Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
63 viewsActivation Energy: The Energy Required To Make The Jump, E, Is The
Activation Energy: The Energy Required To Make The Jump, E, Is The
Uploaded by
Afrah MIonic conductivity is a measure of how easily ions can move through a material. It depends on properties like the number and charge of ions as well as their mobility. The mobility of ions typically increases exponentially with temperature according to an Arrhenius equation, meaning ionic conductivity also rises exponentially with temperature. Plotting the logarithm of conductivity times temperature against the inverse of temperature produces a straight line whose slope equals the activation energy for ion motion.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You might also like
- Water Cooled Chiller ManualDocument52 pagesWater Cooled Chiller Manualkhamsone pengmanivongNo ratings yet
- Zinc Report 2017-06-19 Briol Masson For Zinc OneDocument11 pagesZinc Report 2017-06-19 Briol Masson For Zinc OneLuan BiciNo ratings yet
- Resistance Vs Temperature Experiment Lab ReportDocument7 pagesResistance Vs Temperature Experiment Lab ReportEmily Gatlin67% (3)
- Metals, Semiconductors, and InsulatorsDocument38 pagesMetals, Semiconductors, and InsulatorskaidoqNo ratings yet
- Chapter 5: Carrier Transport Phenomena: Transport The Process by Which Charged Particles (ElectronsDocument32 pagesChapter 5: Carrier Transport Phenomena: Transport The Process by Which Charged Particles (ElectronsAbdullah AbdulhameedNo ratings yet
- CH 3 Current Electricity Notes XiiDocument10 pagesCH 3 Current Electricity Notes Xiisharmanehasandeep1No ratings yet
- 3 Current ElectricityDocument21 pages3 Current ElectricitySakshi KantNo ratings yet
- CLS Aipmt 16 17 XII Phy Study Package 5 SET 1 Chapter 3Document28 pagesCLS Aipmt 16 17 XII Phy Study Package 5 SET 1 Chapter 3Kareena Gupta17% (6)
- EOPM Part1 PDFDocument29 pagesEOPM Part1 PDFRoy VeseyNo ratings yet
- ElectricityDocument44 pagesElectricityKirancivilNo ratings yet
- Materials For ElectronicsDocument22 pagesMaterials For ElectronicsEngr Haseena JabbarNo ratings yet
- XII - Physics - Chapter 3 - Current ElectricityDocument15 pagesXII - Physics - Chapter 3 - Current ElectricityHADINo ratings yet
- Current Electricty by Baba GangDocument7 pagesCurrent Electricty by Baba GangKushagra RajNo ratings yet
- Physics 20 PDFDocument30 pagesPhysics 20 PDFShivani Shree SundaramoorthyNo ratings yet
- Carrier Transport RevisedDocument65 pagesCarrier Transport RevisedShivani GuptaNo ratings yet
- Electrical and Thermal Conduction-2Document3 pagesElectrical and Thermal Conduction-2Sajjad HossainNo ratings yet
- GT 65 QJPSR 66 L VITCxp 02Document8 pagesGT 65 QJPSR 66 L VITCxp 02Sahil SinghNo ratings yet
- PH Gokul Sharma: Current ElectricityDocument15 pagesPH Gokul Sharma: Current ElectricityWillis ChekovNo ratings yet
- Chapter 2-Type of ElectroceramicsDocument33 pagesChapter 2-Type of ElectroceramicsDP DianaNo ratings yet
- CURRENT ELECTRICITY 27-09-2017 RevisedDocument16 pagesCURRENT ELECTRICITY 27-09-2017 RevisedRamesh R ReddyNo ratings yet
- Ele. ConDocument33 pagesEle. ConKomal KambleNo ratings yet
- Chapter 3 Current ElectricityDocument31 pagesChapter 3 Current ElectricitySajjan BalasubramanyanNo ratings yet
- I DQ DT Q T: Chapter - 3 Current ElectricityDocument9 pagesI DQ DT Q T: Chapter - 3 Current ElectricityRahul Kumar SahuNo ratings yet
- 25 - Current, Resistance, and Electromotive Force - R K ParidaDocument11 pages25 - Current, Resistance, and Electromotive Force - R K ParidaMonicaNo ratings yet
- Mid 20-21Document17 pagesMid 20-21Marjuk RahibNo ratings yet
- Current ElectricityDocument44 pagesCurrent Electricityvarshasaindane8640No ratings yet
- 3 Quantum Theory of SolidsDocument15 pages3 Quantum Theory of SolidsyomamaNo ratings yet
- 1a.current Electricity Synopsis (1 32)Document32 pages1a.current Electricity Synopsis (1 32)h21891965No ratings yet
- Physics Notes Xii Current ElectricityDocument27 pagesPhysics Notes Xii Current ElectricitySuman RathiNo ratings yet
