres Agudo Bryant Et Al (2008)Document9 pagesEstres Agudo Bryant Et Al (2008)Eleoner RamirezNo ratings yet
- What Does The PANSS Mean?Document8 pagesWhat Does The PANSS Mean?Farin MauliaNo ratings yet
- Asian Journal of PsychiatryDocument8 pagesAsian Journal of PsychiatryalbdapplimNo ratings yet
- Original PaperDocument9 pagesOriginal PaperSharvari ShahNo ratings yet
- Programme 1615Document29 pagesProgramme 1615Matias RizzoneNo ratings yet
- Treatment-Resistant Schizophrenia: Genetic and Neuroimaging CorrelatesDocument16 pagesTreatment-Resistant Schizophrenia: Genetic and Neuroimaging CorrelatesBianca CaterinalisendraNo ratings yet
- Pregabalina GADDocument7 pagesPregabalina GADGabriel LemosNo ratings yet
- Davidson 2004Document9 pagesDavidson 2004rf6yssdry2No ratings yet
- Pharmacological Treatment of Schizophrenia: Japanese Expert ConsensusDocument8 pagesPharmacological Treatment of Schizophrenia: Japanese Expert ConsensusArif IrpanNo ratings yet
- AripiprazolDocument15 pagesAripiprazolFabrício de Souza XavierNo ratings yet
- 7.tardive Dyskinesia Prevalence in The Period of Second-Generation Antipsychotic UseDocument15 pages7.tardive Dyskinesia Prevalence in The Period of Second-Generation Antipsychotic UseDebit SophasingNo ratings yet
- Whitton Et Al., 2021Document10 pagesWhitton Et Al., 2021Jonathan PitreNo ratings yet
- Predictors of Long-Term Improvement Following Cognitive Remediation in A Sample With Elevated Depressive SymptomsDocument11 pagesPredictors of Long-Term Improvement Following Cognitive Remediation in A Sample With Elevated Depressive SymptomsNerea Montero GarcíaNo ratings yet
- Kato 2005Document3 pagesKato 2005Huyền MiNo ratings yet
- Treatment of Acute Stress DisorderDocument9 pagesTreatment of Acute Stress DisorderAlessandra LacerdaNo ratings yet
- Metaanalysis of Efficacy and Safety of SGADocument12 pagesMetaanalysis of Efficacy and Safety of SGAdr.stojiljkovicNo ratings yet
- Fnhum 16 1029256Document22 pagesFnhum 16 1029256Krikunov KaNo ratings yet
- Translational Psychodelic Science RdocDocument39 pagesTranslational Psychodelic Science RdocRodrigo SosaNo ratings yet
- Ji-Hua Xu 2018Document3 pagesJi-Hua Xu 2018Mary FallNo ratings yet
- Pitman 2002 Biological-PsychiatryDocument4 pagesPitman 2002 Biological-PsychiatryQwerty QwertyNo ratings yet
- Add-On Pharmacotherapy For PatientsDocument2 pagesAdd-On Pharmacotherapy For PatientsmarcoNo ratings yet
- Cognitive Discernible Factors Between Schizophrenia and Schizoavective DisorderDocument4 pagesCognitive Discernible Factors Between Schizophrenia and Schizoavective DisorderdarkarotNo ratings yet
- NIH Public Access: Author ManuscriptDocument18 pagesNIH Public Access: Author ManuscriptAnonymous hvOuCjNo ratings yet
- Cranial Electrotherapy Stimulation For The Treatment of Chronically Symptomatic Bipolar PatientsDocument2 pagesCranial Electrotherapy Stimulation For The Treatment of Chronically Symptomatic Bipolar PatientsAna Maria AmanaturalisNo ratings yet
- Barret, 2006Document7 pagesBarret, 2006Elisabet GobelliNo ratings yet
