Professional Documents
Culture Documents
Results and Discussion: Antibacterial Activity of Silver Nanoparticles
Results and Discussion: Antibacterial Activity of Silver Nanoparticles
Uploaded by
Afrah MCopyright:
Available Formats
You might also like
- Spectrophotometry - PPT 18.07.08Document51 pagesSpectrophotometry - PPT 18.07.08MohammadS.Al-DarabsehNo ratings yet
- Group C Rice Chapter 1 3 UpdatedDocument39 pagesGroup C Rice Chapter 1 3 Updateddeevonc100% (1)
- Silver Nitrate ConcentrationDocument3 pagesSilver Nitrate ConcentrationAfrah MNo ratings yet
- 12 SupportDocument5 pages12 SupportBé Kem Xưởng MayNo ratings yet
- 7332 - Kfa2 Furosemid Kel 5aDocument3 pages7332 - Kfa2 Furosemid Kel 5aSekarayu KusumawNo ratings yet
- Andres - Adsorption Process Using Activated Carbon - Envi Chem Lab (Autorecovered)Document7 pagesAndres - Adsorption Process Using Activated Carbon - Envi Chem Lab (Autorecovered)Andres, Andrea Lyn M.No ratings yet
- Water Models: Work Group: Jorge Madias Sebastian Sylvestre Begnis Wadi Paul ChiapparoliDocument18 pagesWater Models: Work Group: Jorge Madias Sebastian Sylvestre Begnis Wadi Paul ChiapparoliJorge MadiasNo ratings yet
- Figure 2: UV-Vis Spectra Recorded As A Function of Reaction Time of Silver NanoparticlesDocument1 pageFigure 2: UV-Vis Spectra Recorded As A Function of Reaction Time of Silver NanoparticlesAfrah MNo ratings yet
- Nanoparticulas:: Sintesis, Caracterizacion y AplicacionesDocument62 pagesNanoparticulas:: Sintesis, Caracterizacion y AplicacionesruizfacundoNo ratings yet
- Fundamentals of Nuclear Magnetic Resonance (NMR) LoggingDocument25 pagesFundamentals of Nuclear Magnetic Resonance (NMR) LoggingMónica Alexandra Sedano DuarteNo ratings yet
- KFA Derivatif 5-6 ADocument11 pagesKFA Derivatif 5-6 AElvina Iskandar TanurahardjaNo ratings yet
- Foster Cole 101230199 Malaïka Zarrouki 2021-01-29Document7 pagesFoster Cole 101230199 Malaïka Zarrouki 2021-01-29Cole FosterNo ratings yet
- NMR Compatibility ModeDocument25 pagesNMR Compatibility ModeSergioNo ratings yet
- Experimental: Experimental Velocidades Calculadas L-B Calculado (S) (MM) V0 (MM/S) V0 (MM/S)Document6 pagesExperimental: Experimental Velocidades Calculadas L-B Calculado (S) (MM) V0 (MM/S) V0 (MM/S)jhon robert ortiz gilNo ratings yet
- Miller Indices (HKL) Are Necessary To Be Assigned For Each: XRD AnalysisDocument4 pagesMiller Indices (HKL) Are Necessary To Be Assigned For Each: XRD AnalysisAfrah MNo ratings yet
- Annual Session of The Indian Ceramic Society VisakhapatnamDocument12 pagesAnnual Session of The Indian Ceramic Society VisakhapatnamshajeshNo ratings yet
- Investigation 31 Determine The Concentration of CuSO4 Using SpectrometerDocument5 pagesInvestigation 31 Determine The Concentration of CuSO4 Using SpectrometerliaojingNo ratings yet
- Insulation Resistance Value Measurement (1.8.23)Document9 pagesInsulation Resistance Value Measurement (1.8.23)sandeep_bcplNo ratings yet
- A Multi-Modal Sweat Sensing Patch For Cross-Verification of Sweat Rate, Total Ionic Charge, and Na ConcentrationDocument14 pagesA Multi-Modal Sweat Sensing Patch For Cross-Verification of Sweat Rate, Total Ionic Charge, and Na ConcentrationThilini ApsaraNo ratings yet
- Costa, V. y Thickett, D. Metals Coupons Environmental Sensors. 2010Document34 pagesCosta, V. y Thickett, D. Metals Coupons Environmental Sensors. 2010Trinidad Pasíes Arqueología-ConservaciónNo ratings yet
- Insulation Resistance Value Measurement (1.1.17)Document9 pagesInsulation Resistance Value Measurement (1.1.17)ankitNo ratings yet
- CUSO4 PostlabDocument8 pagesCUSO4 PostlabRuwanthika Fernando100% (1)
- Insulation Resistance Value Measurement (1.1.19)Document9 pagesInsulation Resistance Value Measurement (1.1.19)Abdulwahed alsafanyNo ratings yet
- Enzyme Kinetic ReportDocument7 pagesEnzyme Kinetic ReportHalil Onur AltayNo ratings yet
- Antioksidan FRAP FIXDocument4 pagesAntioksidan FRAP FIXizzaaaaawNo ratings yet
- Lo Wry ProcedureDocument1 pageLo Wry ProcedurevijaygovindarajNo ratings yet
- Experiment No1 - Spectrophotometry ManualDocument2 pagesExperiment No1 - Spectrophotometry ManualJERI ANN CAPULONGNo ratings yet
