Professional Documents
Culture Documents
List of Documents To Be Submitted For Grant of Manufacturing Licence Drug Forms
List of Documents To Be Submitted For Grant of Manufacturing Licence Drug Forms
Uploaded by
Kevin albuquerqueOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
List of Documents To Be Submitted For Grant of Manufacturing Licence Drug Forms
List of Documents To Be Submitted For Grant of Manufacturing Licence Drug Forms
Uploaded by
Kevin albuquerqueCopyright:
Available Formats
Procedure for obtaining Manufacturing Drug Licences
Any person who wants to start Manufacturing of drugs should obtain licences from
this Directorate under the provisions of Drugs & Cosmetics Act 1940 & Rules
thereunder by following the procedure given below.
1. For obtaining Licence in Form 25 (non Biological Drugs) and Form 28 (Biological
Drugs) applicant should fill Form 24 and Form 27 respectively along with
additional information data. The Forms and the list of all the documents that needs
to be submitted may be obtained from the Food & Drugs Administration
department or it can also be downloaded from link below.
List of documents to be submitted for Grant of manufacturing Licence
Drug Forms
2. Once all the documents are ready the applicant should verify the documents from
area Drugs Inspector or any Drugs Inspector of Food & Drugs Administration.
3. After documents are verified and found ok, applicant may pay the necessary fees
vide E-challan to be paid to Head of accounts, Demand No. 53; 201 Medical and
Public Health, 04 Public Health, 104 Fees and Fines, 01 Fines. Fee structure can
also be downloaded from link : Fee Structure
4. For generation of E- challan applicant needs to fill the challan form available with
the department for requisite fees and get it verified from Drugs Inspector and
submit it to accounts section where E-challan will be generated and issued to the
applicant. Generated E-challan to be paid in the Treasury branch of State Bank of
India.
5. Applicant needs to submit all the documents mentioned in the list including
original copy of challan paid towards the fees to entry clerk in the inward section
along with covering letter.
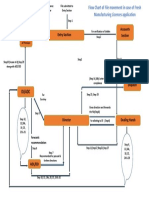
File movement once it is submitted in entry section is as under:
1. Hard copy of application received in the inward Entry section.
2. Entry clerk forwards the file to Accounts section of the Department for verification
of amount paid through challan.
3. The Accounts Section after verification of amount forwards the file to concerned
dealing hand.
4. The dealing hand registers the application, generates the file number and
scrutinises all the documents and puts up to the Director for alloting to the Drugs
Inspector.
5. The Director peruses the application and marks it to any Drugs Inspector/Asst.
Drugs Controller for further processes.
6. The Drugs Inspector/ADC of the area scrutinises the documents submitted and
also scrutinises the product permission requested under the said licences; and
puts comments in the noting.The concerned Drugs Inspector forwards the file to
the next supervisory Officer that is either Assistant Drugs Controller (ADC) or the
Dy. Director, (Dy. Dir) as the case may be.
7. The ADC or the Dy. Director; reviews the comments / note submitted by the Drug
Inspector and puts additional comments depending on his/her observations if any
and forwards the scrutinised application to the Director; who is the Licensing
Authority; with recommendation; depending upon the observation made such as:
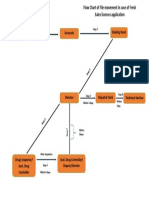
a. The deficient documents to be called.
b. Certain clarifications required in the plan of premises submitted.
c. Certain clarifications required in the product permission sought.
d. Premises to be inspected to verify whether the facilities provided
are in compliance to schedule M, MI, MII, MIII of the Drugs &
Cosmetics Rules (as applicable).
8. Director (Licensing Authority) on perusal agrees to the recommendations made;
and application is sent to the concerned dealing hand.
9. In case of recommendations such as a, b, c the concerned dealing hand, puts up
a draft of letter conveying the observation and calling up for compliance from the
applicant and forwards to the concerned Drugs Inspectors. In case of
recommendations at (d) dealing hand puts up the application to the concerned
Drugs Inspector for inspection of the premises.
10. In case of recommendations such as a, b, c the Drugs Inspector forwards the
draft to the next superior i.e. the ADC/Director; who in turn forwards it to the
Director who approves the draft and sends back the application file to the
concerned dealing hand. In case of observation at d; the Drugs Inspector along
with the supervisory Officer, ADC/Dy. Director conducts the inspection of the
premises for manufacturing premises and submits the report to the Director.
11. In case of recommendations such as a, b, c the dealing hand puts up the file with
letter communicating the deficiency; to the Drugs Inspector.