- Basic Laws and Electrical Properties of MetalsDocument5 pagesBasic Laws and Electrical Properties of MetalsHritik LalNo ratings yet
- Current ElectricityDocument7 pagesCurrent Electricitylakhbhat2020No ratings yet
- Mse Electrical Properties 1aDocument34 pagesMse Electrical Properties 1aSWAGATAM BAZNo ratings yet
- Current Electricity CH3 Part 1Document26 pagesCurrent Electricity CH3 Part 1Rishab SharmaNo ratings yet
- Wa0002.Document19 pagesWa0002.nakshathramoorayilNo ratings yet
- Current & Resistance: - Current and Current Density - Ohm's Law - Resistivity - ResistanceDocument17 pagesCurrent & Resistance: - Current and Current Density - Ohm's Law - Resistivity - ResistancePedroNo ratings yet
- ELK364E Lecture 1 ConductivityDocument64 pagesELK364E Lecture 1 ConductivitySemih YILDIRIMNo ratings yet
- Module 1Document20 pagesModule 1Shobha AnchanNo ratings yet
- XII - Physics - Chapter 3 - Current Electricity - Saju - Hsslive PDFDocument15 pagesXII - Physics - Chapter 3 - Current Electricity - Saju - Hsslive PDFArpit TyagiNo ratings yet
- A) Drude Theory of Metals B) Sommerfeld Theory of Metals.: July 12 1863 July 5 1906 German PhysicistDocument30 pagesA) Drude Theory of Metals B) Sommerfeld Theory of Metals.: July 12 1863 July 5 1906 German PhysicistAPNo ratings yet
- Engineering PhysicsDocument2 pagesEngineering PhysicsThilshathNo ratings yet
- Electrical and Thermal Conduction in SolidsDocument81 pagesElectrical and Thermal Conduction in SolidsAniket Sujay100% (1)
- Chapter 23 & 24: DC CircuitsDocument61 pagesChapter 23 & 24: DC CircuitsjackNo ratings yet
- Current ElectricityDocument25 pagesCurrent Electricityphysicskn92No ratings yet
- Current ElectricityDocument39 pagesCurrent ElectricitySUBHRANIL CHOWDHURYNo ratings yet
- Electrical Conductivity - Notes (November-2017) PDFDocument22 pagesElectrical Conductivity - Notes (November-2017) PDFskacNo ratings yet
- Current Electricity McqsDocument31 pagesCurrent Electricity McqsM.Tharun KumarNo ratings yet
- 2 - Current Electricity PDFDocument27 pages2 - Current Electricity PDFthinkiitNo ratings yet
- Intro. To Conducting Materials-Drude - Lorentz Classical Free Electron Theory of MetalsDocument16 pagesIntro. To Conducting Materials-Drude - Lorentz Classical Free Electron Theory of Metalsmades samiNo ratings yet
- Electrochemistry 21pDocument21 pagesElectrochemistry 21pMalise KaswagaNo ratings yet
- 9 - Current Electricity-01-TheoryDocument27 pages9 - Current Electricity-01-TheoryRaju SinghNo ratings yet
- Current ElectricityDocument73 pagesCurrent ElectricityPiyush JainNo ratings yet
- Dielectric Properties-22Document41 pagesDielectric Properties-22Satya JithNo ratings yet
- Chapter - 6 General Physics 2 Electricity and MagnestismDocument6 pagesChapter - 6 General Physics 2 Electricity and MagnestismAtahan AltayNo ratings yet
- OHM's Law: Length L Is The Distance Between The Two Points at Which The Voltage Is Measured and ADocument95 pagesOHM's Law: Length L Is The Distance Between The Two Points at Which The Voltage Is Measured and Asriya bonkuriNo ratings yet
- Electrical ConductivityDocument19 pagesElectrical ConductivityMahesh Lohith K.S80% (5)
- 4 Metals HandOutDocument22 pages4 Metals HandOutKey ConceptsNo ratings yet
- 1 Current Flow Mechanism-1Document11 pages1 Current Flow Mechanism-1DEEPIKA PAVUNDOSS 20BEC0285No ratings yet
- Free Electron TheoryDocument44 pagesFree Electron TheoryAllen MarananNo ratings yet
- CH 3 Current Electricity 12th PhysicsDocument112 pagesCH 3 Current Electricity 12th PhysicsSNo ratings yet
- Feynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterFrom EverandFeynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterNo ratings yet
- Green Synthesis of Silver Nanoparticles Using Plant ExtractsDocument2 pagesGreen Synthesis of Silver Nanoparticles Using Plant ExtractsAfrah MNo ratings yet
- 4green SynthesisDocument2 pages4green SynthesisAfrah MNo ratings yet
- Staphylococcus Aureus (Gram-Posi-Tive) by Disc DiffusionDocument1 pageStaphylococcus Aureus (Gram-Posi-Tive) by Disc DiffusionAfrah MNo ratings yet