- Insulation Resistance MeasurementDocument9 pagesInsulation Resistance Measurementishwer kushwahNo ratings yet
- Experiment 1: The Visible Spectra of Soft Drinks: A. Pre-Laboratory QuestionsDocument5 pagesExperiment 1: The Visible Spectra of Soft Drinks: A. Pre-Laboratory QuestionsMuhd Mirza HizamiNo ratings yet
- T-Interno DR Munive (Balance Metalurgico Lix Dinamica) 03-07-2019Document40 pagesT-Interno DR Munive (Balance Metalurgico Lix Dinamica) 03-07-2019GTNo ratings yet
- Experiment 1 CHM260 PDFDocument4 pagesExperiment 1 CHM260 PDFTifa IbrahimNo ratings yet
- Design of Shotcrete ConcreteDocument8 pagesDesign of Shotcrete ConcreteDevinder SokhiNo ratings yet
- Simulación Reacción EDocument67 pagesSimulación Reacción EMilagros Morales VergarayNo ratings yet
- Reactions of The Citric Acid Cycle Succinate Dehydrogenase, Fumarase and Malate DehydrogenaseDocument10 pagesReactions of The Citric Acid Cycle Succinate Dehydrogenase, Fumarase and Malate Dehydrogenase2023422047No ratings yet
- YesssDocument6 pagesYesssashNo ratings yet
- Nche211 Exp1Document8 pagesNche211 Exp1Mbali MdlaloseNo ratings yet
- Photometry: International Class Program Faculty of Medicine University of IndonesiaDocument29 pagesPhotometry: International Class Program Faculty of Medicine University of Indonesiawendyjemmy8gmailcomNo ratings yet
- Materi 5 Selectivitas BioleachingDocument8 pagesMateri 5 Selectivitas BioleachingemiaNo ratings yet
- Molecules: Sonochemical Degradation Kinetics of Methyl Violet in Aqueous SolutionsDocument5 pagesMolecules: Sonochemical Degradation Kinetics of Methyl Violet in Aqueous SolutionsThomas CharmNo ratings yet
- Simultaneous Determination of The Concentrations of Cobalt ( - ) and Nickel ( - ) by UV/Vis SpectrosDocument8 pagesSimultaneous Determination of The Concentrations of Cobalt ( - ) and Nickel ( - ) by UV/Vis SpectrosJassim123 SabtNo ratings yet
- ENV Laboratory Report Permanganat ValueDocument19 pagesENV Laboratory Report Permanganat ValueJulio DavidNo ratings yet
- IC Application Note S-368: (MG/L) (MG/L)Document2 pagesIC Application Note S-368: (MG/L) (MG/L)Mai NhựtNo ratings yet
- Color LabDocument9 pagesColor LabssweatNo ratings yet
- Bom BoqDocument4 pagesBom BoqRenzel EstebanNo ratings yet
- The Potential Application of Periwinkle Shell Ash As-Group4 (Autosaved)Document26 pagesThe Potential Application of Periwinkle Shell Ash As-Group4 (Autosaved)Abraham wisdomNo ratings yet
- Ultrasensitive Colorimetric Chromium Chemosensor Based On Dye Color Switching Under The CR (VI) - Stimulated Au NPs Catalytic Activity KeternganDocument26 pagesUltrasensitive Colorimetric Chromium Chemosensor Based On Dye Color Switching Under The CR (VI) - Stimulated Au NPs Catalytic Activity KeternganDesy PermatasariNo ratings yet
- Norris-Steel-Sucker-Rods - Pony-Rods - P001-V02-100908 - 5Document1 pageNorris-Steel-Sucker-Rods - Pony-Rods - P001-V02-100908 - 5Mohamed GhareebNo ratings yet
- Potato Osmolarity IA - ) ) 2Document11 pagesPotato Osmolarity IA - ) ) 2Kanika KumarNo ratings yet
- PembahaasanDocument10 pagesPembahaasanrerenNo ratings yet
- CaffeieneDocument8 pagesCaffeieneHawta AbdullaNo ratings yet
- Figures and TablesDocument3 pagesFigures and TablesAngeline RabuyoNo ratings yet
- Enzimas Actividad y KM EjemploDocument7 pagesEnzimas Actividad y KM Ejemploeymer alberto contreras mirandaNo ratings yet
- ENZIMAS ACTIVIDAD Y KM EJEMPLO Ultimo LabDocument7 pagesENZIMAS ACTIVIDAD Y KM EJEMPLO Ultimo Labeymer alberto contreras mirandaNo ratings yet
- Nor Iftiha Binti Abdul Aziz - 2019217292 - Ras1205eDocument26 pagesNor Iftiha Binti Abdul Aziz - 2019217292 - Ras1205eNor Iftiha AzizNo ratings yet
- CD: Cadmium: 1. Analytical ConditionsDocument2 pagesCD: Cadmium: 1. Analytical ConditionsVương AnhNo ratings yet
- Papverin HCL 5A-1Document3 pagesPapverin HCL 5A-1Elvina Iskandar TanurahardjaNo ratings yet
- Seminar 1 PDFDocument29 pagesSeminar 1 PDFDiana Andrade LarrañagaNo ratings yet
