Professional Documents
Culture Documents
Chapter 8 - Real Gases
Chapter 8 - Real Gases
Uploaded by
carleston thurgoodOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter 8 - Real Gases
Chapter 8 - Real Gases
Uploaded by
carleston thurgoodCopyright:
Available Formats
Chapter 8
Real Gases
Chemical Engineering Thermodynamics - 2019
Equations of State
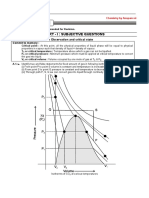
The Ideal gas law is inadequate to explain the behaviour of real gases.
At low molar volumes or high pressures, the molecules come very close to each other and
molecular interactions cannot be neglected.
Many equations of state have been proposed to explain the actual behaviour of gases; These
include but are not limited to:
Van Der Waals equation
Redlich-Kwong Equation
Virial Equation
Chemical Engineering Thermodynamics - 2019
Van Der Waals Equation
Van der Waals proposed the following equation to explain the P-V-T behaviour of real
gases:
a
P V b RT
2
V
Where a and b are van der Waals constants which can be evaluated by:
27 R 2Tc2 RTc
a ; b
64 Pc 8Pc
Where Tc is the critical temperature and Pc is the critical pressure.
R is the gas constant.
Chemical Engineering Thermodynamics - 2019
Example 8.1
One kilo mole CO2 occupies a volume of 0.381 m3 at 313 K. Calculate the pressure
using:
(a) Ideal gas equation;
(b) Van der Waals equation
Take the van der Waals constants to be a = 0.365 Nm4/mol2 and b = 4.28x10-5 m3/mol.
Example 8.2
Calculate the volume occupied by one mole of oxygen at 300 K and 100 bar using:
(a) Ideal gas law;
(b) The van der Waals equation
Take a = 0.1378 Nm4/mol2 and b = 3.18x10-5 m3/mol.
Chemical Engineering Thermodynamics - 2019
The Virial Equation
The ratio PV/RT is called the compressibility factor (Z).
It is the ratio of the volume of a real gas (V) to the volume if the gas behaved ideally at
stated temperature and pressure (RT/P).
Virial equations express the compressibility factor of a gas or vapour as a power series
expansion in P or 1/V.
PV
Z 1 BP C P 2 DP3
RT
PV B C D
Z 1
RT V V 2 V 3
Chemical Engineering Thermodynamics - 2019
The above equations are known as virial equations and constants B, C, D and B', C',
D' are known as virial coefficients.
B and B' are called second virial coefficients; C and C' are called third virial
coefficients, and so on.
For a given gas, these coefficients are functions of temperature only.
The two sets of coefficients are related as:
B C B2
B C
RT RT 2
Chemical Engineering Thermodynamics - 2019
𝑃𝑉 𝐵𝑃
𝑍= =1+
𝑅𝑇 𝑅𝑇
This equation expresses a direct proportionality between Z and P, and is often applied
to vapours at subcritical temperatures up to their saturation pressures
𝑃𝑉 𝐵
𝑍= =1+
𝑅𝑇 𝑉
The equation below allows you to solve for volume using the virial equation
𝑃𝑉 𝐵 𝐶
𝑍= =1+ + 2
𝑅𝑇 𝑉 𝑉
Chemical Engineering Thermodynamics - 2019
Example 8.3
Reported values for the virial coefficients of isopropanol vapour at 200 deg cel are:
B = -388 cm3/mol
C = -26000 cm6/mol2
Calculate V and Z for isopropanol vapour at 200 deg cel and 10 bar by
(a) The ideal gas law
𝑃𝑉 𝐵𝑃
(b) 𝑍 = =1+
𝑅𝑇 𝑅𝑇
𝑃𝑉 𝐵 𝐶
(c) 𝑍 = =1+ + 2
𝑅𝑇 𝑉 𝑉
Chemical Engineering Thermodynamics - 2019
Redlich-Kwong Equation
A two-parameter equation of state widely used in engineering calculations.
RT a
P
V b T 0.5V V b
The constants a and b are evaluated as:
0.4278R 2Tc2.5 0.0867 RTc
a ; b
Pc Pc
Chemical Engineering Thermodynamics - 2019
Example 8.4
Calculate the compressibility factor and molar volume for methanol vapour at 500 K
and 10 bar by using the following equations.