12. The Drugs Inspector countersigns the same and puts up to the ADC/Dy.
Director.
13. The ADC/Dy. Dir. again countersigns and forwards it to the Director for sign.
14. The Director signs the letter and sends the file to Dispatch clerk for dispatch.
15. The Dispatch clerk gives the outward no.; prepares and sends it to the post for
delivery.
16. In continuation to step 10; Director peruses the report and then agrees to the
recommendation which is either; it is recommended to either
(a) Grant the licence; or
(b) To call for compliance of certain deficiencies.
17. In case of (b) the process from step no. 9 to 15 is repeated.
18. In continuation to step 16; if grant of licence is recommended; then the dealing
hand puts up the draft of the licence to be issued; along with the covering letter;
and the list of products permitted with endorsement; to the Drugs Inspector.
19. The Drugs Inspector verifies the draft for correction and puts up to ADC/Dy. Dir.
20. The ADC/Dy. Dir. Countersigns and puts up to the Director.
21. The Director approves the draft and sends to the dealing hand.
22. The dealing hand prepares the fair licence along with the list of products and
puts up to Drugs Inspector.
23. Steps from 12, 13, 14 and 15 are repeated.
24. After compliance of the deficiencies as regards steps 16(b) are submitted by the
applicant; dealing hand puts up the file to the concerned Drugs Inspector.
25. The Drugs Inspector verifies the compliance submitted and puts up the noting
depending upon the observations made and puts up the file to the ADC/Dy. Dir
with recommendation either for grant of licence or for verification of the
compliance submitted by visiting the premises.
26. The ADC/Dy. Dir. Puts up their comments and forwards the file to DFDA.
27. The DFDA agrees to the recommendations and sends back the file to the
dealing hand.
28. The dealing hand depending upon whether it is recommended for grant of
licence or for re-inspection; puts up either the draft of the licence or the file
directly to the Drugs Inspector.
29. The Drugs Inspector along with ADC/Dy. Dir. Re-inspect the premises and
submit their report to the Director with recommendation for grant of licences.
30. Steps from 18 to 22 are repeated.
31. The Drugs Inspector countersigns the licence and puts up to ADC/Dy. Dir.
32. The ADC/Dy. Dir. Countersigns the licence and puts up to DFDA for sign.
33. The DFDA signs the licence and sends it to Dispatch clerk for dispatch.
Note: 1) Validity of licence - valid upto 5 years from issue of licence subject to the
conditions specified in the licence & to the provisions of the Drugs &
Cosmetics Act, 1940 and the Rules thereunder.
2) Click on the link below for timelines:
Citizen charter
You might also like
- Procedure For Obtaining Retail (Medical Store) Drug Licences / Wholesale Drug LicencesDocument2 pagesProcedure For Obtaining Retail (Medical Store) Drug Licences / Wholesale Drug LicencesKevin albuquerqueNo ratings yet
- Grant of Drug and Cosmetic Manufacturing Licence in OwnDocument4 pagesGrant of Drug and Cosmetic Manufacturing Licence in Owngo2pakadeNo ratings yet
- Procedure To Obtain Drug Manufacturing License (Fresh)Document2 pagesProcedure To Obtain Drug Manufacturing License (Fresh)Jaiprakash SharmaNo ratings yet
- Grant of Drug and Cosmetic Manufacturing License in Own PremDocument3 pagesGrant of Drug and Cosmetic Manufacturing License in Own PremAbhilash NarayananNo ratings yet
- IDL ChinaDocument2 pagesIDL ChinaRuchi VoraNo ratings yet
- Procedure To Obtain Manufacturing License For Medical DevicesDocument2 pagesProcedure To Obtain Manufacturing License For Medical DevicesSachin PisalNo ratings yet
- Loan LicenseDocument2 pagesLoan Licensejohnancy19No ratings yet
- Adip QCDocument4 pagesAdip QCYktashNo ratings yet