- Kondisi Ruang Awal Akhir Rata-Rata P (MMHG) Temperatur (°C) : Penentuan Panjang Gelombang MaksimumDocument83 pagesKondisi Ruang Awal Akhir Rata-Rata P (MMHG) Temperatur (°C) : Penentuan Panjang Gelombang MaksimumTaniadi SuriaNo ratings yet
- Staphylococcus Aureus (Gram-Posi-Tive) by Disc DiffusionDocument1 pageStaphylococcus Aureus (Gram-Posi-Tive) by Disc DiffusionAfrah MNo ratings yet
- 4green SynthesisDocument2 pages4green SynthesisAfrah MNo ratings yet
- Green Synthesis of Silver Nanoparticles Using Plant ExtractsDocument2 pagesGreen Synthesis of Silver Nanoparticles Using Plant ExtractsAfrah MNo ratings yet
- Results and Discussion: Silver Nanoparticles CharacterizationDocument1 pageResults and Discussion: Silver Nanoparticles CharacterizationAfrah MNo ratings yet
- 1department of Medical NanotechnologyDocument2 pages1department of Medical NanotechnologyAfrah MNo ratings yet
- 1department of Medical NanotechnologyDocument2 pages1department of Medical NanotechnologyAfrah MNo ratings yet
- Morphology Study: BiosynthesisDocument2 pagesMorphology Study: BiosynthesisAfrah MNo ratings yet
- Free Radical Scavenging Activity: 1. Results and DiscussionDocument2 pagesFree Radical Scavenging Activity: 1. Results and DiscussionAfrah MNo ratings yet
- FT-IR Chemical AnalysisDocument1 pageFT-IR Chemical AnalysisAfrah MNo ratings yet
- 4green SynthesisDocument2 pages4green SynthesisAfrah MNo ratings yet
- Abstract: Green: Curcuma LongaDocument1 pageAbstract: Green: Curcuma LongaAfrah MNo ratings yet
- Conclusion: C. Longa Compounds (Figure 7B)Document2 pagesConclusion: C. Longa Compounds (Figure 7B)Afrah MNo ratings yet
- 1department of Medical NanotechnologyDocument3 pages1department of Medical NanotechnologyAfrah MNo ratings yet
- 2 ChengDocument2 pages2 ChengAfrah MNo ratings yet
- C. Longa Compounds (Figure 7B) .: Physicochem Probl Miner Process. 2010 45:85-98Document2 pagesC. Longa Compounds (Figure 7B) .: Physicochem Probl Miner Process. 2010 45:85-98Afrah MNo ratings yet
- 5the Bioreduction of The AgDocument2 pages5the Bioreduction of The AgAfrah MNo ratings yet
- 3physical ApproachesDocument3 pages3physical ApproachesAfrah MNo ratings yet
- 5the Bioreduction of The AgDocument3 pages5the Bioreduction of The AgAfrah MNo ratings yet
- Synthesis of Ag/C. Longa Emulsion: Extraction PreparationDocument4 pagesSynthesis of Ag/C. Longa Emulsion: Extraction PreparationAfrah MNo ratings yet
- Figure 6: XRD Pattern of Silver Nanoparticles: Angle (2 )Document2 pagesFigure 6: XRD Pattern of Silver Nanoparticles: Angle (2 )Afrah MNo ratings yet
- 1department of Medical NanotechnologyDocument1 page1department of Medical NanotechnologyAfrah MNo ratings yet
- 5the Bioreduction of The AgDocument2 pages5the Bioreduction of The AgAfrah MNo ratings yet
- Figure 2: UV-Vis Spectra Recorded As A Function of Reaction Time of Silver NanoparticlesDocument1 pageFigure 2: UV-Vis Spectra Recorded As A Function of Reaction Time of Silver NanoparticlesAfrah MNo ratings yet
- Geissus Latifolia) and Its Biological Activity. Org Med Chem LettDocument1 pageGeissus Latifolia) and Its Biological Activity. Org Med Chem LettAfrah MNo ratings yet
- Caulerpa Racemosa Var. Cylin-Dracea. J Hazard MaterDocument3 pagesCaulerpa Racemosa Var. Cylin-Dracea. J Hazard MaterAfrah MNo ratings yet
- Polygonum Glabrum Willd. Leaf Extract Mediated Green Synthesis of Silver Nanoparticles and Their Assessment of Antimicrobial ActivityDocument1 pagePolygonum Glabrum Willd. Leaf Extract Mediated Green Synthesis of Silver Nanoparticles and Their Assessment of Antimicrobial ActivityAfrah MNo ratings yet
- Fiber Optic Test OTDRDocument37 pagesFiber Optic Test OTDRalbert_f42100% (3)
- Notess CBSE Class 12 Physics Notes - Wave OpticsDocument10 pagesNotess CBSE Class 12 Physics Notes - Wave OpticsPrashant KoreNo ratings yet
- HSSC I Send Up Key ChemistryDocument5 pagesHSSC I Send Up Key Chemistrymymegaacc111No ratings yet
- PH8201 Physics For Civil Engineering Notes PDFDocument92 pagesPH8201 Physics For Civil Engineering Notes PDFperiasamy_nano100% (1)
- General AwarenessDocument159 pagesGeneral AwarenessNiladri GoswamiNo ratings yet
