Professional Documents
Culture Documents
UV-Vis Application - Quantitative Analysis Using Second-Order Derivative Spectrum No A349
UV-Vis Application - Quantitative Analysis Using Second-Order Derivative Spectrum No A349
Uploaded by
Ramon Trinidad De la OOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
UV-Vis Application - Quantitative Analysis Using Second-Order Derivative Spectrum No A349
UV-Vis Application - Quantitative Analysis Using Second-Order Derivative Spectrum No A349
Uploaded by
Ramon Trinidad De la OCopyright:
Available Formats
LAAN-A-UV-E000
SHIMADZU APPLICATION NEWS
● SPECTROPHOTOMETRIC ANALYSIS
No. A349
Quantitative Analysis using Second-order Derivative Spectrum
Derivative spectral analysis is often used for the peak concentration levels have a linear relationship,
identification due to its advantages in differentiating quantitation is easily performed even when
closely adjacent absorption peaks, identifying weak background absorption exists.
absorption peaks obscured by sharp peaks, and This Application News presents an example of
identifying wavelength at maximum absorbance for measurement using derivative spectrum with a UV-Vis
broad spectra. Because the derivative values and spectrophotometer.
■ Derivative Spectrum
Derivative spectral analysis refers to taking the (4) There is a linear relationship between the derivative
derivative with respect to absorbance wavelengths. values and the concentration levels, so quantitative
Depending on the application, 1st, 2nd, 3rd or 4th- analysis is easily performed even when
order derivative is conducted. In the past, derivative background absorption exists.
spectra were obtained using electronic methods
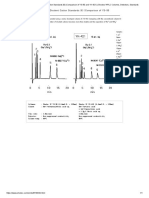
(memory shift or analog differentiation) or optical Fig. 1 shows a comparison of the original and second-
methods (adjacent wavelength method or wavelength order derivative spectra for two absorption peaks
modulation method). However, currently, derivative overlapping at extremely close wavelengths. (a) is the
spectra can be easily obtained by processing data on original spectrum, where absorption peak A overlaps
a Personal Computer. with B to form the spectrum A + B. Here, it is
impossible to identify absorption peak B. (b) is the
The benefits of derivative spectral analysis include the second-order derivative spectrum. Sometimes the
following. second-order derivative spectrum is flipped vertically
(1) Absorption bands can be identified even when two to make it easier to see. In spectrum (b), absorption
or more absorption peaks overlap in an extremely peak B, which was obscured by peak A, can be clearly
small wavelength range. identified. The peak-valley absorbance difference in
(2) Weak absorption bands can be identified even the derivative spectrum relates linearly to
when obscured by a sharp absorbance peak. concentration levels, making quantitation possible.
(3) A particular wavelength at maximum absorbance
can be identified from a broad absorption spectrum.
Fig.1 Original and 2nd-order Derivative Spectra
No.A349
■ Quantitation of Paraquat and Diquat in Blood Plasma
If herbicides paraquat or diquat is ingested by mistake, results were obtained for paraquat. Although not
they are absorbed by the body and can be detected in shown below, good results were also obtained for
the blood plasma and urine. When measuring diquat. The measurement involved obtaining an
paraquat and diquat, their absorption peaks overlap in absorption spectrum for the test sample and
a small wavelength range. However, these two peaks processing the data to calculate the second-order
can be differentiated by using the second-order derivative spectrum. Based on the obtained second-
derivative spectrum.1) A measurement of paraquat order derivative spectrum, paraquat was quantified
and diquat contained in blood plasma is shown below. using the height between inflection points near 396nm
and 403nm. Similarly, diquat was quantitated using the
Fig. 2 shows the analysis flow chart. Fig. 3 and 4 show height between 454 nm and 464 nm.
the measurement results. Excellent quantitation
Calibration Curve
0.3500
1.0mL blood plasma
0.3000
Add 1.0mL of 100mg/mL sulfosalicylic acid solution
0.2000
Centrifugal separation (for 5min. at 3000rpm)
Abs.