- Central Drugs Standard Control Organization: Guidance Document For Test LicenceDocument31 pagesCentral Drugs Standard Control Organization: Guidance Document For Test LicenceVikram UllalNo ratings yet
- Loan License Agreement A. OverviewDocument3 pagesLoan License Agreement A. Overviewyash100% (2)
- Quezon City Health DepartmentDocument3 pagesQuezon City Health DepartmentGennelyn Lozano Israel100% (1)
- Veterinary Biological 2Document63 pagesVeterinary Biological 2Gourav BhardwajNo ratings yet
- D.1.2 PR Procedure For Organic CertificationDocument4 pagesD.1.2 PR Procedure For Organic Certificationmilctf1No ratings yet
- Notices For The Application of Plant Master FileDocument7 pagesNotices For The Application of Plant Master FileAnandharaj AsaithambiNo ratings yet
- Export Development PromotionDocument22 pagesExport Development PromotionshrikantNo ratings yet
- Guideline For Drug Registration Applicants 20JAN2018 1Document41 pagesGuideline For Drug Registration Applicants 20JAN2018 1fyraghNo ratings yet
- A Guide To Application For Factory Canteen Licence: Food and Environmental Hygiene Department (March 2012 Edition)Document23 pagesA Guide To Application For Factory Canteen Licence: Food and Environmental Hygiene Department (March 2012 Edition)aujourdNo ratings yet
- Co PPDocument15 pagesCo PPVikas JhawatNo ratings yet
- Guiding Documents For Zonal & Sub-Zonal & Port Offices 17.06.2011Document483 pagesGuiding Documents For Zonal & Sub-Zonal & Port Offices 17.06.2011rk7bpsNo ratings yet
- A Guide To Application For Food Factory Licence: Food and Environmental Hygiene Department (March 2012 Edition)Document14 pagesA Guide To Application For Food Factory Licence: Food and Environmental Hygiene Department (March 2012 Edition)aujourdNo ratings yet
- Guidelines For Licensing Manufacturing Plant For Human Medicines Herbal Medicines and Medical Devices - EnglishDocument13 pagesGuidelines For Licensing Manufacturing Plant For Human Medicines Herbal Medicines and Medical Devices - EnglishtesteNo ratings yet
- Microsoft Word - Terms and ConditionsDocument3 pagesMicrosoft Word - Terms and ConditionsIsaac SamuelNo ratings yet
- Commissioner General Instruction On Electronic Billing Machine CertificationDocument32 pagesCommissioner General Instruction On Electronic Billing Machine CertificationVenkata Sriramachandra Santosh KumarNo ratings yet
- Standard Operating Procedure: 2 Floor, OPF Building G-5/2 IslamabadDocument17 pagesStandard Operating Procedure: 2 Floor, OPF Building G-5/2 IslamabadShahid SiddiqueNo ratings yet
- New Dispensing Licence Application FormDocument7 pagesNew Dispensing Licence Application FormkavithaNo ratings yet
- Family Planning Manual Indemnity SchemeDocument10 pagesFamily Planning Manual Indemnity SchemeRamdas SalveNo ratings yet
- The Procedures Are Subjected To Changes With Respect To Enclosed G.O. Gazette NotificationDocument4 pagesThe Procedures Are Subjected To Changes With Respect To Enclosed G.O. Gazette NotificationVanittharaniRamasamyNo ratings yet
- Export Development PromotionDocument9 pagesExport Development PromotionDSNo ratings yet
- Guideline On Drug Registration Application in Myanmar - 2014Document51 pagesGuideline On Drug Registration Application in Myanmar - 2014Kush MukherjiNo ratings yet
- Drugs and Cosmetics Act Part-II (BP-505T) (U-1, P-2)Document12 pagesDrugs and Cosmetics Act Part-II (BP-505T) (U-1, P-2)Anand deshmukhNo ratings yet
- Guidelines For PharmacistsDocument19 pagesGuidelines For PharmacistsebayNo ratings yet
- Guidance For eCTD SubmissionDocument18 pagesGuidance For eCTD SubmissionSrikanth SunkaraNo ratings yet
- App Instructions PDFDocument6 pagesApp Instructions PDFjspectorNo ratings yet
- Weight and Measurement License ProcedureDocument1 pageWeight and Measurement License ProcedureMiteshNo ratings yet
- Be Informed That Invitation No 95/2014/39 Canceled &replaced by Invitation No. 95/2014/39 /R For Following ItemsDocument17 pagesBe Informed That Invitation No 95/2014/39 Canceled &replaced by Invitation No. 95/2014/39 /R For Following ItemsketanmunjaniNo ratings yet