- Optical Instrument: By: Sariyati, S.TDocument21 pagesOptical Instrument: By: Sariyati, S.TparamitaNo ratings yet
- How Rainbow Is FormedDocument15 pagesHow Rainbow Is FormedLaksmita Zhafira DisaNo ratings yet
- Mcqs For Mock TestDocument7 pagesMcqs For Mock TestHira Enayatullah Khan100% (1)
- OLD Human Eye and The Colourful WorldDocument11 pagesOLD Human Eye and The Colourful WorldRadhika GargNo ratings yet
- Atomic Emission Spectroscopy AsaDocument16 pagesAtomic Emission Spectroscopy AsaMark Cliffton Badlon100% (1)
- Patch AntennaDocument8 pagesPatch Antennajoseph vijayNo ratings yet
- VHF JRC JHS-800SDocument4 pagesVHF JRC JHS-800Sxuantruong0212No ratings yet
- Fundamentals of PhotographyDocument29 pagesFundamentals of PhotographyAndrew Richard Thompson100% (2)
- 23 Electronic TheodolitesDocument4 pages23 Electronic TheodolitesEmaNo ratings yet
- 3X-Rvv-65A-R9: 18-Port Sector Antenna, 6X694-960 and 12X1695-2690 MHZ, 65° HPBW, 9xretDocument5 pages3X-Rvv-65A-R9: 18-Port Sector Antenna, 6X694-960 and 12X1695-2690 MHZ, 65° HPBW, 9xretoaguilar83No ratings yet
- Technical Details: The World InnovationDocument4 pagesTechnical Details: The World InnovationTBS Máy Phát ĐiệnNo ratings yet
- Qau Course Outlines PDFDocument11 pagesQau Course Outlines PDFphooolNo ratings yet
- Vs Main Catalogue 2012 2013katalogDocument564 pagesVs Main Catalogue 2012 2013katalogDanka DukanacNo ratings yet
- BIS StandardsDocument3 pagesBIS Standardsjannumits100% (1)
- Pacer JA Vol 17 ReplacementDocument475 pagesPacer JA Vol 17 ReplacementJeff TaylorNo ratings yet
- Fresnel BiprismDocument12 pagesFresnel BiprismShubham JaswalNo ratings yet
- Stefan M Hoek Omicron PDFDocument7 pagesStefan M Hoek Omicron PDFmersiumNo ratings yet
- Light 9 QPDocument14 pagesLight 9 QPYeasir Arham MahirNo ratings yet
- X Final Grand Test KitDocument188 pagesX Final Grand Test KitSana DanishNo ratings yet
- Q-Smart850 Specs EN 052014Document2 pagesQ-Smart850 Specs EN 052014déborah_rosalesNo ratings yet
- XX. Introductory Physics, High SchoolDocument25 pagesXX. Introductory Physics, High SchoolramjdNo ratings yet
- Photoluminescence and Optical Absorption in Cds Se NanocrystalsDocument3 pagesPhotoluminescence and Optical Absorption in Cds Se NanocrystalsSIMETNo ratings yet
- Pix 4 Dmapper Camera Requirements For Precision AGDocument10 pagesPix 4 Dmapper Camera Requirements For Precision AGColleen CallahanNo ratings yet
- Post Task - Ingrid Gissela GonzálezDocument12 pagesPost Task - Ingrid Gissela GonzálezJawin Ricardo Perez AcevedoNo ratings yet
Results and Discussion: Antibacterial Activity of Silver Nanoparticles
Results and Discussion: Antibacterial Activity of Silver Nanoparticles
Uploaded by
Afrah MOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Results and Discussion: Antibacterial Activity of Silver Nanoparticles
Results and Discussion: Antibacterial Activity of Silver Nanoparticles
Uploaded by
Afrah MCopyright:
Available Formats
XRD measurements
The crystallite size of synthesized product was determined using
X- ray diffractometer (D8 Advanced BRUKER AXS GmbH,
Germany) operating at a voltage of 40 kV and a current of 30 mA
with CuKα radiation operating between 10 and 80° of 2θ angles
at scanning rate of 2° per min.
Antibacterial activity of silver nanoparticles
Antibacterial activity of test samples was determined against S.
aureus and E. coli as test micro-organisms, using agar well
diffusion assay method. Muller Hinton Agar (Hi-Media Lab. Pvt.
Ltd., India) was prepared, poured in sterile petri dishes and
allowed to solidify (Gopalakrishnan et al., 2016). A strain of S.
aureus and
E. coli was swabbed uniformly on individual plates using sterile
cotton swab. Holes were made in each plate with the help of
sterilized stainless steel cork borer. Each hole was filled with 14
μl of different concentrations (25, 50, 75 and 100 µg/ml) of test
samples using micropipette. All the Petri dish plates containing
micro-organism and test samples were incubated at 37°C for 24 h
at room temperature. The diameter of zone of inhibition was
measured in millimeter around each well using a ruler.