1.0mL supernatant 0.1000
Add 0.25mL of 0.05 M Na2S2O4-5N NaOH solution
0.0000
0.0000 5.0000 10.0000 12.0000
Concentration (µg/mL)
UV-Vis Spectrophotometer Calibration curve function : Conc.=36.64414×Abs.-0.04150
Correlation coefficient : 0.99915
Fig.2 Flow Chart of Analytical Method Fig.3 Determination of Paraquat in Plasma
for Paraquat and Diquat in Plasma
Fig.4 2nd Derivative Spectrum of Paraquat and Diquat in Plasma
Reference:
1) Chiaki Fukuie, Kiyoshi Ameno, Setsuko Ameno, et al.:
Simultaneous Analysis of Paraquat and Diquat Contained in Blood Serum and Urine using Second
Derivative Spectrophotometry. Igaku No Ayumi, No. 7, Vol. 143, 1998, 657 - 658.
SHIMADZU CORPORATION. International Marketing Division
3. Kanda-Nishikicho 1-chome, Chiyoda-ku, Tokyo 101-8448, Japan Phone: 81(3)3219-5641 Fax. 81(3)3219-5710
Cable Add.:SHIMADZU TOKYO
Printed in Japan 3100-06401-10A-IK
You might also like
- Analy Food Colour Uv-VisDocument13 pagesAnaly Food Colour Uv-VisNoor Zarif100% (1)
- Chm580 Experiment 2Document8 pagesChm580 Experiment 2ohhi100% (1)
- Lambda Max KMnO4Document9 pagesLambda Max KMnO4Rayeha90% (10)
- EXP2 UV-Visible Deteermination of An Unknown Concentration of Kmno4 Solution PDFDocument5 pagesEXP2 UV-Visible Deteermination of An Unknown Concentration of Kmno4 Solution PDFRaidah AfiqahNo ratings yet
- Report UV-VIS YayaDocument13 pagesReport UV-VIS YayaInfra Nadya67% (3)
- Andres Anal Chem CalibrationDocument5 pagesAndres Anal Chem CalibrationAndres, Andrea Lyn M.No ratings yet
- CHM260 Lab Report SubmissionDocument38 pagesCHM260 Lab Report Submissionasta yuno100% (1)
- 2017 Nano Letts Continuous In-Flight Synthesis Supplementary SIDocument14 pages2017 Nano Letts Continuous In-Flight Synthesis Supplementary SIpdmagNo ratings yet
- Chemy 310 Experiment 4Document8 pagesChemy 310 Experiment 4Faisal MumtazNo ratings yet
- AIU-section 2Document11 pagesAIU-section 2pansiehaNo ratings yet
- UV-Vis LabDocument5 pagesUV-Vis LabCesar GutierrezNo ratings yet
- Uv-V ApplicationDocument42 pagesUv-V ApplicationTare ye TesfuNo ratings yet
- Exp 2 CHM 260Document8 pagesExp 2 CHM 2602023637002No ratings yet
- Lecture 18Document35 pagesLecture 18Hadia SAULATNo ratings yet
- Spectrophotometer: DR Fadhl Alakwaa 2013-2014 Third Year Biomedical Engineering DepartmentDocument52 pagesSpectrophotometer: DR Fadhl Alakwaa 2013-2014 Third Year Biomedical Engineering Departmentabdulla ateeqNo ratings yet
- LE18-PI-FR-FA20Document35 pagesLE18-PI-FR-FA20junaidkhan565613No ratings yet
- Spektrofotometri UV-VIS AriDocument44 pagesSpektrofotometri UV-VIS AriMiqdad Abdul MuizNo ratings yet
- The Power of Slope SpectrosDocument2 pagesThe Power of Slope SpectrosJohn SiricoNo ratings yet
- DATE: May 28, 2014. Ultra-Violet Visible Spectroscopy Practical Report: Aim: To Apply The Beer-Lambert Relationship To An Aqueous Solution ContainingDocument14 pagesDATE: May 28, 2014. Ultra-Violet Visible Spectroscopy Practical Report: Aim: To Apply The Beer-Lambert Relationship To An Aqueous Solution ContainingNeelam MalikNo ratings yet
- Use of The Spectrophotometer-2Document7 pagesUse of The Spectrophotometer-2Tori Andika BukitNo ratings yet
- Trabalho 1 (Versão Final)Document11 pagesTrabalho 1 (Versão Final)ritacostajoanarodrigues754No ratings yet