- 1department of Medical NanotechnologyDocument2 pages1department of Medical NanotechnologyAfrah MNo ratings yet
- 1department of Medical NanotechnologyDocument2 pages1department of Medical NanotechnologyAfrah MNo ratings yet
- Results and Discussion: Silver Nanoparticles CharacterizationDocument1 pageResults and Discussion: Silver Nanoparticles CharacterizationAfrah MNo ratings yet
- Morphology Study: BiosynthesisDocument2 pagesMorphology Study: BiosynthesisAfrah MNo ratings yet
- Conclusion: C. Longa Compounds (Figure 7B)Document2 pagesConclusion: C. Longa Compounds (Figure 7B)Afrah MNo ratings yet
- FT-IR Chemical AnalysisDocument1 pageFT-IR Chemical AnalysisAfrah MNo ratings yet
- 5the Bioreduction of The AgDocument2 pages5the Bioreduction of The AgAfrah MNo ratings yet
- 4green SynthesisDocument2 pages4green SynthesisAfrah MNo ratings yet
- 3physical ApproachesDocument3 pages3physical ApproachesAfrah MNo ratings yet
- 5the Bioreduction of The AgDocument3 pages5the Bioreduction of The AgAfrah MNo ratings yet
- 1department of Medical NanotechnologyDocument3 pages1department of Medical NanotechnologyAfrah MNo ratings yet
- Free Radical Scavenging Activity: 1. Results and DiscussionDocument2 pagesFree Radical Scavenging Activity: 1. Results and DiscussionAfrah MNo ratings yet
- Abstract: Green: Curcuma LongaDocument1 pageAbstract: Green: Curcuma LongaAfrah MNo ratings yet
- C. Longa Compounds (Figure 7B) .: Physicochem Probl Miner Process. 2010 45:85-98Document2 pagesC. Longa Compounds (Figure 7B) .: Physicochem Probl Miner Process. 2010 45:85-98Afrah MNo ratings yet
- Caulerpa Racemosa Var. Cylin-Dracea. J Hazard MaterDocument3 pagesCaulerpa Racemosa Var. Cylin-Dracea. J Hazard MaterAfrah MNo ratings yet
- Synthesis of Ag/C. Longa Emulsion: Extraction PreparationDocument4 pagesSynthesis of Ag/C. Longa Emulsion: Extraction PreparationAfrah MNo ratings yet
- 2 ChengDocument2 pages2 ChengAfrah MNo ratings yet
- 1department of Medical NanotechnologyDocument1 page1department of Medical NanotechnologyAfrah MNo ratings yet
- Figure 6: XRD Pattern of Silver Nanoparticles: Angle (2 )Document2 pagesFigure 6: XRD Pattern of Silver Nanoparticles: Angle (2 )Afrah MNo ratings yet
- Figure 2: UV-Vis Spectra Recorded As A Function of Reaction Time of Silver NanoparticlesDocument1 pageFigure 2: UV-Vis Spectra Recorded As A Function of Reaction Time of Silver NanoparticlesAfrah MNo ratings yet
- 5the Bioreduction of The AgDocument2 pages5the Bioreduction of The AgAfrah MNo ratings yet
- Polygonum Glabrum Willd. Leaf Extract Mediated Green Synthesis of Silver Nanoparticles and Their Assessment of Antimicrobial ActivityDocument1 pagePolygonum Glabrum Willd. Leaf Extract Mediated Green Synthesis of Silver Nanoparticles and Their Assessment of Antimicrobial ActivityAfrah MNo ratings yet
- Geissus Latifolia) and Its Biological Activity. Org Med Chem LettDocument1 pageGeissus Latifolia) and Its Biological Activity. Org Med Chem LettAfrah MNo ratings yet
- Nissan Throttle Body Relearn ProcedureDocument2 pagesNissan Throttle Body Relearn ProcedureWilliam DroolNo ratings yet
- Booklet 1 Methods For in Vivo Dosimetry in External RadiotherapyDocument87 pagesBooklet 1 Methods For in Vivo Dosimetry in External RadiotherapyNesly M. MateoNo ratings yet
- PRIUS D Katalog (EMEC Dosing Pump Made in Italy)Document18 pagesPRIUS D Katalog (EMEC Dosing Pump Made in Italy)hary fadlyNo ratings yet
- Mid Term Paper - Thermodynamics For TechnologistsDocument1 pageMid Term Paper - Thermodynamics For TechnologistsSafwan HaiderNo ratings yet
- TM2500 and TM2500+ Intro PagesDocument24 pagesTM2500 and TM2500+ Intro Pagesavryone0% (1)
- Alfa Laval Separator Manual FOPX-609Document220 pagesAlfa Laval Separator Manual FOPX-609enriquezrafael75% (4)
- Bunker CalculationDocument10 pagesBunker CalculationNimesh PereraNo ratings yet
- Smart Storage Company FileDocument10 pagesSmart Storage Company Filetham phungNo ratings yet
- MS-SV Zawory SafetyDocument61 pagesMS-SV Zawory SafetyImieNo ratings yet
