Professional Documents
Culture Documents
MTN-105 Tutorial 2
MTN-105 Tutorial 2
Uploaded by
guddu guptaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
MTN-105 Tutorial 2
MTN-105 Tutorial 2
Uploaded by
guddu guptaCopyright:
Available Formats
Indian Institute of Technology Roorkee
Department of Metallurgical and Materials Engineering
MT-105 Electrical and Electronic Materials
Assignment 2
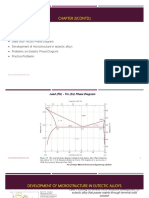
1. Consider 50% Pb- 50% Sn solder alloy:
f13_07_pg196
Sketch the microstructure of the alloy at various stages as it is cooled from the melt. What
i. is the importance of this alloy in electrical applications?
ii. At what temperature does the solid melt? What is the significance of this temperature?
iii. What is the temperature range over which the alloy is a mixture of melt and solid? What is
the microstructure of the solid?
iv. Consider the solder at room temperature following cooling from 1830C. Assume that the
rate of cooling from 1830C to room temperature is faster than the atomic diffusion rates
needed to change the compositions of the α and β phases in the solid. Assuming the alloy
is 1 kg. Calculate the masses of the following components in the solid.
a) The primary α (proeutectic), b) α in the whole alloy, c) α in the eutectic solid and
d) β in the alloy (where is the β phase?)
e) For Pb-40Sn, find the degree of freedom at,
i) liquid region, ii) liquidus, iii) two phase mushy region, iv) solidus and v) at room
temperature.
2. i. Consider a multicomponent alloy containing N elements. If w1, w2, w3,…..,wN are the
weight fractions of the components 1, 2, 3, …..,N in the alloy and M1, M2, M3,……..,MN
are the respective atomic masses of the elements, show that the atomic fraction of the i th
component is given by,
ni = wi ∕ Mi
------------------------------
w1 ∕M1+w2 ∕M2+------------+wN ∕MN
ii. Consider the semiconducting II-VI compound cadmium selenide, CdSe. Given the atomic
masses of Cd and Se, find the weight fraction of Cd and Se in the compound and grams of
Cd and Se needed to make 100 grams of CdSe.
You might also like
- Electro Chemistry FinalDocument51 pagesElectro Chemistry FinalManoj50% (2)
- Lectut-MTN-105-Doc-MT 201A-Tutorial - CH 1 (4 Files Merged)Document9 pagesLectut-MTN-105-Doc-MT 201A-Tutorial - CH 1 (4 Files Merged)Vikhyath KstNo ratings yet
- Chapter 1: Structure: Universiti Teknologi MaraDocument16 pagesChapter 1: Structure: Universiti Teknologi MaraRasyidi AhmadNo ratings yet
- Chemistry Olympiad 2015 Tutorial Worksheet (ACJC Prelims 2014/P3/2 (B), (C) )Document5 pagesChemistry Olympiad 2015 Tutorial Worksheet (ACJC Prelims 2014/P3/2 (B), (C) )Timothy HandokoNo ratings yet
- Platinum (II) : Nickel (II), Palladium (II)Document4 pagesPlatinum (II) : Nickel (II), Palladium (II)Alexandra BarthNo ratings yet
- Assignment PhaseDiaDocument5 pagesAssignment PhaseDiaAnshu Kumar GuptaNo ratings yet
- (CC - 1) 22Document2 pages(CC - 1) 22Pralay MaitiNo ratings yet
- Assignment 2 Pool PDFDocument2 pagesAssignment 2 Pool PDFSambhav JainNo ratings yet
- Assignment 2 Pool PDFDocument2 pagesAssignment 2 Pool PDFSambhav JainNo ratings yet
- Tutorial-2024 240305 131855Document4 pagesTutorial-2024 240305 131855hiepcon1216No ratings yet
- Chemistry QuestionsDocument2 pagesChemistry QuestionsSudeep NayakNo ratings yet
- Xu Et Al-2012-Angewandte ChemieDocument3 pagesXu Et Al-2012-Angewandte ChemieAasiya ShaikhNo ratings yet
- No 137 Alfirano 3 MMTADocument10 pagesNo 137 Alfirano 3 MMTAJason LangNo ratings yet
- Transmutation of Iodine: Results of The EFTTRA-T1 Irradiation TestDocument6 pagesTransmutation of Iodine: Results of The EFTTRA-T1 Irradiation TestWalid BadrNo ratings yet
- Chemistry PSPM 1 2008/2009Document3 pagesChemistry PSPM 1 2008/2009Viknish Arumugam50% (2)
- Srivastava 2020 J. Phys. Conf. Ser. 1531 012031Document7 pagesSrivastava 2020 J. Phys. Conf. Ser. 1531 012031gaurav senNo ratings yet
- Electronic Structure, Bonding, and Ground-State Properties of Alb - Type Transition-Metal DiboridesDocument12 pagesElectronic Structure, Bonding, and Ground-State Properties of Alb - Type Transition-Metal DiboridesimprincesssNo ratings yet