(a) Truncated form of virial equation
(b) Redlich-Kwong equation
Experimental values of virial coefficients are, B = -2.19x10-4 m3/mol;
C = -1.73x10-8 m6/mol2. The critical temperature and pressure of methanol are 512.6 K
and 81 bar.
Chemical Engineering Thermodynamics - 2019
You might also like
- APT300S Tutorial - RefrigerationDocument2 pagesAPT300S Tutorial - Refrigerationcarleston thurgood0% (1)
- Problem Set 3 Due: 10/1/2021 Problem 1:: P RT V B A V A V B VDocument3 pagesProblem Set 3 Due: 10/1/2021 Problem 1:: P RT V B A V A V B VSaúl Guerra RazoNo ratings yet
- GPG347 Installation and Commissioning of Refrigeration Systems PDFDocument20 pagesGPG347 Installation and Commissioning of Refrigeration Systems PDFIppiNo ratings yet
- Module 4. Lesson 3 Gaseous FuelsDocument6 pagesModule 4. Lesson 3 Gaseous FuelsVJ CarbonellNo ratings yet
- Lesson 6-Real GasesDocument11 pagesLesson 6-Real GasesOrley G FadriquelNo ratings yet
- Thermodynamics 1: Volumetric Properties of Pure FluidsDocument24 pagesThermodynamics 1: Volumetric Properties of Pure FluidsHabib Faisal Yahya100% (1)
- Chemical Engineering 301 Lecture Notes: (Revised 9/04)Document9 pagesChemical Engineering 301 Lecture Notes: (Revised 9/04)shiv kr dubeyNo ratings yet
- 728Document2 pages728tanisha guptaNo ratings yet
- Equations of StateDocument33 pagesEquations of StateDevika BharathanNo ratings yet
- HW Assignment 4 S2011Document1 pageHW Assignment 4 S2011Joanne ChangNo ratings yet
- ps1 QuestionsDocument2 pagesps1 QuestionsdjambulazizNo ratings yet
- Tutorials For ChemicalthermodynamicsDocument20 pagesTutorials For Chemicalthermodynamicselisee tsokezoNo ratings yet
- Other Thermodynamic Relations: Real Gas Behavior & Equations of StateDocument10 pagesOther Thermodynamic Relations: Real Gas Behavior & Equations of StateJamesBanglaganNo ratings yet
- Thermo VOLUMETRIC PROPERTIESDocument41 pagesThermo VOLUMETRIC PROPERTIESKhairunnisarumiNo ratings yet
- CH 353 Problem Set 1Document3 pagesCH 353 Problem Set 1jon jonNo ratings yet
- Main ISM Ch07Document14 pagesMain ISM Ch07Shoja Sammy RahimianNo ratings yet
- Topic 16 - Compressibility Factor and Compressibility ChartsDocument14 pagesTopic 16 - Compressibility Factor and Compressibility ChartswevansNo ratings yet
- Termodinamica GeneralizadaDocument28 pagesTermodinamica GeneralizadaSebastian BonanniNo ratings yet
- Properties of Gases & Gas MixturesDocument11 pagesProperties of Gases & Gas MixtureshemantNo ratings yet
- Thesis 71Document214 pagesThesis 71Pérez Castillo GabrielaNo ratings yet
- Homework TermodinamicaDocument4 pagesHomework Termodinamicacarlosprez212No ratings yet
- Unit8 1 TNSDocument11 pagesUnit8 1 TNSSylvesterMcLaneNo ratings yet
- Gas IdealDocument10 pagesGas IdealAlexis CGNo ratings yet
- Volumetric Properties of Pure FluidsDocument38 pagesVolumetric Properties of Pure Fluidsrezarizqi09No ratings yet
- Cet IiDocument2 pagesCet IiVivek ParmarNo ratings yet
- Gaseous State (Real Gas) ExerciseDocument13 pagesGaseous State (Real Gas) ExercisemikcNo ratings yet
- Week4 - Chapter3-Ideal Gas ModelDocument28 pagesWeek4 - Chapter3-Ideal Gas ModelNadiaNo ratings yet
- Preparation of Psychrometric Charts For Water Vapour in Martian AtmosphereDocument12 pagesPreparation of Psychrometric Charts For Water Vapour in Martian AtmosphereRiska MirandaNo ratings yet