- Installation and Operation Manual XL-3 Oil-Fired Pool and Spa HeaterDocument32 pagesInstallation and Operation Manual XL-3 Oil-Fired Pool and Spa HeaterFilomeno FernandezNo ratings yet
- Test - 02: Class: XI - 2022 (Term-02)Document10 pagesTest - 02: Class: XI - 2022 (Term-02)DhruvJainNo ratings yet
- KGE Catalogue.36165930 PDFDocument2 pagesKGE Catalogue.36165930 PDFAshish MishraNo ratings yet
- Home Call For Proposals - 8th UNESCO Youth Forum Webform ResultsDocument12 pagesHome Call For Proposals - 8th UNESCO Youth Forum Webform ResultsJoseph MulabbiNo ratings yet
- AWS Guide To Abbreviations Certified WelderDocument1 pageAWS Guide To Abbreviations Certified Weldersigurdur hannessonNo ratings yet
- Buletinul Institutului Politehnic DIN Iaşi: Tomul LIV (LVIII) Fasc. 2Document68 pagesBuletinul Institutului Politehnic DIN Iaşi: Tomul LIV (LVIII) Fasc. 2Nutri ZemaNo ratings yet
- 10 RT-flex Design PDFDocument15 pages10 RT-flex Design PDFMarijaŽaperNo ratings yet
- Top Gun I-ARC 180Document9 pagesTop Gun I-ARC 180Tom PleysierNo ratings yet
- 2Q13 Power Transactions and TrendsDocument8 pages2Q13 Power Transactions and TrendsEuglena VerdeNo ratings yet
- Flow Measuring DevicesDocument39 pagesFlow Measuring DevicesAbdul Moeed Kalson100% (1)
- 16 Project Cost EstimateDocument6 pages16 Project Cost EstimateBhuneshwar Chelak100% (2)
- 6 Centrifugal Compressor IDocument40 pages6 Centrifugal Compressor IBassam AmirNo ratings yet
- MIPS EPC CatalogDocument17 pagesMIPS EPC CatalogPrasanthNo ratings yet
- Internal Combustion Engines NotesDocument12 pagesInternal Combustion Engines NotesrgowdamithunpvtNo ratings yet
- Jungian AstrologyDocument21 pagesJungian AstrologyLuiza NeumayerNo ratings yet
- DLL G7 Q3 Lesson 15Document3 pagesDLL G7 Q3 Lesson 15Jay-ar AlzonaNo ratings yet
- Periodic Trends: Name Class DateDocument18 pagesPeriodic Trends: Name Class DateAya LutfiNo ratings yet
- Capillary TubeDocument8 pagesCapillary Tubeziko23100% (2)
- Question Bank MechDocument102 pagesQuestion Bank MechKaradam PatelNo ratings yet
Activation Energy: The Energy Required To Make The Jump, E, Is The
Activation Energy: The Energy Required To Make The Jump, E, Is The
Uploaded by
Afrah M0 ratings0% found this document useful (0 votes)
63 views3 pagesIonic conductivity is a measure of how easily ions can move through a material. It depends on properties like the number and charge of ions as well as their mobility. The mobility of ions typically increases exponentially with temperature according to an Arrhenius equation, meaning ionic conductivity also rises exponentially with temperature. Plotting the logarithm of conductivity times temperature against the inverse of temperature produces a straight line whose slope equals the activation energy for ion motion.
Original Description:
Original Title
8
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentIonic conductivity is a measure of how easily ions can move through a material. It depends on properties like the number and charge of ions as well as their mobility. The mobility of ions typically increases exponentially with temperature according to an Arrhenius equation, meaning ionic conductivity also rises exponentially with temperature. Plotting the logarithm of conductivity times temperature against the inverse of temperature produces a straight line whose slope equals the activation energy for ion motion.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
63 views3 pagesActivation Energy: The Energy Required To Make The Jump, E, Is The
Activation Energy: The Energy Required To Make The Jump, E, Is The
Uploaded by
Afrah MIonic conductivity is a measure of how easily ions can move through a material. It depends on properties like the number and charge of ions as well as their mobility. The mobility of ions typically increases exponentially with temperature according to an Arrhenius equation, meaning ionic conductivity also rises exponentially with temperature. Plotting the logarithm of conductivity times temperature against the inverse of temperature produces a straight line whose slope equals the activation energy for ion motion.