- Guiding Documents For Zonal & Sub-Zonal & Port Offices 17.06.2011 PDFDocument483 pagesGuiding Documents For Zonal & Sub-Zonal & Port Offices 17.06.2011 PDFmedicinalchemistNo ratings yet
- Chapter 2. Additional EU Organic - 3Document14 pagesChapter 2. Additional EU Organic - 3Manuel MariñasNo ratings yet
- FDA Citizen's Charter CFRRDocument92 pagesFDA Citizen's Charter CFRRRaeanne Sabado BangitNo ratings yet
- Guidelines - Legal Profession Act 2004Document4 pagesGuidelines - Legal Profession Act 2004bobby adleNo ratings yet
- DCD Pa Doc Review Process r12Document16 pagesDCD Pa Doc Review Process r12Pref1811No ratings yet
- HFSRB Citizens-Charter-2020Document54 pagesHFSRB Citizens-Charter-2020Jane Therese RoveloNo ratings yet
- Renewal of Drugs Manufacturing License Process Description: Form 1ADocument2 pagesRenewal of Drugs Manufacturing License Process Description: Form 1Anasim zafarNo ratings yet
- Scheme (Pharm D)Document5 pagesScheme (Pharm D)ashokNo ratings yet
- Certification OnionsDocument24 pagesCertification OnionservaishaliNo ratings yet
- Licensing ProceduresDocument37 pagesLicensing ProceduresDlamini SiceloNo ratings yet
- CDSCO Medical Devices PDFDocument41 pagesCDSCO Medical Devices PDFSantosh KadamNo ratings yet
- BE GuidelineDocument3 pagesBE GuidelinePrince PavanNo ratings yet
- Drug License in MaharashtraDocument8 pagesDrug License in MaharashtraRsankar GmNo ratings yet
- Instructions To Tenderers Earnest Money:: H:/Dist Trans/Tender DocumentDocument10 pagesInstructions To Tenderers Earnest Money:: H:/Dist Trans/Tender DocumentPrateek BandyopadhyayNo ratings yet
- Appl Format Mid Term Review Anti Dumping DutyDocument7 pagesAppl Format Mid Term Review Anti Dumping DutySrinivasa KirankumarNo ratings yet
- Instructions EngDocument12 pagesInstructions EngHashan H MarasingheNo ratings yet
- FDA Act 1945 Blood BankDocument14 pagesFDA Act 1945 Blood BankWaheed SiddiquiNo ratings yet
- AEO Model Appeal Procedures (E)Document2 pagesAEO Model Appeal Procedures (E)yayobinaNo ratings yet
- Coa M2015-007Document12 pagesCoa M2015-007Abraham Junio80% (5)
- Decree 4 Provisions For Medical Device RegistrationDocument22 pagesDecree 4 Provisions For Medical Device RegistrationALEJANDRONo ratings yet
- Gop216 Rspo Information On Complaint Handling - 20191202Document3 pagesGop216 Rspo Information On Complaint Handling - 20191202Rizwan MohammadNo ratings yet
- 7 Faceless Asst and DRCDocument6 pages7 Faceless Asst and DRCGaurav VermaNo ratings yet
- The Contractor Payment Application Audit: Guidance for Auditing AIA Documents G702 & G703From EverandThe Contractor Payment Application Audit: Guidance for Auditing AIA Documents G702 & G703No ratings yet
- Federal Rules of Civil Procedure: Hyperlinked, #2From EverandFederal Rules of Civil Procedure: Hyperlinked, #2Rating: 5 out of 5 stars5/5 (1)
- DHDJDHDJDJDHSHDocument4 pagesDHDJDHDJDJDHSHKevin albuquerqueNo ratings yet
- International Journal of School and Cognitive PsychologyDocument4 pagesInternational Journal of School and Cognitive PsychologyKevin albuquerqueNo ratings yet
- SgdjdjhehjdhdhhdjDocument4 pagesSgdjdjhehjdhdhhdjKevin albuquerqueNo ratings yet
- Dysjshdjdh2 - (20 04 2022)Document18 pagesDysjshdjdh2 - (20 04 2022)Kevin albuquerqueNo ratings yet
- Operational Plan For Transporation of Government Servents Kadamba Transport Corporation LimitedDocument4 pagesOperational Plan For Transporation of Government Servents Kadamba Transport Corporation LimitedKevin albuquerqueNo ratings yet
- Procedure For Obtaining Retail (Medical Store) Drug Licences / Wholesale Drug LicencesDocument2 pagesProcedure For Obtaining Retail (Medical Store) Drug Licences / Wholesale Drug LicencesKevin albuquerqueNo ratings yet