- Lab Week 2 - Spectrophotometry: Basic Laws of Light Absorption. For A Uniform Absorbing Medium (Solution: Solvent andDocument7 pagesLab Week 2 - Spectrophotometry: Basic Laws of Light Absorption. For A Uniform Absorbing Medium (Solution: Solvent andfrhslmnNo ratings yet
- Microcontroller Based Visible Light Spectrometer: Keshav Dhikale, Abhijeet Shimpi, Rushikesh Raut D.A.GhogareDocument4 pagesMicrocontroller Based Visible Light Spectrometer: Keshav Dhikale, Abhijeet Shimpi, Rushikesh Raut D.A.GhogareAnthropology BooksNo ratings yet
- Spectrophotometry: An Analytical ToolDocument25 pagesSpectrophotometry: An Analytical ToolDian Ayu ChotimahNo ratings yet
- Bristol Myers Squibb CTechnologies Bioprocess International PaperDocument8 pagesBristol Myers Squibb CTechnologies Bioprocess International PaperLeS BrunNo ratings yet
- Andres - Adsorption Process Using Activated Carbon - Envi Chem Lab (Autorecovered)Document7 pagesAndres - Adsorption Process Using Activated Carbon - Envi Chem Lab (Autorecovered)Andres, Andrea Lyn M.No ratings yet
- Aspirin Lab 012714Document22 pagesAspirin Lab 012714David SepulvedaNo ratings yet
- CHM260 Lab Report SubmissionDocument37 pagesCHM260 Lab Report Submissionasta yuno100% (1)
- 01.ex Name Spectrophotometric Determination of Iron.Document4 pages01.ex Name Spectrophotometric Determination of Iron.Md Sohel RanaNo ratings yet
- Suman Sir Lab ReportDocument7 pagesSuman Sir Lab ReportRonak YaduvanshiNo ratings yet
- 7 Spectrophotometry Loops - ManualDocument5 pages7 Spectrophotometry Loops - ManualJerry WangNo ratings yet
- Braken Hoff 1986Document10 pagesBraken Hoff 1986Annisa HayatiNo ratings yet
- Espectrofotômetro UV3600 ShimadzuDocument22 pagesEspectrofotômetro UV3600 ShimadzuVilmar Bertotti JuniorNo ratings yet
- Spectrophotometry in The Visible and UltravioletDocument4 pagesSpectrophotometry in The Visible and Ultravioletrnd labNo ratings yet
- Analytical Chemistry - Exp 1Document8 pagesAnalytical Chemistry - Exp 1Zharifah Bari'ah Basa'ah100% (1)
- Chem Exp-2Document6 pagesChem Exp-2aanika roshniNo ratings yet
- TechniquesDocument32 pagesTechniquesRoro RageyNo ratings yet
- DNA QuantificationDocument3 pagesDNA QuantificationKanishk AgrawalNo ratings yet
- EpmaDocument4 pagesEpmaSANKET SINGHNo ratings yet
- 01216129Document2 pages01216129kirubhaaNo ratings yet
- 2.2.24. Absorption Spectrophotometry, InfraredDocument4 pages2.2.24. Absorption Spectrophotometry, InfraredBelen RodriguezNo ratings yet
- UltrosoundDocument3 pagesUltrosounddevanarayan2000No ratings yet
- PNP Spec Lab RPTDocument14 pagesPNP Spec Lab RPTAlisa Dearth100% (1)
- Anintroduction Tomulti-Component Peakpurity: Marcv. Gorenstein, Ph.D.And Jeanneb. Li, PH.DDocument4 pagesAnintroduction Tomulti-Component Peakpurity: Marcv. Gorenstein, Ph.D.And Jeanneb. Li, PH.DEsteban FernandezmNo ratings yet
- Uvvis Absorption SpectrosDocument16 pagesUvvis Absorption SpectrosMugurNo ratings yet
- 11 Chapter 11Document8 pages11 Chapter 11Smita SahooNo ratings yet
- SpectrophotometerDocument12 pagesSpectrophotometerPatricia JaneNo ratings yet
- Determination of Copper Concentration Using UV Vis SpectrophotometeryDocument6 pagesDetermination of Copper Concentration Using UV Vis SpectrophotometeryLoeyNo ratings yet