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 3
, is the
The energy required to make the jump, Ea
activation energy.
Ionic Conductivity
Ionic Conductivity, σ, is defined the same as electrical
conductivity:
σ = nZem
where n is the number of charge carriers per unit volume, Ze is
the charge (e = 1.602189×10-19 C), and m is the mobility, which
is a measure of the drift velocity in a constant electric field.
Material Conductivity / (S m-1)
Ionic Conductors Ionic crystals <10-16 – 10-2
Solid electrolytes 10-1 – 103
Strong (liquid) electrolytes 10-1 – 103
Electronic conductors Metals 103 – 107
Semiconductors 10-3 – 104
Insulators <10-10
Ionic Conductivity
The temperature dependence of the mobility of the ions
can be expressed by an Arrhenius equation.
Ea
m
where m is a proportionality constant
Ea
exp m m0 exp 0
kT or known as the pre-exponential factor
kT
m0 depends on the attempt frequency (frequency of vibration of
the lattice 1012-1013 Hz), distance moved by ion, and the size of
the external field.
If the external field is small (up to 300 V cm-1), a temperature
dependence of 1/T is present in the pre exponential factor.
0 Ea
An expression for the variation of ionic conductivity: o exp
The term σ0 contains n and Ze as well as the attempt frequency
and jump distance. Taking logs… T – Ea
ln
0
T
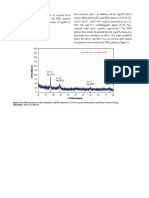
Plotting lnσT vs 1/T should produce a straight line with a slope of –E a.
lnσ vs 1/T is also used
Conductivities of Solid Electrolytes vs Temperature
NaCl
You might also like
- Water Cooled Chiller ManualDocument52 pagesWater Cooled Chiller Manualkhamsone pengmanivongNo ratings yet
- Zinc Report 2017-06-19 Briol Masson For Zinc OneDocument11 pagesZinc Report 2017-06-19 Briol Masson For Zinc OneLuan BiciNo ratings yet
- Resistance Vs Temperature Experiment Lab ReportDocument7 pagesResistance Vs Temperature Experiment Lab ReportEmily Gatlin67% (3)
- Metals, Semiconductors, and InsulatorsDocument38 pagesMetals, Semiconductors, and InsulatorskaidoqNo ratings yet
- Chapter 5: Carrier Transport Phenomena: Transport The Process by Which Charged Particles (ElectronsDocument32 pagesChapter 5: Carrier Transport Phenomena: Transport The Process by Which Charged Particles (ElectronsAbdullah AbdulhameedNo ratings yet
- CH 3 Current Electricity Notes XiiDocument10 pagesCH 3 Current Electricity Notes Xiisharmanehasandeep1No ratings yet
- 3 Current ElectricityDocument21 pages3 Current ElectricitySakshi KantNo ratings yet
- CLS Aipmt 16 17 XII Phy Study Package 5 SET 1 Chapter 3Document28 pagesCLS Aipmt 16 17 XII Phy Study Package 5 SET 1 Chapter 3Kareena Gupta17% (6)
- EOPM Part1 PDFDocument29 pagesEOPM Part1 PDFRoy VeseyNo ratings yet
- ElectricityDocument44 pagesElectricityKirancivilNo ratings yet
- Materials For ElectronicsDocument22 pagesMaterials For ElectronicsEngr Haseena JabbarNo ratings yet
- XII - Physics - Chapter 3 - Current ElectricityDocument15 pagesXII - Physics - Chapter 3 - Current ElectricityHADINo ratings yet
- Current Electricty by Baba GangDocument7 pagesCurrent Electricty by Baba GangKushagra RajNo ratings yet
- Physics 20 PDFDocument30 pagesPhysics 20 PDFShivani Shree SundaramoorthyNo ratings yet
- Carrier Transport RevisedDocument65 pagesCarrier Transport RevisedShivani GuptaNo ratings yet
- Electrical and Thermal Conduction-2Document3 pagesElectrical and Thermal Conduction-2Sajjad HossainNo ratings yet
- GT 65 QJPSR 66 L VITCxp 02Document8 pagesGT 65 QJPSR 66 L VITCxp 02Sahil SinghNo ratings yet
- PH Gokul Sharma: Current ElectricityDocument15 pagesPH Gokul Sharma: Current ElectricityWillis ChekovNo ratings yet
- Chapter 2-Type of ElectroceramicsDocument33 pagesChapter 2-Type of ElectroceramicsDP DianaNo ratings yet