- r05310803 Chemical Engineering Thermodynamics IIDocument8 pagesr05310803 Chemical Engineering Thermodynamics IISrinivasa Rao GNo ratings yet
- Real GasesDocument13 pagesReal GasesShubh GuptaNo ratings yet
- Ideal and Real GasDocument7 pagesIdeal and Real GasAnastasia Iin LNo ratings yet
- Unit 2Document13 pagesUnit 2pedro_erguetaNo ratings yet
- Meyer 2015Document41 pagesMeyer 2015vghs072No ratings yet
- LECTURE 2: Supplementary Notes Example: Comparison of Van Der Waals Forces For Pure SpeciesDocument14 pagesLECTURE 2: Supplementary Notes Example: Comparison of Van Der Waals Forces For Pure SpeciesAnıl KahvecioğluNo ratings yet
- Week/day 3: Properties of Pure SubstancesDocument57 pagesWeek/day 3: Properties of Pure Substancesronni bermudezNo ratings yet
- Article (Tord Martinsen's Conflicted Copy 2015-06-09)Document10 pagesArticle (Tord Martinsen's Conflicted Copy 2015-06-09)Tord MartinsenNo ratings yet
- Chm3410hwk01-Soln 249211458Document7 pagesChm3410hwk01-Soln 249211458Jerika ArceoNo ratings yet
- Chapter 4 Single-Phase SystemDocument38 pagesChapter 4 Single-Phase SystemRenu SekaranNo ratings yet
- Realistic Equations of StateDocument94 pagesRealistic Equations of Stateaman09752No ratings yet
- A Comparison of Equations of StateDocument8 pagesA Comparison of Equations of StateDarren Sean HoNo ratings yet
- Generalized Correlation For Liquids Lect-7Document9 pagesGeneralized Correlation For Liquids Lect-7Junaid100% (1)
- Gas Laws HandoutDocument10 pagesGas Laws HandoutVenu ReddyNo ratings yet
- Compressor Handbook 2Document7 pagesCompressor Handbook 2mssj87No ratings yet
- Chemical Engineering ThermodynamicsDocument5 pagesChemical Engineering ThermodynamicsP P DNo ratings yet
- 11 GasesDocument17 pages11 Gasespuja ritongaNo ratings yet
- IPUE 208 (Jan-April) : Introduction To Process and Utilities EngineeringDocument29 pagesIPUE 208 (Jan-April) : Introduction To Process and Utilities EngineeringRandy SooknananNo ratings yet
- Virial Equation of StateDocument9 pagesVirial Equation of StateSaba ArifNo ratings yet
- B.E. Poling, J.M. Prausnitz, J.P. O'Connell, 'The Properties of Gases and Liquids' 5ht Ed. Property Data Bank. Appendix ADocument61 pagesB.E. Poling, J.M. Prausnitz, J.P. O'Connell, 'The Properties of Gases and Liquids' 5ht Ed. Property Data Bank. Appendix AIsaac A Vazquez MedranoNo ratings yet
- ThermoII Exercise 1Document53 pagesThermoII Exercise 1Batuhan KalyoncuNo ratings yet
- Physical Chemistry Reference 2Document33 pagesPhysical Chemistry Reference 2Kuo SarongNo ratings yet
- 04 - 2022 GS TaDocument15 pages04 - 2022 GS Ta2022 BALAKRISHNAN ADHITHINo ratings yet
- Introduction To Raoults LawDocument8 pagesIntroduction To Raoults Lawdesi_parisNo ratings yet
- TME 304unit 2 - LEC 3Document14 pagesTME 304unit 2 - LEC 3BoweNo ratings yet
- Funda Iron & SteelDocument124 pagesFunda Iron & SteelpecmettNo ratings yet
- W-15E-AUFDocument6 pagesW-15E-AUFHuy NguyễnNo ratings yet
- Thermo Test 1 SolutionDocument5 pagesThermo Test 1 SolutionBernie TanNo ratings yet
- Gas Behaviour EOSDocument59 pagesGas Behaviour EOSMurugavel ChandranNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Carbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from -20° to 250°C and Pressures up to 1000 BarFrom EverandCarbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from -20° to 250°C and Pressures up to 1000 BarNo ratings yet