- Asl WorkbookDocument11 pagesAsl WorkbookSonal SinglaNo ratings yet
- Class Test (Atomic Structure) : Academic Session: 2019-2020Document5 pagesClass Test (Atomic Structure) : Academic Session: 2019-2020GM Ali KawsarNo ratings yet
- TransitionDocument39 pagesTransitioniratuzipacifique2No ratings yet
- Unit 3 ElectrochemisrtyDocument7 pagesUnit 3 ElectrochemisrtyRahgul M.S.50% (2)
- 2.2.1 Electron Structure QPDocument5 pages2.2.1 Electron Structure QPshraddhabakshi12No ratings yet
- Revision - Metals and Electrolysis - AdjustedDocument10 pagesRevision - Metals and Electrolysis - AdjustedLiew You tong (Unityss)No ratings yet
- Electro Chemistry Ol Chem 2023Document20 pagesElectro Chemistry Ol Chem 2023rameezshyamaleeNo ratings yet
- Unit 3 ElectrochemistryDocument7 pagesUnit 3 ElectrochemistrySapna 2704No ratings yet
- Prepared by Beehive Digital Concepts Cochin For Mahatma Gandhi University KottayamDocument21 pagesPrepared by Beehive Digital Concepts Cochin For Mahatma Gandhi University KottayamSuraj SawadekarNo ratings yet
- DMX 5204 - Materials Engineering Tma-01 - 2021Document4 pagesDMX 5204 - Materials Engineering Tma-01 - 2021cba.plutoNo ratings yet
- Energetic Zinc Ion ChemistryDocument3 pagesEnergetic Zinc Ion ChemistryMarco Miranda RodríguezNo ratings yet
- Ment 120 ExamDocument5 pagesMent 120 ExamnattydreadfathelahNo ratings yet
- Chemistry Quarter ExamDocument7 pagesChemistry Quarter ExamAhmed NasrNo ratings yet
- Electrochemistry Nerst EquationDocument7 pagesElectrochemistry Nerst EquationsumathiNo ratings yet
- Chemistry BRIDGE COURSE Chem 12thDocument16 pagesChemistry BRIDGE COURSE Chem 12thaasthashakya02032008No ratings yet
- Bond Length and Bond Length Distortion in Zn-Series of II-VI Group of SemiconductorsDocument5 pagesBond Length and Bond Length Distortion in Zn-Series of II-VI Group of SemiconductorsIJRASETPublicationsNo ratings yet
- Electrochemistry Uti Module 3Document8 pagesElectrochemistry Uti Module 3arunarajeshwaryNo ratings yet
- 5.3.1 Transition MetalsDocument37 pages5.3.1 Transition MetalsMalak AlqaidoomNo ratings yet
- Markscheme HL Paper3Document60 pagesMarkscheme HL Paper3dilemNo ratings yet
- Ijirt148302 PaperDocument4 pagesIjirt148302 PaperHemant ParmarNo ratings yet
- Class Test 1 (Atomic Structure) : Academic Session: 2018-2019Document6 pagesClass Test 1 (Atomic Structure) : Academic Session: 2018-2019GM Ali KawsarNo ratings yet
- MSE 260 Assignment 5Document2 pagesMSE 260 Assignment 5michael ananaNo ratings yet
- Week 2 1 IPE 2203-LecturesDocument38 pagesWeek 2 1 IPE 2203-LecturesMD Al-AminNo ratings yet
- Questions On Transition MetalsDocument3 pagesQuestions On Transition MetalscpliamNo ratings yet
- Ch1105 Materials Science For Chemical EngineersDocument2 pagesCh1105 Materials Science For Chemical EngineersWeimingTanNo ratings yet
- Naming Compounds, Molar Mass, The Mole Workshop Activity - Sept 12th - 13thDocument37 pagesNaming Compounds, Molar Mass, The Mole Workshop Activity - Sept 12th - 13thAlen buiNo ratings yet
- Answer Both The Question. Write Your Answers in The Spaces ProvidedDocument5 pagesAnswer Both The Question. Write Your Answers in The Spaces ProvidedThilagaNo ratings yet
- Estructura y Propiedades de Materiales: Capítulo 2. Estructura AtómicaDocument3 pagesEstructura y Propiedades de Materiales: Capítulo 2. Estructura AtómicaDavid StrokeysNo ratings yet
- Chapter 2 (Contd.)Document10 pagesChapter 2 (Contd.)mohansaiNo ratings yet
- Acids, Bases & Salts 1 QP PDFDocument9 pagesAcids, Bases & Salts 1 QP PDFSatria HalimNo ratings yet
- All Kerala Bhavans Chemistry 2010Document16 pagesAll Kerala Bhavans Chemistry 2010SajeevNo ratings yet
- Tutorial 1Document4 pagesTutorial 1karanNo ratings yet
- Act03 Exploring ElectrochemistryDocument13 pagesAct03 Exploring ElectrochemistryRenNo ratings yet
- MYP Year: 5 Subject: Chemistry Unit: Electrochemistry Global Context: Orientation in Space and Time Revision Sheet-2Document4 pagesMYP Year: 5 Subject: Chemistry Unit: Electrochemistry Global Context: Orientation in Space and Time Revision Sheet-2mayana agarwalNo ratings yet