- A Comparison of The Effectiveness of Three Drug Regimens On Cognitive Performance of Patients With Parkinson's DiseaseDocument12 pagesA Comparison of The Effectiveness of Three Drug Regimens On Cognitive Performance of Patients With Parkinson's DiseaseGolita emsakiNo ratings yet
- Tea Com LaserDocument20 pagesTea Com LaserPaloma ChagasNo ratings yet
- Physical Activity Reduces Clinical Symptoms and Restores Neuroplasticity in Major DepressionDocument15 pagesPhysical Activity Reduces Clinical Symptoms and Restores Neuroplasticity in Major DepressionVerônica DuquinnaNo ratings yet
- Clinical Implications of Brief Psychiatric Rating Scale ScoresDocument6 pagesClinical Implications of Brief Psychiatric Rating Scale Scores주병욱No ratings yet
- Fourth International Conference On Alzheimer'S Disease s137Document2 pagesFourth International Conference On Alzheimer'S Disease s137Sergiy Prokofiy MontanoNo ratings yet
- Variance in Neurocognitive Performance Is Associated With Dysbindin-1 in Schizophrenia: A Preliminary StudyDocument5 pagesVariance in Neurocognitive Performance Is Associated With Dysbindin-1 in Schizophrenia: A Preliminary Studyfajar tampubolonNo ratings yet
- ZolpidemProductLabel 0819 PDFDocument7 pagesZolpidemProductLabel 0819 PDFLeslie CitromeNo ratings yet
- Restoril™ (Temazepam) Capsules USP RX Only Warning: Risks From Concomitant Use With OpioidsDocument14 pagesRestoril™ (Temazepam) Capsules USP RX Only Warning: Risks From Concomitant Use With OpioidsLeslie CitromeNo ratings yet
- See Full Prescribing Information For Complete Boxed WarningDocument14 pagesSee Full Prescribing Information For Complete Boxed WarningLeslie CitromeNo ratings yet
- DiabetesEpidemiologySlidesForDistribution CITROME AdaptedPsychServ2006Document8 pagesDiabetesEpidemiologySlidesForDistribution CITROME AdaptedPsychServ2006Leslie CitromeNo ratings yet
- WhatIsTranscranialMagneticStimulation CITROME KlineLine1999Document1 pageWhatIsTranscranialMagneticStimulation CITROME KlineLine1999Leslie CitromeNo ratings yet
- CATIENNTEditorialRegardingCITROME KERWIN IntJClinPract2006Document2 pagesCATIENNTEditorialRegardingCITROME KERWIN IntJClinPract2006Leslie CitromeNo ratings yet
- IncidencePrevalenceSurveillanceDiabetesMellitusInpatientsPoster CITROME NCDEU2006Document1 pageIncidencePrevalenceSurveillanceDiabetesMellitusInpatientsPoster CITROME NCDEU2006Leslie CitromeNo ratings yet
- Leslie Citrome, MD, MPH, Richard Josiassen, PHD, Nigel Bark, MD, Karen S Brown, MS, Suresh Mallikaarjun, PHD, Daniel E Salazar, PHDDocument1 pageLeslie Citrome, MD, MPH, Richard Josiassen, PHD, Nigel Bark, MD, Karen S Brown, MS, Suresh Mallikaarjun, PHD, Daniel E Salazar, PHDLeslie CitromeNo ratings yet
- DiabetesEpidemiologyFiguresSupplement CITROME PsychServ2006onlineDocument5 pagesDiabetesEpidemiologyFiguresSupplement CITROME PsychServ2006onlineLeslie CitromeNo ratings yet
- OlanzapineEarlyPredictorsWeightGainBipolarDisorder LIPKOVICH JClinPsychopharm2006Document5 pagesOlanzapineEarlyPredictorsWeightGainBipolarDisorder LIPKOVICH JClinPsychopharm2006Leslie CitromeNo ratings yet
- DiabetesSchizophreniaInterview CITROME BehavHealthCare2006Document8 pagesDiabetesSchizophreniaInterview CITROME BehavHealthCare2006Leslie CitromeNo ratings yet