Antioxidant activity of silver nanoparticles
The ability of AgNPs to scavenge hydrogen peroxide (30% H 2O2,
Image chemicals) was determined by standard method (Thampi
and Shalini, 2015b). A solution of hydrogen peroxide (40 mM)
was prepared in phosphate buffer saline (pH=7.4). Different
concentrations of test samples (10, 20, 30, 40 and 50 µg/ml) were
prepared, from each concentration 4 ml of test sample was mixed
with 0.6 ml of previously prepared H 2O2 solution. Absorbance of
solution was measured at 230 nm after 10 min against blank
solution using UV-Vis spectrophotometer.
RESULTS AND DISCUSSION
Optimization of synthesis parameters
Heating time of extract
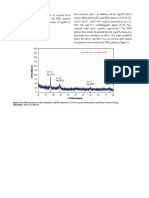
Maximum absorbance of the synthesized AgNPs
increased gradually with increase in heating time of
M. stenopetala leaves from 5 min up to 15 min at
60°C and began to decline until it reached 30 min
(Figure 2a).
0.35
0.30 15 min
0.25 20 min
Absorbance
0.20 10 min
0.15 25 min
0.10 30 min
0.05
5 min
0.00
300 400 500 600 700
Wavelength (nm)
a
a
0.35 1 mM
1.25 mM
0.30
0.25 0.75 mM
Absorbance
0.20
0.5 mM
0.15
0.10
0.25 mM
0.05
0.00
300 400 500 600 700
Wavelength (nm)
b
Figure 2. (a) Absorption spectra of AgNPs with varying extract heating time. (b)
Absorption spectra of AgNPs with varying silver nitrate concentration as prepared.
You might also like
- Spectrophotometry - PPT 18.07.08Document51 pagesSpectrophotometry - PPT 18.07.08MohammadS.Al-DarabsehNo ratings yet
- Group C Rice Chapter 1 3 UpdatedDocument39 pagesGroup C Rice Chapter 1 3 Updateddeevonc100% (1)
- Silver Nitrate ConcentrationDocument3 pagesSilver Nitrate ConcentrationAfrah MNo ratings yet
- 12 SupportDocument5 pages12 SupportBé Kem Xưởng MayNo ratings yet
- 7332 - Kfa2 Furosemid Kel 5aDocument3 pages7332 - Kfa2 Furosemid Kel 5aSekarayu KusumawNo ratings yet
- Andres - Adsorption Process Using Activated Carbon - Envi Chem Lab (Autorecovered)Document7 pagesAndres - Adsorption Process Using Activated Carbon - Envi Chem Lab (Autorecovered)Andres, Andrea Lyn M.No ratings yet
- Water Models: Work Group: Jorge Madias Sebastian Sylvestre Begnis Wadi Paul ChiapparoliDocument18 pagesWater Models: Work Group: Jorge Madias Sebastian Sylvestre Begnis Wadi Paul ChiapparoliJorge MadiasNo ratings yet
- Figure 2: UV-Vis Spectra Recorded As A Function of Reaction Time of Silver NanoparticlesDocument1 pageFigure 2: UV-Vis Spectra Recorded As A Function of Reaction Time of Silver NanoparticlesAfrah MNo ratings yet
- Nanoparticulas:: Sintesis, Caracterizacion y AplicacionesDocument62 pagesNanoparticulas:: Sintesis, Caracterizacion y AplicacionesruizfacundoNo ratings yet
- Fundamentals of Nuclear Magnetic Resonance (NMR) LoggingDocument25 pagesFundamentals of Nuclear Magnetic Resonance (NMR) LoggingMónica Alexandra Sedano DuarteNo ratings yet
- KFA Derivatif 5-6 ADocument11 pagesKFA Derivatif 5-6 AElvina Iskandar TanurahardjaNo ratings yet
- Foster Cole 101230199 Malaïka Zarrouki 2021-01-29Document7 pagesFoster Cole 101230199 Malaïka Zarrouki 2021-01-29Cole FosterNo ratings yet
- NMR Compatibility ModeDocument25 pagesNMR Compatibility ModeSergioNo ratings yet
- Experimental: Experimental Velocidades Calculadas L-B Calculado (S) (MM) V0 (MM/S) V0 (MM/S)Document6 pagesExperimental: Experimental Velocidades Calculadas L-B Calculado (S) (MM) V0 (MM/S) V0 (MM/S)jhon robert ortiz gilNo ratings yet
- Miller Indices (HKL) Are Necessary To Be Assigned For Each: XRD AnalysisDocument4 pagesMiller Indices (HKL) Are Necessary To Be Assigned For Each: XRD AnalysisAfrah MNo ratings yet
- Annual Session of The Indian Ceramic Society VisakhapatnamDocument12 pagesAnnual Session of The Indian Ceramic Society VisakhapatnamshajeshNo ratings yet
- Investigation 31 Determine The Concentration of CuSO4 Using SpectrometerDocument5 pagesInvestigation 31 Determine The Concentration of CuSO4 Using SpectrometerliaojingNo ratings yet