- Entry Section Accounts Section: Di/AdcDocument1 pageEntry Section Accounts Section: Di/AdcKevin albuquerqueNo ratings yet
- Entry Stage Accounts Dealing Hand: Application Submitted Step 1Document1 pageEntry Stage Accounts Dealing Hand: Application Submitted Step 1Kevin albuquerqueNo ratings yet
- The Impact of Undergraduate Degrees On Early-Career Earnings PDFDocument82 pagesThe Impact of Undergraduate Degrees On Early-Career Earnings PDFKevin albuquerqueNo ratings yet
- Ssip GDocument6 pagesSsip GDevie Anne BiscarraNo ratings yet
- Public Borrowing and Debt Management by Mario RanceDocument47 pagesPublic Borrowing and Debt Management by Mario RanceWo Rance100% (2)
- Electronic Reservation Slip (ERS) Electronic Reservation Slip (ERS) - (B2B)Document2 pagesElectronic Reservation Slip (ERS) Electronic Reservation Slip (ERS) - (B2B)MUNNA SKNo ratings yet
- (PDF) Ombudsman v. Rodriguez DigestDocument3 pages(PDF) Ombudsman v. Rodriguez DigestKathlyn DacudaoNo ratings yet
- E-WAY BILL DetailsDocument1 pageE-WAY BILL DetailsAnkit guptaNo ratings yet
- PAF Citizen's Charter Handbook 1st Edition - 2019Document130 pagesPAF Citizen's Charter Handbook 1st Edition - 2019HAS PAFCMOGNo ratings yet
- Republic of The Philippines National Police Commission Philippine National Police Barangay Li-Ong, Guinsiliban, CamiguinDocument3 pagesRepublic of The Philippines National Police Commission Philippine National Police Barangay Li-Ong, Guinsiliban, CamiguinCPMFP CAMPPONo ratings yet
- Client CounsellingDocument4 pagesClient CounsellingRanvidsNo ratings yet
- Dicoese of Bacolod v. COMELECDocument45 pagesDicoese of Bacolod v. COMELECAnsai CaluganNo ratings yet
- Forms of Business OwnershipDocument26 pagesForms of Business OwnershipRitesh yadavNo ratings yet
- Sales Agreement AdamawaDocument4 pagesSales Agreement AdamawaPeter DurbaNo ratings yet
- Minor Literature and The Translation of The M OtherDocument19 pagesMinor Literature and The Translation of The M OtherkamlaNo ratings yet
- Quiz Mas 4Document2 pagesQuiz Mas 4Cheese ButterNo ratings yet
- Role of Military in Turkish PoliticsDocument5 pagesRole of Military in Turkish PoliticsHamza Bilal100% (4)
- Sample Annotated BibliographyDocument6 pagesSample Annotated BibliographyHassan RazaNo ratings yet
- PressconDocument4 pagesPressconSamantha Vhiel VicenteNo ratings yet
- The Legislative BranchDocument20 pagesThe Legislative BranchBANGTAN AMIEENo ratings yet
- Virtual Work Integration TableDocument1 pageVirtual Work Integration Tablefloi dNo ratings yet
- How Internet Affect LifeDocument2 pagesHow Internet Affect Lifesalwa najlaaNo ratings yet
- Not Economy But Politics Is A Key To Success PDFDocument7 pagesNot Economy But Politics Is A Key To Success PDFumeerzNo ratings yet
- A Grain of WheatDocument6 pagesA Grain of WheatShikah IkaNo ratings yet
- 2023 24 Uefa CL RulesDocument104 pages2023 24 Uefa CL Rulesnewsitaquera0No ratings yet
- HCS 455 - Policy Process - Part 1 - Health Care Reform - Week 3Document8 pagesHCS 455 - Policy Process - Part 1 - Health Care Reform - Week 3Dawn Wrightington0% (1)
- The Role of Citizens in Intelligence Gathering and SharingDocument17 pagesThe Role of Citizens in Intelligence Gathering and SharingDon OkerekeNo ratings yet
- GLGR LawsuitDocument9 pagesGLGR LawsuitScott McClallenNo ratings yet
- MSW SocialWorkCAS Professional Transcript Entry OverviewDocument1 pageMSW SocialWorkCAS Professional Transcript Entry OverviewfatimaNo ratings yet
- RF 2 Non Net Fellowship FormDocument1 pageRF 2 Non Net Fellowship Formsanju BarmanNo ratings yet
- 1 - Misterio v. Cebu State College of Science and Technology (2005) PDFDocument10 pages1 - Misterio v. Cebu State College of Science and Technology (2005) PDFGeorgina Mia GatoNo ratings yet
- Manish R. Kapadiya ProfileDocument1 pageManish R. Kapadiya ProfileMANISH KAPADIYANo ratings yet
- Republic of The PhilippinesDocument10 pagesRepublic of The PhilippinesSalva MariaNo ratings yet