- Chapter 6 - UV - Vis - PPT Compatibility ModeDocument15 pagesChapter 6 - UV - Vis - PPT Compatibility ModeNavin RajNo ratings yet
- Spectrophotometric Determination of Iron: Experiment No: 02 Experiment NameDocument7 pagesSpectrophotometric Determination of Iron: Experiment No: 02 Experiment NameRafid JawadNo ratings yet
- Laser Flash Photolysis Spectrometer: Pride in PrecisionDocument20 pagesLaser Flash Photolysis Spectrometer: Pride in PrecisionsukinsynNo ratings yet
- Plasma Diagnostics: Course Notes: Prof. F.F. Chen P A7: P DDocument23 pagesPlasma Diagnostics: Course Notes: Prof. F.F. Chen P A7: P Ddmp130No ratings yet
- Absorption Edge and Band GapDocument0 pagesAbsorption Edge and Band GapBasharat AhmadNo ratings yet
- Cuvette Guide 09-13Document27 pagesCuvette Guide 09-13Ramon Trinidad De la ONo ratings yet
- UV VIS Phenol LabDocument6 pagesUV VIS Phenol LabJoão Paulo FioriNo ratings yet
- Spline and Spline Wavelet Methods with Applications to Signal and Image Processing: Volume III: Selected TopicsFrom EverandSpline and Spline Wavelet Methods with Applications to Signal and Image Processing: Volume III: Selected TopicsNo ratings yet
- Liquid Chromatography - Mass Spectrometry: An IntroductionFrom EverandLiquid Chromatography - Mass Spectrometry: An IntroductionNo ratings yet
- Standard and Super-Resolution Bioimaging Data Analysis: A PrimerFrom EverandStandard and Super-Resolution Bioimaging Data Analysis: A PrimerNo ratings yet
- Nexera Series SpecificationsDocument8 pagesNexera Series SpecificationsRamon Trinidad De la ONo ratings yet
- AXIMA AssuranceDocument4 pagesAXIMA AssuranceRamon Trinidad De la ONo ratings yet
- Monovalent and Divalent Cation Standards (6) (Comparison of YS-50 and YK-421) - Shodex - HPLC Columns, Detectors, StandardsDocument1 pageMonovalent and Divalent Cation Standards (6) (Comparison of YS-50 and YK-421) - Shodex - HPLC Columns, Detectors, StandardsRamon Trinidad De la ONo ratings yet
- AXIMA PerformanceDocument6 pagesAXIMA PerformanceRamon Trinidad De la ONo ratings yet
- TOC Application - TOC-L - TOC - Determination According To USP 643Document2 pagesTOC Application - TOC-L - TOC - Determination According To USP 643Ramon Trinidad De la ONo ratings yet
- TOC Application - TOC-L - TOC - Control Samples and Control ChartsDocument2 pagesTOC Application - TOC-L - TOC - Control Samples and Control ChartsRamon Trinidad De la ONo ratings yet
- TOC Application - TOC-L - USP-Specified TOC System Suitability Test by TOC-LCPHDocument2 pagesTOC Application - TOC-L - USP-Specified TOC System Suitability Test by TOC-LCPHRamon Trinidad De la ONo ratings yet
- TOC Application - TOC-L - Characterization of Algae by TOCDocument2 pagesTOC Application - TOC-L - Characterization of Algae by TOCRamon Trinidad De la ONo ratings yet
- Analytical Chemistry 602037-Chapter 8 PDFDocument30 pagesAnalytical Chemistry 602037-Chapter 8 PDFNguyễn Trần PhúNo ratings yet
- Studi Kajian Pembuatan Asam Oksalat Dengan Variasi Kecepatan Pengadukan Dan Lama Waktu Pengadukan Dari Bahan Dasar Ampas TebuDocument7 pagesStudi Kajian Pembuatan Asam Oksalat Dengan Variasi Kecepatan Pengadukan Dan Lama Waktu Pengadukan Dari Bahan Dasar Ampas TebuMuhammad RizkyNo ratings yet
- Flash (Equilibrium) DistillationDocument1 pageFlash (Equilibrium) DistillationTimothy JonesNo ratings yet