- Insulation Resistance Value Measurement (1.8.23)Document9 pagesInsulation Resistance Value Measurement (1.8.23)sandeep_bcplNo ratings yet
- A Multi-Modal Sweat Sensing Patch For Cross-Verification of Sweat Rate, Total Ionic Charge, and Na ConcentrationDocument14 pagesA Multi-Modal Sweat Sensing Patch For Cross-Verification of Sweat Rate, Total Ionic Charge, and Na ConcentrationThilini ApsaraNo ratings yet
- Costa, V. y Thickett, D. Metals Coupons Environmental Sensors. 2010Document34 pagesCosta, V. y Thickett, D. Metals Coupons Environmental Sensors. 2010Trinidad Pasíes Arqueología-ConservaciónNo ratings yet
- Insulation Resistance Value Measurement (1.1.17)Document9 pagesInsulation Resistance Value Measurement (1.1.17)ankitNo ratings yet
- CUSO4 PostlabDocument8 pagesCUSO4 PostlabRuwanthika Fernando100% (1)
- Insulation Resistance Value Measurement (1.1.19)Document9 pagesInsulation Resistance Value Measurement (1.1.19)Abdulwahed alsafanyNo ratings yet
- Enzyme Kinetic ReportDocument7 pagesEnzyme Kinetic ReportHalil Onur AltayNo ratings yet
- Antioksidan FRAP FIXDocument4 pagesAntioksidan FRAP FIXizzaaaaawNo ratings yet
- Lo Wry ProcedureDocument1 pageLo Wry ProcedurevijaygovindarajNo ratings yet
- Experiment No1 - Spectrophotometry ManualDocument2 pagesExperiment No1 - Spectrophotometry ManualJERI ANN CAPULONGNo ratings yet
- Insulation Resistance MeasurementDocument9 pagesInsulation Resistance Measurementishwer kushwahNo ratings yet
- Experiment 1: The Visible Spectra of Soft Drinks: A. Pre-Laboratory QuestionsDocument5 pagesExperiment 1: The Visible Spectra of Soft Drinks: A. Pre-Laboratory QuestionsMuhd Mirza HizamiNo ratings yet
- T-Interno DR Munive (Balance Metalurgico Lix Dinamica) 03-07-2019Document40 pagesT-Interno DR Munive (Balance Metalurgico Lix Dinamica) 03-07-2019GTNo ratings yet
- Experiment 1 CHM260 PDFDocument4 pagesExperiment 1 CHM260 PDFTifa IbrahimNo ratings yet
- Design of Shotcrete ConcreteDocument8 pagesDesign of Shotcrete ConcreteDevinder SokhiNo ratings yet
- Simulación Reacción EDocument67 pagesSimulación Reacción EMilagros Morales VergarayNo ratings yet
- Reactions of The Citric Acid Cycle Succinate Dehydrogenase, Fumarase and Malate DehydrogenaseDocument10 pagesReactions of The Citric Acid Cycle Succinate Dehydrogenase, Fumarase and Malate Dehydrogenase2023422047No ratings yet
- YesssDocument6 pagesYesssashNo ratings yet
- Nche211 Exp1Document8 pagesNche211 Exp1Mbali MdlaloseNo ratings yet
- Photometry: International Class Program Faculty of Medicine University of IndonesiaDocument29 pagesPhotometry: International Class Program Faculty of Medicine University of Indonesiawendyjemmy8gmailcomNo ratings yet
- Materi 5 Selectivitas BioleachingDocument8 pagesMateri 5 Selectivitas BioleachingemiaNo ratings yet
- Molecules: Sonochemical Degradation Kinetics of Methyl Violet in Aqueous SolutionsDocument5 pagesMolecules: Sonochemical Degradation Kinetics of Methyl Violet in Aqueous SolutionsThomas CharmNo ratings yet
- Simultaneous Determination of The Concentrations of Cobalt ( - ) and Nickel ( - ) by UV/Vis SpectrosDocument8 pagesSimultaneous Determination of The Concentrations of Cobalt ( - ) and Nickel ( - ) by UV/Vis SpectrosJassim123 SabtNo ratings yet
- ENV Laboratory Report Permanganat ValueDocument19 pagesENV Laboratory Report Permanganat ValueJulio DavidNo ratings yet
- IC Application Note S-368: (MG/L) (MG/L)Document2 pagesIC Application Note S-368: (MG/L) (MG/L)Mai NhựtNo ratings yet
- Color LabDocument9 pagesColor LabssweatNo ratings yet
- Bom BoqDocument4 pagesBom BoqRenzel EstebanNo ratings yet
- The Potential Application of Periwinkle Shell Ash As-Group4 (Autosaved)Document26 pagesThe Potential Application of Periwinkle Shell Ash As-Group4 (Autosaved)Abraham wisdomNo ratings yet
- Ultrasensitive Colorimetric Chromium Chemosensor Based On Dye Color Switching Under The CR (VI) - Stimulated Au NPs Catalytic Activity KeternganDocument26 pagesUltrasensitive Colorimetric Chromium Chemosensor Based On Dye Color Switching Under The CR (VI) - Stimulated Au NPs Catalytic Activity KeternganDesy PermatasariNo ratings yet