- OlanzapineHighDoseRCTHGLFPoster KINON CINP2006Document19 pagesOlanzapineHighDoseRCTHGLFPoster KINON CINP2006Leslie CitromeNo ratings yet
- Sscchhiizzoopphhrreenniiaa: Ccuurrrreenntt Ttrreeaattm Meenntt CcoonnssiiddeerraattiioonnssDocument4 pagesSscchhiizzoopphhrreenniiaa: Ccuurrrreenntt Ttrreeaattm Meenntt CcoonnssiiddeerraattiioonnssLeslie CitromeNo ratings yet
- MedicalTrainingUnitedStatesAddendum CITROME CMAJ1992Document2 pagesMedicalTrainingUnitedStatesAddendum CITROME CMAJ1992Leslie CitromeNo ratings yet
- ZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME APA2006Document1 pageZiprasidoneHaloperidolHostilitySchizophreniaPoster CITROME APA2006Leslie CitromeNo ratings yet
- Benefits of A Second Dose of Intramuscular (IM) Aripiprazole To Control Agitation in Patients With Schizophrenia or Bipolar I DisorderDocument1 pageBenefits of A Second Dose of Intramuscular (IM) Aripiprazole To Control Agitation in Patients With Schizophrenia or Bipolar I DisorderLeslie CitromeNo ratings yet
- ZiprasidoneHaloperidolAgitationlPostHocPoster CITROME ACNP2004Document1 pageZiprasidoneHaloperidolAgitationlPostHocPoster CITROME ACNP2004Leslie CitromeNo ratings yet
- UpdateBiologicalTreatmentAggression VOLAVKA ActaEspPsiquiatr2006Document13 pagesUpdateBiologicalTreatmentAggression VOLAVKA ActaEspPsiquiatr2006Leslie CitromeNo ratings yet
- DivalproexDRtoERClinicalTrialAPA Poster CITROME APA2004Document1 pageDivalproexDRtoERClinicalTrialAPA Poster CITROME APA2004Leslie CitromeNo ratings yet
- AtypicalAntipsychoticsDiabetesMellitusCaseControlACNPPoster CITROME ACNP2003Document1 pageAtypicalAntipsychoticsDiabetesMellitusCaseControlACNPPoster CITROME ACNP2003Leslie CitromeNo ratings yet
- MoodStabilizerUtilizationAbbottAPAPoster CITROME 2004Document1 pageMoodStabilizerUtilizationAbbottAPAPoster CITROME 2004Leslie CitromeNo ratings yet
- AtypicalAntipsychoticsDiabetesMellitusCaseControlAPAPoster CITROME APA2004Document1 pageAtypicalAntipsychoticsDiabetesMellitusCaseControlAPAPoster CITROME APA2004Leslie CitromeNo ratings yet
- DosingSGAPosterWCBPHandout CITROME 2005Document4 pagesDosingSGAPosterWCBPHandout CITROME 2005Leslie CitromeNo ratings yet
- NewTreatmentsAgitationReview CITROME PsychQuarterly2004Document18 pagesNewTreatmentsAgitationReview CITROME PsychQuarterly2004Leslie CitromeNo ratings yet
- Mood Stabilizer and Antipsychotic Medication Coprescribing (Polypharmacy)Document1 pageMood Stabilizer and Antipsychotic Medication Coprescribing (Polypharmacy)Leslie CitromeNo ratings yet
- Antipsychotic Drugs in EpilepsyDocument5 pagesAntipsychotic Drugs in EpilepsyAttaufiq IrawanNo ratings yet
- Treatment in Schizophrenia: Factors For Adherence: Francisca Caiado de BragançaDocument39 pagesTreatment in Schizophrenia: Factors For Adherence: Francisca Caiado de BragançaArif IrpanNo ratings yet
- Schizophrenia - Wikipedia, The Free EncyclopediaDocument20 pagesSchizophrenia - Wikipedia, The Free EncyclopediaChitransh SaxenaNo ratings yet
- Agitation After TBIDocument66 pagesAgitation After TBIerika_mak23No ratings yet