- CURRENT ELECTRICITY 27-09-2017 RevisedDocument16 pagesCURRENT ELECTRICITY 27-09-2017 RevisedRamesh R ReddyNo ratings yet
- Ele. ConDocument33 pagesEle. ConKomal KambleNo ratings yet
- Chapter 3 Current ElectricityDocument31 pagesChapter 3 Current ElectricitySajjan BalasubramanyanNo ratings yet
- I DQ DT Q T: Chapter - 3 Current ElectricityDocument9 pagesI DQ DT Q T: Chapter - 3 Current ElectricityRahul Kumar SahuNo ratings yet
- 25 - Current, Resistance, and Electromotive Force - R K ParidaDocument11 pages25 - Current, Resistance, and Electromotive Force - R K ParidaMonicaNo ratings yet
- Mid 20-21Document17 pagesMid 20-21Marjuk RahibNo ratings yet
- Current ElectricityDocument44 pagesCurrent Electricityvarshasaindane8640No ratings yet
- 3 Quantum Theory of SolidsDocument15 pages3 Quantum Theory of SolidsyomamaNo ratings yet
- 1a.current Electricity Synopsis (1 32)Document32 pages1a.current Electricity Synopsis (1 32)h21891965No ratings yet
- Physics Notes Xii Current ElectricityDocument27 pagesPhysics Notes Xii Current ElectricitySuman RathiNo ratings yet
- Basic Laws and Electrical Properties of MetalsDocument5 pagesBasic Laws and Electrical Properties of MetalsHritik LalNo ratings yet
- Current ElectricityDocument7 pagesCurrent Electricitylakhbhat2020No ratings yet
- Mse Electrical Properties 1aDocument34 pagesMse Electrical Properties 1aSWAGATAM BAZNo ratings yet
- Current Electricity CH3 Part 1Document26 pagesCurrent Electricity CH3 Part 1Rishab SharmaNo ratings yet
- Wa0002.Document19 pagesWa0002.nakshathramoorayilNo ratings yet
- Current & Resistance: - Current and Current Density - Ohm's Law - Resistivity - ResistanceDocument17 pagesCurrent & Resistance: - Current and Current Density - Ohm's Law - Resistivity - ResistancePedroNo ratings yet
- ELK364E Lecture 1 ConductivityDocument64 pagesELK364E Lecture 1 ConductivitySemih YILDIRIMNo ratings yet
- Module 1Document20 pagesModule 1Shobha AnchanNo ratings yet
- XII - Physics - Chapter 3 - Current Electricity - Saju - Hsslive PDFDocument15 pagesXII - Physics - Chapter 3 - Current Electricity - Saju - Hsslive PDFArpit TyagiNo ratings yet
- A) Drude Theory of Metals B) Sommerfeld Theory of Metals.: July 12 1863 July 5 1906 German PhysicistDocument30 pagesA) Drude Theory of Metals B) Sommerfeld Theory of Metals.: July 12 1863 July 5 1906 German PhysicistAPNo ratings yet
- Engineering PhysicsDocument2 pagesEngineering PhysicsThilshathNo ratings yet
- Electrical and Thermal Conduction in SolidsDocument81 pagesElectrical and Thermal Conduction in SolidsAniket Sujay100% (1)
- Chapter 23 & 24: DC CircuitsDocument61 pagesChapter 23 & 24: DC CircuitsjackNo ratings yet
- Current ElectricityDocument25 pagesCurrent Electricityphysicskn92No ratings yet
- Current ElectricityDocument39 pagesCurrent ElectricitySUBHRANIL CHOWDHURYNo ratings yet
- Electrical Conductivity - Notes (November-2017) PDFDocument22 pagesElectrical Conductivity - Notes (November-2017) PDFskacNo ratings yet
- Current Electricity McqsDocument31 pagesCurrent Electricity McqsM.Tharun KumarNo ratings yet
- 2 - Current Electricity PDFDocument27 pages2 - Current Electricity PDFthinkiitNo ratings yet
- Intro. To Conducting Materials-Drude - Lorentz Classical Free Electron Theory of MetalsDocument16 pagesIntro. To Conducting Materials-Drude - Lorentz Classical Free Electron Theory of Metalsmades samiNo ratings yet
- Electrochemistry 21pDocument21 pagesElectrochemistry 21pMalise KaswagaNo ratings yet
- 9 - Current Electricity-01-TheoryDocument27 pages9 - Current Electricity-01-TheoryRaju SinghNo ratings yet
- Current ElectricityDocument73 pagesCurrent ElectricityPiyush JainNo ratings yet
- Dielectric Properties-22Document41 pagesDielectric Properties-22Satya JithNo ratings yet