- Norris-Steel-Sucker-Rods - Pony-Rods - P001-V02-100908 - 5Document1 pageNorris-Steel-Sucker-Rods - Pony-Rods - P001-V02-100908 - 5Mohamed GhareebNo ratings yet
- Potato Osmolarity IA - ) ) 2Document11 pagesPotato Osmolarity IA - ) ) 2Kanika KumarNo ratings yet
- PembahaasanDocument10 pagesPembahaasanrerenNo ratings yet
- CaffeieneDocument8 pagesCaffeieneHawta AbdullaNo ratings yet
- Figures and TablesDocument3 pagesFigures and TablesAngeline RabuyoNo ratings yet
- Enzimas Actividad y KM EjemploDocument7 pagesEnzimas Actividad y KM Ejemploeymer alberto contreras mirandaNo ratings yet
- ENZIMAS ACTIVIDAD Y KM EJEMPLO Ultimo LabDocument7 pagesENZIMAS ACTIVIDAD Y KM EJEMPLO Ultimo Labeymer alberto contreras mirandaNo ratings yet
- Nor Iftiha Binti Abdul Aziz - 2019217292 - Ras1205eDocument26 pagesNor Iftiha Binti Abdul Aziz - 2019217292 - Ras1205eNor Iftiha AzizNo ratings yet
- CD: Cadmium: 1. Analytical ConditionsDocument2 pagesCD: Cadmium: 1. Analytical ConditionsVương AnhNo ratings yet
- Papverin HCL 5A-1Document3 pagesPapverin HCL 5A-1Elvina Iskandar TanurahardjaNo ratings yet
- Seminar 1 PDFDocument29 pagesSeminar 1 PDFDiana Andrade LarrañagaNo ratings yet
- Kondisi Ruang Awal Akhir Rata-Rata P (MMHG) Temperatur (°C) : Penentuan Panjang Gelombang MaksimumDocument83 pagesKondisi Ruang Awal Akhir Rata-Rata P (MMHG) Temperatur (°C) : Penentuan Panjang Gelombang MaksimumTaniadi SuriaNo ratings yet
- Staphylococcus Aureus (Gram-Posi-Tive) by Disc DiffusionDocument1 pageStaphylococcus Aureus (Gram-Posi-Tive) by Disc DiffusionAfrah MNo ratings yet
- 4green SynthesisDocument2 pages4green SynthesisAfrah MNo ratings yet
- Green Synthesis of Silver Nanoparticles Using Plant ExtractsDocument2 pagesGreen Synthesis of Silver Nanoparticles Using Plant ExtractsAfrah MNo ratings yet
- Results and Discussion: Silver Nanoparticles CharacterizationDocument1 pageResults and Discussion: Silver Nanoparticles CharacterizationAfrah MNo ratings yet
- 1department of Medical NanotechnologyDocument2 pages1department of Medical NanotechnologyAfrah MNo ratings yet
- 1department of Medical NanotechnologyDocument2 pages1department of Medical NanotechnologyAfrah MNo ratings yet
- Morphology Study: BiosynthesisDocument2 pagesMorphology Study: BiosynthesisAfrah MNo ratings yet
- Free Radical Scavenging Activity: 1. Results and DiscussionDocument2 pagesFree Radical Scavenging Activity: 1. Results and DiscussionAfrah MNo ratings yet
- FT-IR Chemical AnalysisDocument1 pageFT-IR Chemical AnalysisAfrah MNo ratings yet
- 4green SynthesisDocument2 pages4green SynthesisAfrah MNo ratings yet
- Abstract: Green: Curcuma LongaDocument1 pageAbstract: Green: Curcuma LongaAfrah MNo ratings yet
- Conclusion: C. Longa Compounds (Figure 7B)Document2 pagesConclusion: C. Longa Compounds (Figure 7B)Afrah MNo ratings yet
- 1department of Medical NanotechnologyDocument3 pages1department of Medical NanotechnologyAfrah MNo ratings yet
- 2 ChengDocument2 pages2 ChengAfrah MNo ratings yet
- C. Longa Compounds (Figure 7B) .: Physicochem Probl Miner Process. 2010 45:85-98Document2 pagesC. Longa Compounds (Figure 7B) .: Physicochem Probl Miner Process. 2010 45:85-98Afrah MNo ratings yet
- 5the Bioreduction of The AgDocument2 pages5the Bioreduction of The AgAfrah MNo ratings yet
- 3physical ApproachesDocument3 pages3physical ApproachesAfrah MNo ratings yet
- 5the Bioreduction of The AgDocument3 pages5the Bioreduction of The AgAfrah MNo ratings yet
- Synthesis of Ag/C. Longa Emulsion: Extraction PreparationDocument4 pagesSynthesis of Ag/C. Longa Emulsion: Extraction PreparationAfrah MNo ratings yet
- Figure 6: XRD Pattern of Silver Nanoparticles: Angle (2 )Document2 pagesFigure 6: XRD Pattern of Silver Nanoparticles: Angle (2 )Afrah MNo ratings yet
- 1department of Medical NanotechnologyDocument1 page1department of Medical NanotechnologyAfrah MNo ratings yet
