Professional Documents
Culture Documents
Activation of Rubisco Regulates Photosynthesis at High Temperature and CO
Activation of Rubisco Regulates Photosynthesis at High Temperature and CO
Uploaded by
Haniar MeyOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Activation of Rubisco Regulates Photosynthesis at High Temperature and CO
Activation of Rubisco Regulates Photosynthesis at High Temperature and CO
Uploaded by
Haniar MeyCopyright:
Available Formats
Commentary
Activation of Rubisco regulates photosynthesis at
high temperature and CO2
Richard G. Jensen*
Department of Biochemistry and Molecular Biophysics, University of Arizona, Tucson, AZ 85721
T he enzyme Rubisco, short for ribulose-
1,5-bisphosphate carboxylase兾oxygen-
ase, is the enzyme that incorporates CO2
into plants during photosynthesis. As it con-
stitutes about 30% of the total protein in a
plant leaf, Rubisco is probably the most
abundant protein on earth and a major sink
for plant nitrogen. Rubisco is widely ac-
cepted as the ultimate rate-limiting step in
photosynthetic carbon fixation. Atmo-
spheric oxygen competes with CO2 as a
substrate for Rubisco, giving rise to photo-
respiration. When first purified, the enzyme
appeared to have a poor affinity for CO2
[Km (CO2)] of 450 M (1), whereas air in
equilibrium with water, 25°C, is about 10
M. Later, Lorimer et al. (2) showed that
COMMENTARY
the active site of Rubisco must first be
carbamylated by an activator CO2, separate
from the substrate CO2, and must bind
Mg2⫹ before binding the five-carbon sub-
strate, ribulose-1,5-bisphosphate (RuBP).
Indeed, on addition of RuBP, the measured
Km(CO2) approached the concentrations of
dissolved CO2 in water; however, the rate of
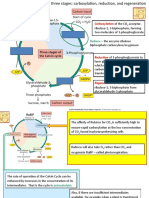
the reaction was sustained for only 5 min- Fig. 1. Activase (blue) switches Rubisco (yellow) from an inactive to an active form by the ATP-dependent
utes and then declined rapidly. This decline release of tight-binding sugar phosphates such RuBP. Only the active form of Rubisco is capable of
catalyzing CO2 fixation, the first step in photosynthesis. In the scheme, a Rubisco active site that has
was shown to be caused by the tight binding
spontaneously lost CO2 (decarbamylation) (step 2) binds RuBP (step 3). The binding of RuBP causes
of RuBP to Rubisco that had lost the acti- conformational changes that produce a dead-end complex consisting of inactive Rubisco and tightly
vator CO2 (Fig. 1). The missing ingredient bound RuBP. Activase physically interacts with Rubisco (step 5), changing the conformation of Rubisco
needed to uncouple Rubisco and RuBP was (step 6) to one that binds RuBP less tightly. ATP hydrolysis by activase is required for these conformational
found in the intact plant with a separate changes, perhaps for priming activase (step 4). Because of the lower affinity, RuBP dissociates from the
protein, called Rubisco activase (3). This active site of Rubisco (step 7), which frees the site for subsequent carbamylation (step 1) or rebinding of
enzyme acts on Rubisco and allows release RuBP (step 3), but probably after dissociation of activase (step 8). Crafts-Brandner and Salvucci (6) show
of the bound RuBP so that the site can bind that an accelerated rate of Rubisco deactivation occurs at high temperature (steps 2 and 3), which is not
matched by a faster rate of activation by activase. ATP hydrolysis (step 4) and兾or activase–Rubisco
the activator CO2 and Mg2⫹. Rubisco acti-
interactions (steps 5 and 6) may limit the rate of Rubisco activation at high temperature.
vase itself requires ATP, and its activity is
related to the energy charge of the chloro-
plast (4). Thus, the proportion of Rubisco
perature response in the test tube under the basis of the kinetics of Rubisco and the
that is active in a leaf (activation state) can
controlled conditions, and by using amount of active enzyme in the leaf, the
vary depending on the effectiveness of
Rubisco and Rubisco activase isolated authors have shown that, under both high
Rubisco activase in removing bound RuBP.
from tobacco, they were able to ascribe temperature and high CO2, photosynthe-
Regulation of Rubisco fine tunes the rate of
the limitation to a specific biochemical sis was constrained by the activity of
CO2 fixation to the rate of photosynthetic
event, the inability of Rubisco activase to Rubisco activase.
electron transport, ensuring that chloroplast Changes in the global environment
metabolites are always optimal for photo- keep pace with a faster deactivation of
Rubisco. Increased CO2 also decreased since the beginning of the Industrial Rev-
Downloaded at Indonesia: PNAS Sponsored on September 25, 2020
synthesis (5). olution have raised concerns about the
The paper by Crafts-Brandner and Sal- the activation state of Rubisco in leaves,
impact on natural and agroecosystems.
vucci (6) in this issue of PNAS provides and the authors conclude that the re-
Increasing CO2 levels and corresponding
evidence that, with plants under heat sponse could be explained by a decreased
stress, the activation state of Rubisco and energy charge in the chloroplast that re-
photosynthesis as measured by CO2 ex- duced the ATPase activity of Rubisco See companion article on page 13430.
change is reduced. By duplicating the tem- activase. By calculating photosynthesis on *E-mail: Jensen@u.arizona.edu.