- Implementation Plan: Kheto Nxumayo Agricultural HDocument13 pagesImplementation Plan: Kheto Nxumayo Agricultural Hcarleston thurgoodNo ratings yet
- Applied Mechanics Exercise 2 Vectors & ResultantsDocument4 pagesApplied Mechanics Exercise 2 Vectors & Resultantscarleston thurgoodNo ratings yet
- Reaction Rates: BIG IdeaDocument34 pagesReaction Rates: BIG Ideacarleston thurgoodNo ratings yet
- Block Flow Diagram: OF Chlorination ProcessDocument2 pagesBlock Flow Diagram: OF Chlorination Processcarleston thurgoodNo ratings yet
- Cet300s Fisa 2014 MemoDocument17 pagesCet300s Fisa 2014 Memocarleston thurgoodNo ratings yet
- Process Streams Around Reactor System Stream Names: Liquid Benzene Chlorine Vapour Product Liquid Product MCB DCB Chlorine HCI Water Benzene TotalDocument6 pagesProcess Streams Around Reactor System Stream Names: Liquid Benzene Chlorine Vapour Product Liquid Product MCB DCB Chlorine HCI Water Benzene Totalcarleston thurgoodNo ratings yet
- Wa Ter F, T Water Vapour Produce D: Assumptions: No Spatial Variation Incompressible FluidDocument4 pagesWa Ter F, T Water Vapour Produce D: Assumptions: No Spatial Variation Incompressible Fluidcarleston thurgoodNo ratings yet
- Tutorial 3Document15 pagesTutorial 3carleston thurgoodNo ratings yet
- Tutorial 2 - LinearizationDocument1 pageTutorial 2 - Linearizationcarleston thurgoodNo ratings yet
- 2018 FisaDocument5 pages2018 Fisacarleston thurgoodNo ratings yet
- Chapter 3. Mathematical Modelling Principles: Prof. TV Ojumu (PHD) E-MailDocument7 pagesChapter 3. Mathematical Modelling Principles: Prof. TV Ojumu (PHD) E-Mailcarleston thurgoodNo ratings yet
- Chapter 3. Mathematical Modelling Principles: Prof. TV Ojumu (PHD) E-MailDocument5 pagesChapter 3. Mathematical Modelling Principles: Prof. TV Ojumu (PHD) E-Mailcarleston thurgoodNo ratings yet
- Chapter 3. Mathematical Modelling Principles: Prof. TV Ojumu (PHD) E-MailDocument9 pagesChapter 3. Mathematical Modelling Principles: Prof. TV Ojumu (PHD) E-Mailcarleston thurgoodNo ratings yet
- Assignment No 1 2015Document1 pageAssignment No 1 2015carleston thurgoodNo ratings yet
- Carleston Mabunda 218304870 Enginnering Mathematics AssignmentDocument2 pagesCarleston Mabunda 218304870 Enginnering Mathematics Assignmentcarleston thurgoodNo ratings yet
- Tut 8 - Multi Stage CompressorsDocument1 pageTut 8 - Multi Stage Compressorscarleston thurgoodNo ratings yet
- Department of Chemical Engineering Sick Test 1 PTY 260S Total Marks: 40 Date: 23rd December 2020 Time: 2:00 PM To 3: 15 PMDocument2 pagesDepartment of Chemical Engineering Sick Test 1 PTY 260S Total Marks: 40 Date: 23rd December 2020 Time: 2:00 PM To 3: 15 PMcarleston thurgoodNo ratings yet
- Faculty of Engineering & The Built Environment Final Assessment: Semester One Course: Diploma in Chemical Engineering (D3CHME)Document6 pagesFaculty of Engineering & The Built Environment Final Assessment: Semester One Course: Diploma in Chemical Engineering (D3CHME)carleston thurgoodNo ratings yet
- Add Author Names and Information Include University or Department Names If NeededDocument1 pageAdd Author Names and Information Include University or Department Names If Neededcarleston thurgoodNo ratings yet
- Typical Questions That CRE May AnswerDocument6 pagesTypical Questions That CRE May Answercarleston thurgoodNo ratings yet
- Heat ExchangersDocument2 pagesHeat Exchangerscarleston thurgoodNo ratings yet