- 5the Bioreduction of The AgDocument2 pages5the Bioreduction of The AgAfrah MNo ratings yet
- Figure 2: UV-Vis Spectra Recorded As A Function of Reaction Time of Silver NanoparticlesDocument1 pageFigure 2: UV-Vis Spectra Recorded As A Function of Reaction Time of Silver NanoparticlesAfrah MNo ratings yet
- Geissus Latifolia) and Its Biological Activity. Org Med Chem LettDocument1 pageGeissus Latifolia) and Its Biological Activity. Org Med Chem LettAfrah MNo ratings yet
- Caulerpa Racemosa Var. Cylin-Dracea. J Hazard MaterDocument3 pagesCaulerpa Racemosa Var. Cylin-Dracea. J Hazard MaterAfrah MNo ratings yet
- Polygonum Glabrum Willd. Leaf Extract Mediated Green Synthesis of Silver Nanoparticles and Their Assessment of Antimicrobial ActivityDocument1 pagePolygonum Glabrum Willd. Leaf Extract Mediated Green Synthesis of Silver Nanoparticles and Their Assessment of Antimicrobial ActivityAfrah MNo ratings yet
- Fiber Optic Test OTDRDocument37 pagesFiber Optic Test OTDRalbert_f42100% (3)
- Notess CBSE Class 12 Physics Notes - Wave OpticsDocument10 pagesNotess CBSE Class 12 Physics Notes - Wave OpticsPrashant KoreNo ratings yet
- HSSC I Send Up Key ChemistryDocument5 pagesHSSC I Send Up Key Chemistrymymegaacc111No ratings yet
- PH8201 Physics For Civil Engineering Notes PDFDocument92 pagesPH8201 Physics For Civil Engineering Notes PDFperiasamy_nano100% (1)
- General AwarenessDocument159 pagesGeneral AwarenessNiladri GoswamiNo ratings yet
- Optical Instrument: By: Sariyati, S.TDocument21 pagesOptical Instrument: By: Sariyati, S.TparamitaNo ratings yet
- How Rainbow Is FormedDocument15 pagesHow Rainbow Is FormedLaksmita Zhafira DisaNo ratings yet
- Mcqs For Mock TestDocument7 pagesMcqs For Mock TestHira Enayatullah Khan100% (1)
- OLD Human Eye and The Colourful WorldDocument11 pagesOLD Human Eye and The Colourful WorldRadhika GargNo ratings yet
- Atomic Emission Spectroscopy AsaDocument16 pagesAtomic Emission Spectroscopy AsaMark Cliffton Badlon100% (1)
- Patch AntennaDocument8 pagesPatch Antennajoseph vijayNo ratings yet
- VHF JRC JHS-800SDocument4 pagesVHF JRC JHS-800Sxuantruong0212No ratings yet
- Fundamentals of PhotographyDocument29 pagesFundamentals of PhotographyAndrew Richard Thompson100% (2)
- 23 Electronic TheodolitesDocument4 pages23 Electronic TheodolitesEmaNo ratings yet
- 3X-Rvv-65A-R9: 18-Port Sector Antenna, 6X694-960 and 12X1695-2690 MHZ, 65° HPBW, 9xretDocument5 pages3X-Rvv-65A-R9: 18-Port Sector Antenna, 6X694-960 and 12X1695-2690 MHZ, 65° HPBW, 9xretoaguilar83No ratings yet
- Technical Details: The World InnovationDocument4 pagesTechnical Details: The World InnovationTBS Máy Phát ĐiệnNo ratings yet
- Qau Course Outlines PDFDocument11 pagesQau Course Outlines PDFphooolNo ratings yet
- Vs Main Catalogue 2012 2013katalogDocument564 pagesVs Main Catalogue 2012 2013katalogDanka DukanacNo ratings yet
- BIS StandardsDocument3 pagesBIS Standardsjannumits100% (1)
- Pacer JA Vol 17 ReplacementDocument475 pagesPacer JA Vol 17 ReplacementJeff TaylorNo ratings yet
- Fresnel BiprismDocument12 pagesFresnel BiprismShubham JaswalNo ratings yet
- Stefan M Hoek Omicron PDFDocument7 pagesStefan M Hoek Omicron PDFmersiumNo ratings yet
- Light 9 QPDocument14 pagesLight 9 QPYeasir Arham MahirNo ratings yet
- X Final Grand Test KitDocument188 pagesX Final Grand Test KitSana DanishNo ratings yet
- Q-Smart850 Specs EN 052014Document2 pagesQ-Smart850 Specs EN 052014déborah_rosalesNo ratings yet
- XX. Introductory Physics, High SchoolDocument25 pagesXX. Introductory Physics, High SchoolramjdNo ratings yet
- Photoluminescence and Optical Absorption in Cds Se NanocrystalsDocument3 pagesPhotoluminescence and Optical Absorption in Cds Se NanocrystalsSIMETNo ratings yet
- Pix 4 Dmapper Camera Requirements For Precision AGDocument10 pagesPix 4 Dmapper Camera Requirements For Precision AGColleen CallahanNo ratings yet
- Post Task - Ingrid Gissela GonzálezDocument12 pagesPost Task - Ingrid Gissela GonzálezJawin Ricardo Perez AcevedoNo ratings yet