- PH Level Research PaperDocument4 pagesPH Level Research Paperc9r5wdf5100% (1)
- Analysis of Cyclic Voltammetry DataDocument7 pagesAnalysis of Cyclic Voltammetry DataSudip SinhaNo ratings yet
- 1: Precipitation Reactions 1.1. DefinitionDocument73 pages1: Precipitation Reactions 1.1. Definitionsuhanizah suhanizahNo ratings yet
- Fundamental Calculations To Convert Intensities Into Concentrations in Optical Emission Spectrochemical AnalysisDocument14 pagesFundamental Calculations To Convert Intensities Into Concentrations in Optical Emission Spectrochemical AnalysisPYDNo ratings yet
- Anachem NeutralizationDocument2 pagesAnachem NeutralizationChristian Ghail MacapagalNo ratings yet
- Analytic Homeworh Lec 5Document3 pagesAnalytic Homeworh Lec 5Nam NguyenNo ratings yet
- Paras A ScribdDocument2 pagesParas A ScribdIra Khryzel GalloNo ratings yet
- 01 Introduction To Instrumental AnalysisDocument15 pages01 Introduction To Instrumental AnalysisMarcoNo ratings yet
- Norma A751Document5 pagesNorma A751Dionisio Hidalgo SanchezNo ratings yet
- ANCH111Document11 pagesANCH111Krizhel Ann Marie DS ZaideNo ratings yet
- Lecture Note Prepared by Dr. Supriyo Saha: Basic Theory and Principle Behind HPLCDocument3 pagesLecture Note Prepared by Dr. Supriyo Saha: Basic Theory and Principle Behind HPLCsupriyoNo ratings yet
- Determination of CalciumDocument7 pagesDetermination of CalciumAhmed IsmailNo ratings yet
- Heparins, Low-Molecular-MassDocument3 pagesHeparins, Low-Molecular-MassArtem KulikovNo ratings yet
- HPLC Calibration Procedure - Pharmaceutical GuidelinesDocument12 pagesHPLC Calibration Procedure - Pharmaceutical Guidelinessahuchem100% (1)
- V Naoh (ML) PH: Otentiometric ItrationDocument9 pagesV Naoh (ML) PH: Otentiometric ItrationradyjrNo ratings yet
- Mass Spectrometry Based Metabolomics A Guide For Annotation, Quantification and Best ReportingDocument10 pagesMass Spectrometry Based Metabolomics A Guide For Annotation, Quantification and Best ReportingsridharNo ratings yet
- NimesulideDocument2 pagesNimesulideThambik DuraiNo ratings yet
- Neutralization IWMDocument22 pagesNeutralization IWMusmansherdin100% (2)
- Anitta Denny IMS12019 Purity and Homogeneity CheckDocument3 pagesAnitta Denny IMS12019 Purity and Homogeneity CheckAnittaDennyNo ratings yet
- HPLC by EktaDocument42 pagesHPLC by EktaEkta SharmaNo ratings yet
- Crystal Defects4 SDocument5 pagesCrystal Defects4 SS.M. Abdul Mannan MahdiNo ratings yet
- Titration of KOH and CH3COOHDocument2 pagesTitration of KOH and CH3COOHEnde WishNo ratings yet
- Demonstration Indicators : Using UniversalDocument3 pagesDemonstration Indicators : Using UniversalJhonattan BaezNo ratings yet
- Quantification of Nucleic Acids: Spectrophotometric AnalysisDocument2 pagesQuantification of Nucleic Acids: Spectrophotometric Analysishely shahNo ratings yet
- Acid TrimesicDocument4 pagesAcid TrimesicGustavo Nazareno MonteiroNo ratings yet
- 1.1.3 AcidsDocument8 pages1.1.3 AcidsKel1209No ratings yet
- Static Simulation Program of Copper Solvent Extraction Configurations Using Microsoft Excel SolverDocument76 pagesStatic Simulation Program of Copper Solvent Extraction Configurations Using Microsoft Excel SolverMichel MNongNo ratings yet