- Tut 1 - Concepts and First LawDocument2 pagesTut 1 - Concepts and First Lawcarleston thurgoodNo ratings yet
- Tut 4 - Real GasesDocument1 pageTut 4 - Real Gasescarleston thurgoodNo ratings yet
- Production Eng (Slide 2)Document13 pagesProduction Eng (Slide 2)carleston thurgoodNo ratings yet
- Gas Laws and Kinetic TheoryDocument2 pagesGas Laws and Kinetic TheoryDhanBahadurNo ratings yet
- Objective: Metallurgy & Materials Engineering Department University of IndonesiaDocument48 pagesObjective: Metallurgy & Materials Engineering Department University of IndonesiaAulia HafidzNo ratings yet
- Chemical Physical Change Webquest PDFDocument4 pagesChemical Physical Change Webquest PDFRobert LackeyNo ratings yet
- Chapter 10 Heat TransferDocument14 pagesChapter 10 Heat TransferaimanrslnNo ratings yet
- Plant Schematic: Flowsheet1Document26 pagesPlant Schematic: Flowsheet1Abdullah N. TahirNo ratings yet
- Reynolds Number ApparatusDocument66 pagesReynolds Number ApparatusEdelMaeNabongGomezNo ratings yet
- Ge F-Class Gek111895Document2 pagesGe F-Class Gek111895gopinathsampath0% (2)
- MPOFF MCQsDocument24 pagesMPOFF MCQsAbhijeet SolatNo ratings yet
- Introduction Natural Gas ProcessingDocument58 pagesIntroduction Natural Gas ProcessingAlexander David100% (1)
- Solutions Chemistry (NM) (Ex-1 & 2)Document11 pagesSolutions Chemistry (NM) (Ex-1 & 2)Harshpreet SinghNo ratings yet
- Density Vs ViscosityDocument1 pageDensity Vs ViscosityKhaleeq AhmedNo ratings yet
- States of Matter Practice TestDocument2 pagesStates of Matter Practice TestSadiaKanwalNo ratings yet
- Data Sheet: Amadeus ProjectDocument7 pagesData Sheet: Amadeus ProjectErikikoNo ratings yet
- The Rashba Hamiltonian and Electron TransportDocument6 pagesThe Rashba Hamiltonian and Electron TransportDiegoNo ratings yet
- Department of ChemistryDocument15 pagesDepartment of ChemistryWorcPrimerNo ratings yet
- Chapter 08Document43 pagesChapter 08Sigmund PohanNo ratings yet
- Hall EffectDocument15 pagesHall EffectHardik GuptaNo ratings yet
- Gas Storage in Aquifers and Salt CavernsDocument58 pagesGas Storage in Aquifers and Salt Cavernssaladinayubi1234100% (2)
- 3-SCH 403 Colagative PropertiesDocument25 pages3-SCH 403 Colagative PropertiesAnn KiamaNo ratings yet
- Entrained-Bed Gasification Processes: Koppers-Totzek (K-T) ProcessDocument8 pagesEntrained-Bed Gasification Processes: Koppers-Totzek (K-T) ProcessSarita ShitNo ratings yet
- Introduction To N.G Sheet 1Document2 pagesIntroduction To N.G Sheet 1Eng Said ElsayedNo ratings yet
- Forced Convection Boiling Inside Helically-Coiled TubesDocument15 pagesForced Convection Boiling Inside Helically-Coiled TubesAmoul DhahriNo ratings yet
- Topic 4.3 - Covalent Structures MCQDocument7 pagesTopic 4.3 - Covalent Structures MCQDonal GrayNo ratings yet
- Air Conditioning 2022-2023Document127 pagesAir Conditioning 2022-2023Ali BandarNo ratings yet
- Physics Tut Sheet 3 Dielectric PropertiesDocument2 pagesPhysics Tut Sheet 3 Dielectric PropertiesHarshit SoniNo ratings yet
- OCECO - Sizing Pressure Vacuum Relief ValvesDocument4 pagesOCECO - Sizing Pressure Vacuum Relief ValvesVasile PopescuNo ratings yet
- Pchem10e Solutions ch04Document10 pagesPchem10e Solutions ch04이호준No ratings yet
- 3 Separator Design and Construction - UpdateDocument36 pages3 Separator Design and Construction - Updateمصطفى العباديNo ratings yet