- Chapter - 6 General Physics 2 Electricity and MagnestismDocument6 pagesChapter - 6 General Physics 2 Electricity and MagnestismAtahan AltayNo ratings yet
- OHM's Law: Length L Is The Distance Between The Two Points at Which The Voltage Is Measured and ADocument95 pagesOHM's Law: Length L Is The Distance Between The Two Points at Which The Voltage Is Measured and Asriya bonkuriNo ratings yet
- Electrical ConductivityDocument19 pagesElectrical ConductivityMahesh Lohith K.S80% (5)
- 4 Metals HandOutDocument22 pages4 Metals HandOutKey ConceptsNo ratings yet
- 1 Current Flow Mechanism-1Document11 pages1 Current Flow Mechanism-1DEEPIKA PAVUNDOSS 20BEC0285No ratings yet
- Free Electron TheoryDocument44 pagesFree Electron TheoryAllen MarananNo ratings yet
- CH 3 Current Electricity 12th PhysicsDocument112 pagesCH 3 Current Electricity 12th PhysicsSNo ratings yet
- Feynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterFrom EverandFeynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterNo ratings yet
- Green Synthesis of Silver Nanoparticles Using Plant ExtractsDocument2 pagesGreen Synthesis of Silver Nanoparticles Using Plant ExtractsAfrah MNo ratings yet
- 4green SynthesisDocument2 pages4green SynthesisAfrah MNo ratings yet
- Staphylococcus Aureus (Gram-Posi-Tive) by Disc DiffusionDocument1 pageStaphylococcus Aureus (Gram-Posi-Tive) by Disc DiffusionAfrah MNo ratings yet
- 1department of Medical NanotechnologyDocument2 pages1department of Medical NanotechnologyAfrah MNo ratings yet
- 1department of Medical NanotechnologyDocument2 pages1department of Medical NanotechnologyAfrah MNo ratings yet
- Results and Discussion: Silver Nanoparticles CharacterizationDocument1 pageResults and Discussion: Silver Nanoparticles CharacterizationAfrah MNo ratings yet
- Morphology Study: BiosynthesisDocument2 pagesMorphology Study: BiosynthesisAfrah MNo ratings yet
- Conclusion: C. Longa Compounds (Figure 7B)Document2 pagesConclusion: C. Longa Compounds (Figure 7B)Afrah MNo ratings yet
- FT-IR Chemical AnalysisDocument1 pageFT-IR Chemical AnalysisAfrah MNo ratings yet
- 5the Bioreduction of The AgDocument2 pages5the Bioreduction of The AgAfrah MNo ratings yet
- 4green SynthesisDocument2 pages4green SynthesisAfrah MNo ratings yet
- 3physical ApproachesDocument3 pages3physical ApproachesAfrah MNo ratings yet
- 5the Bioreduction of The AgDocument3 pages5the Bioreduction of The AgAfrah MNo ratings yet
- 1department of Medical NanotechnologyDocument3 pages1department of Medical NanotechnologyAfrah MNo ratings yet
- Free Radical Scavenging Activity: 1. Results and DiscussionDocument2 pagesFree Radical Scavenging Activity: 1. Results and DiscussionAfrah MNo ratings yet
- Abstract: Green: Curcuma LongaDocument1 pageAbstract: Green: Curcuma LongaAfrah MNo ratings yet
- C. Longa Compounds (Figure 7B) .: Physicochem Probl Miner Process. 2010 45:85-98Document2 pagesC. Longa Compounds (Figure 7B) .: Physicochem Probl Miner Process. 2010 45:85-98Afrah MNo ratings yet
- Caulerpa Racemosa Var. Cylin-Dracea. J Hazard MaterDocument3 pagesCaulerpa Racemosa Var. Cylin-Dracea. J Hazard MaterAfrah MNo ratings yet
- Synthesis of Ag/C. Longa Emulsion: Extraction PreparationDocument4 pagesSynthesis of Ag/C. Longa Emulsion: Extraction PreparationAfrah MNo ratings yet
- 2 ChengDocument2 pages2 ChengAfrah MNo ratings yet
- 1department of Medical NanotechnologyDocument1 page1department of Medical NanotechnologyAfrah MNo ratings yet
- Figure 6: XRD Pattern of Silver Nanoparticles: Angle (2 )Document2 pagesFigure 6: XRD Pattern of Silver Nanoparticles: Angle (2 )Afrah MNo ratings yet
- Figure 2: UV-Vis Spectra Recorded As A Function of Reaction Time of Silver NanoparticlesDocument1 pageFigure 2: UV-Vis Spectra Recorded As A Function of Reaction Time of Silver NanoparticlesAfrah MNo ratings yet