- Chapter 18. Mood StabilizersDocument12 pagesChapter 18. Mood StabilizersAlfen PranataNo ratings yet
- Pharmacology (Psychosis and Mania)Document53 pagesPharmacology (Psychosis and Mania)lavanyakakarlaNo ratings yet
- SchizophreniaDocument46 pagesSchizophreniaEmilyne Joy Mendoza CabayaNo ratings yet
- Practical Points of Psychotropic Drugs - 19!7!2021Document51 pagesPractical Points of Psychotropic Drugs - 19!7!2021HayaaaNo ratings yet
- Drug Induced Parkinsonism: A Common Cause of Parkinsonism in Older PeopleDocument6 pagesDrug Induced Parkinsonism: A Common Cause of Parkinsonism in Older Peoplekung_pauNo ratings yet
- CH 16Document3 pagesCH 16Venice LopezNo ratings yet
- Review ArticleDocument9 pagesReview ArticleSamuel MillerNo ratings yet
- Prevalensi Tardive Dyskinesia Pada Pasien Skizofrenia Yang Mendapat Terapi Antipsikotik Di RSJ HB Saanin Padang Wenny Sagita Dita HasniDocument7 pagesPrevalensi Tardive Dyskinesia Pada Pasien Skizofrenia Yang Mendapat Terapi Antipsikotik Di RSJ HB Saanin Padang Wenny Sagita Dita HasniArif IrpanNo ratings yet
- MCQ II March 09 PDFDocument9 pagesMCQ II March 09 PDFSpacetoon DaysNo ratings yet
- 39207618Document6 pages39207618api-301074100% (1)
- Perceived Awareness of Clozapine Associated With Socio - Demographic Status, Clinical, and Side Effect Profile Among Patients From Mental Health Hospital, Taif, Saudi ArabiaDocument8 pagesPerceived Awareness of Clozapine Associated With Socio - Demographic Status, Clinical, and Side Effect Profile Among Patients From Mental Health Hospital, Taif, Saudi ArabiaJAVED ATHER SIDDIQUINo ratings yet
- Neurobiologic Theories and PsychopharmacologyDocument7 pagesNeurobiologic Theories and PsychopharmacologyhoneyNo ratings yet
- Dip 2Document129 pagesDip 2Raju Teach KapsNo ratings yet
- TG Chapter15Document12 pagesTG Chapter15ibrahim muashiNo ratings yet
- Passmedicine Mcqs PsychiatryDocument54 pagesPassmedicine Mcqs PsychiatryMayar Wael100% (3)
- Mood-Stabilizing AgentsDocument9 pagesMood-Stabilizing AgentsRoci ArceNo ratings yet
- Schizophrenia: Sattish Harbance-Singh 2013Document18 pagesSchizophrenia: Sattish Harbance-Singh 2013Aaron WallaceNo ratings yet
- Psychiatry Uworld A4Document133 pagesPsychiatry Uworld A4farheennnahmedNo ratings yet
- Canadian GPC AnsiedadDocument83 pagesCanadian GPC AnsiedadFabiana FloresNo ratings yet
- What Is SchizophreniaDocument18 pagesWhat Is SchizophreniachristnavNo ratings yet
- B2B Psychopharmacology 2015Document128 pagesB2B Psychopharmacology 2015Soleil DaddouNo ratings yet
- Lec 11-Psychotic Disorders p1Document6 pagesLec 11-Psychotic Disorders p1AnmarNo ratings yet
- History of Community PsychiatryDocument19 pagesHistory of Community PsychiatryNoremozachhom JahidNo ratings yet
- Physical and Chemical Restraint (An Update)Document15 pagesPhysical and Chemical Restraint (An Update)Alifvia IntanNo ratings yet
- Determination of Packaging Efficiency of Norflu (Fluphenazine-Nortriptyline) in Case of Normal PackagingDocument74 pagesDetermination of Packaging Efficiency of Norflu (Fluphenazine-Nortriptyline) in Case of Normal PackagingRinta MoonNo ratings yet