- Peka - Chemistry Form 4 - Student's and Teacher's Manual - 01 - ElectrochemistryDocument4 pagesPeka - Chemistry Form 4 - Student's and Teacher's Manual - 01 - ElectrochemistryWong Wai Lun75% (8)
- Applied Surface ScienceDocument16 pagesApplied Surface ScienceNamNo ratings yet
- Paper Vii Model 2Document11 pagesPaper Vii Model 2Monica SrinivasanNo ratings yet
- Chapter 20: Electrochemistry: Homework QuestionsDocument2 pagesChapter 20: Electrochemistry: Homework Questionservaldi0% (1)
- Orbital Contribution To Magnetic MomentDocument25 pagesOrbital Contribution To Magnetic MomentSonali MahuleNo ratings yet
- Cy4202 20-21 EndDocument5 pagesCy4202 20-21 EndAakash BanerjeeNo ratings yet
- Properties of Semiconductor Alloys: Group-IV, III-V and II-VI SemiconductorsFrom EverandProperties of Semiconductor Alloys: Group-IV, III-V and II-VI SemiconductorsNo ratings yet
- Poster Contest Rule Book - FinalDocument8 pagesPoster Contest Rule Book - Finalguddu guptaNo ratings yet
- Form For Reporting Unfair MeansDocument2 pagesForm For Reporting Unfair Meansguddu guptaNo ratings yet
- 2021-22 Nutanix FAQsDocument16 pages2021-22 Nutanix FAQsguddu guptaNo ratings yet
- Power System Harmonics Research: A Survey: G. K. SinghDocument22 pagesPower System Harmonics Research: A Survey: G. K. Singhguddu guptaNo ratings yet
- Lecture 005Document30 pagesLecture 005guddu guptaNo ratings yet
- 8085 - Instruction - Set - Operation Codes - HexaDocument2 pages8085 - Instruction - Set - Operation Codes - Hexaguddu guptaNo ratings yet
- Tutorial Sheet - 7 2020Document1 pageTutorial Sheet - 7 2020guddu guptaNo ratings yet
- Tutorial Sheet No.8 1. Derive The Transfer Functions of The Following Filter Circuits: (A) + + + 1 A + 1 1+ RDocument2 pagesTutorial Sheet No.8 1. Derive The Transfer Functions of The Following Filter Circuits: (A) + + + 1 A + 1 1+ Rguddu guptaNo ratings yet
- Physics Handwritten Notes Part 1Document271 pagesPhysics Handwritten Notes Part 1guddu guptaNo ratings yet
- Tutorial Sheet - 1 2020Document1 pageTutorial Sheet - 1 2020guddu guptaNo ratings yet
- Tutorial Sheet - 9 2020Document2 pagesTutorial Sheet - 9 2020guddu guptaNo ratings yet
- N2019-20 Power T and D L17Document30 pagesN2019-20 Power T and D L17guddu guptaNo ratings yet
- Pre Board 1 (2020-21)Document7 pagesPre Board 1 (2020-21)guddu guptaNo ratings yet
- Tutorial Sheet - 2 2020Document2 pagesTutorial Sheet - 2 2020guddu guptaNo ratings yet
- IMG Winter Assignment A New HopeDocument1 pageIMG Winter Assignment A New Hopeguddu guptaNo ratings yet
- Tutorial Sheet - 3 2020Document2 pagesTutorial Sheet - 3 2020guddu guptaNo ratings yet
- Pre Board 1 (2020-21)Document7 pagesPre Board 1 (2020-21)guddu guptaNo ratings yet
- Lecture - 10 Lecture - 10: EEN-206: Power Transmission and Distribution EEN-206: Power Transmission and DistributionDocument17 pagesLecture - 10 Lecture - 10: EEN-206: Power Transmission and Distribution EEN-206: Power Transmission and Distributionguddu guptaNo ratings yet
- Lecture - 13 Lecture - 13: EEN-206: Power Transmission and Distribution EEN-206: Power Transmission and DistributionDocument20 pagesLecture - 13 Lecture - 13: EEN-206: Power Transmission and Distribution EEN-206: Power Transmission and Distributionguddu guptaNo ratings yet
- Fan Clubs Portal: Last Date of Submission: 20th March 2021Document3 pagesFan Clubs Portal: Last Date of Submission: 20th March 2021guddu guptaNo ratings yet
- Lecture - 11 Lecture - 11: EEN-206: Power Transmission and Distribution EEN-206: Power Transmission and DistributionDocument23 pagesLecture - 11 Lecture - 11: EEN-206: Power Transmission and Distribution EEN-206: Power Transmission and Distributionguddu guptaNo ratings yet
- Lecture - 12 Lecture - 12: EEN-206: Power Transmission and Distribution EEN-206: Power Transmission and DistributionDocument23 pagesLecture - 12 Lecture - 12: EEN-206: Power Transmission and Distribution EEN-206: Power Transmission and Distributionguddu guptaNo ratings yet