- 5the Bioreduction of The AgDocument2 pages5the Bioreduction of The AgAfrah MNo ratings yet
- Polygonum Glabrum Willd. Leaf Extract Mediated Green Synthesis of Silver Nanoparticles and Their Assessment of Antimicrobial ActivityDocument1 pagePolygonum Glabrum Willd. Leaf Extract Mediated Green Synthesis of Silver Nanoparticles and Their Assessment of Antimicrobial ActivityAfrah MNo ratings yet
- Geissus Latifolia) and Its Biological Activity. Org Med Chem LettDocument1 pageGeissus Latifolia) and Its Biological Activity. Org Med Chem LettAfrah MNo ratings yet
- Nissan Throttle Body Relearn ProcedureDocument2 pagesNissan Throttle Body Relearn ProcedureWilliam DroolNo ratings yet
- Booklet 1 Methods For in Vivo Dosimetry in External RadiotherapyDocument87 pagesBooklet 1 Methods For in Vivo Dosimetry in External RadiotherapyNesly M. MateoNo ratings yet
- PRIUS D Katalog (EMEC Dosing Pump Made in Italy)Document18 pagesPRIUS D Katalog (EMEC Dosing Pump Made in Italy)hary fadlyNo ratings yet
- Mid Term Paper - Thermodynamics For TechnologistsDocument1 pageMid Term Paper - Thermodynamics For TechnologistsSafwan HaiderNo ratings yet
- TM2500 and TM2500+ Intro PagesDocument24 pagesTM2500 and TM2500+ Intro Pagesavryone0% (1)
- Alfa Laval Separator Manual FOPX-609Document220 pagesAlfa Laval Separator Manual FOPX-609enriquezrafael75% (4)
- Bunker CalculationDocument10 pagesBunker CalculationNimesh PereraNo ratings yet
- Smart Storage Company FileDocument10 pagesSmart Storage Company Filetham phungNo ratings yet
- MS-SV Zawory SafetyDocument61 pagesMS-SV Zawory SafetyImieNo ratings yet
- Installation and Operation Manual XL-3 Oil-Fired Pool and Spa HeaterDocument32 pagesInstallation and Operation Manual XL-3 Oil-Fired Pool and Spa HeaterFilomeno FernandezNo ratings yet
- Test - 02: Class: XI - 2022 (Term-02)Document10 pagesTest - 02: Class: XI - 2022 (Term-02)DhruvJainNo ratings yet
- KGE Catalogue.36165930 PDFDocument2 pagesKGE Catalogue.36165930 PDFAshish MishraNo ratings yet
- Home Call For Proposals - 8th UNESCO Youth Forum Webform ResultsDocument12 pagesHome Call For Proposals - 8th UNESCO Youth Forum Webform ResultsJoseph MulabbiNo ratings yet
- AWS Guide To Abbreviations Certified WelderDocument1 pageAWS Guide To Abbreviations Certified Weldersigurdur hannessonNo ratings yet
- Buletinul Institutului Politehnic DIN Iaşi: Tomul LIV (LVIII) Fasc. 2Document68 pagesBuletinul Institutului Politehnic DIN Iaşi: Tomul LIV (LVIII) Fasc. 2Nutri ZemaNo ratings yet
- 10 RT-flex Design PDFDocument15 pages10 RT-flex Design PDFMarijaŽaperNo ratings yet
- Top Gun I-ARC 180Document9 pagesTop Gun I-ARC 180Tom PleysierNo ratings yet
- 2Q13 Power Transactions and TrendsDocument8 pages2Q13 Power Transactions and TrendsEuglena VerdeNo ratings yet
- Flow Measuring DevicesDocument39 pagesFlow Measuring DevicesAbdul Moeed Kalson100% (1)
- 16 Project Cost EstimateDocument6 pages16 Project Cost EstimateBhuneshwar Chelak100% (2)
- 6 Centrifugal Compressor IDocument40 pages6 Centrifugal Compressor IBassam AmirNo ratings yet
- MIPS EPC CatalogDocument17 pagesMIPS EPC CatalogPrasanthNo ratings yet
- Internal Combustion Engines NotesDocument12 pagesInternal Combustion Engines NotesrgowdamithunpvtNo ratings yet
- Jungian AstrologyDocument21 pagesJungian AstrologyLuiza NeumayerNo ratings yet
- DLL G7 Q3 Lesson 15Document3 pagesDLL G7 Q3 Lesson 15Jay-ar AlzonaNo ratings yet
- Periodic Trends: Name Class DateDocument18 pagesPeriodic Trends: Name Class DateAya LutfiNo ratings yet
- Capillary TubeDocument8 pagesCapillary Tubeziko23100% (2)
- Question Bank MechDocument102 pagesQuestion Bank MechKaradam PatelNo ratings yet