PNAS 兩 November 21, 2000 兩 vol. 97 兩 no. 24 兩 12937–12938
changes in temperature will directly affect Crafts-Brandner and Salvucci (6) high- plants contains 16 subunits—8 large chlo-
the earth’s carbon balance by altering lights the importance of Rubisco activa- roplast-encoded subunits and 8 small nucle-
photosynthetic carbon fixation. Thus, it is tion as a determinant of photosynthetic ar-encoded subunits. Some details about
essential to develop an understanding of performance under conditions associated the binding of activase are known (4), but
the mechanistic response of photosynthe- with a changing global climate. The clear how its binding disturbs the protein struc-
sis to environmental change. Models of conclusion of these experiments is that ture to allow release of RuBP is not under-
photosynthesis (7, 8) have been widely Rubisco activation is the major limitation stood. With the three-dimensional structure
adapted to predict how environmental to CO2 fixation during photosynthesis, of Rubisco at hand, attempts are being
made to improve the reaction kinetics of the
changes will influence photosynthesis and certainly under high CO2, high tempera- enzyme. Whether it is possible to decrease
to pinpoint biochemical limitations in the ture, and optimal light. the affinity of the activated Rubisco–RuBP
process. These models are based primarily Although some of the major details are complex for oxygen and thus increase
on the kinetics of fully activated Rubisco, known, there is still much to be learned carboxylation over oxygenation is still un-
and they make a clear distinction between about the regulation of Rubisco. The struc- known. Attempts to alter specificity by mu-
limitations attributable to the enzyme ture of Rubisco is known from several plant tagenesis or interspecific genetic replace-
amount and those limiting the regenera- sources (9). Structure determination has ment must consider interactions between
tion of the substrate, RuBP. The report by been a difficult feat: Rubisco from higher Rubisco and Rubisco activase.
1. Weissbach, A., Horecker, B. L. & Hurwitz, J. 4. Portis, A. R., Jr. (1995) J Exp. Bot. 46, 1285–1291. 7. Farquhar, G. D., von Caemmerer, S. & Berry J. A.
(1956) J. Biol. Chem. 218, 795–810. 5. Perchorowicz, J. T., Raynes, D. A. & Jensen, (1980) Planta 149, 178–190.
2. Lorimer, G. H., Badger, M. R. & Andrews, T. J. R. G. (1981) Proc. Natl. Acad. Sci. USA 78, 8. Sharkey, T. D. (1985) Bot. Rev. 51,
(1976) Biochemistry 15, 529–536. 2985–2989. 53–105.
3. Salvucci, M. E., Portis, A. R., Jr. & Ogren, W. L. 6. Crafts-Brandner, S. J. & Salvucci, M. E. (2000) 9. Spreitzer, R. J. (1999) Photosynthesis Res. 60,
(1985) Photosynthesis Res. 7, 193–201. Proc. Natl. Acad. Sci. USA 97, 13430–13435. 29–42.
Downloaded at Indonesia: PNAS Sponsored on September 25, 2020
12938 兩 www.pnas.org Jensen
You might also like
- Biomaterials Quick Study SheetDocument2 pagesBiomaterials Quick Study SheetWilliam ChanNo ratings yet
- German Monograph For CannabisDocument7 pagesGerman Monograph For CannabisAngel Cvetanov100% (1)
- C72 Steel - 2Document4 pagesC72 Steel - 2Bruno Cavalcante0% (1)
- Microsoft Word - P and P Paper1Document11 pagesMicrosoft Word - P and P Paper1HankNo ratings yet
- Rubisco PDFDocument3 pagesRubisco PDFHans EdwardNo ratings yet
- Rubisco Encyclopedie EnvironnementDocument4 pagesRubisco Encyclopedie EnvironnementArmando TrentelmanNo ratings yet
- Calvin CycleDocument14 pagesCalvin CycleWahyu ArifNo ratings yet
- A227 RuBP CarboxylaseDocument4 pagesA227 RuBP Carboxylaseramloghun veer100% (1)
- Photo RespirationDocument4 pagesPhoto RespirationKasun WekasingheNo ratings yet
- DarkreactionsDocument10 pagesDarkreactionsdhonaNo ratings yet
- RuBisCO - WikipediaDocument86 pagesRuBisCO - WikipediaBashiir NuurNo ratings yet
- Biol1Xx7 Photosynthesis II Calvin Cycle & CO Fixation: Assoc Prof Charles WarrenDocument28 pagesBiol1Xx7 Photosynthesis II Calvin Cycle & CO Fixation: Assoc Prof Charles WarrenRachaelNo ratings yet
- Photosynthetic Carbohydrate synthesis (Calvin Cycle) : Lectured by Dr. Qin Yongmei (秦咏梅) :Nov. 28, 2007Document55 pagesPhotosynthetic Carbohydrate synthesis (Calvin Cycle) : Lectured by Dr. Qin Yongmei (秦咏梅) :Nov. 28, 2007bbaaddaarrNo ratings yet
- PhotorespirationDocument1 pagePhotorespirationAnis AzwaNo ratings yet
- 02 Plant EcophysiologyDocument49 pages02 Plant Ecophysiologylam lamNo ratings yet
- The Calvin Cycle and The Pentose Phosphate PathwayDocument26 pagesThe Calvin Cycle and The Pentose Phosphate PathwayAnthonyPacoGómezNo ratings yet
- C3 C4 CAM Short No AnswersDocument1 pageC3 C4 CAM Short No AnswersAntony ThabesanNo ratings yet
- Rubisco ChatGPTExp2022Document2 pagesRubisco ChatGPTExp2022Elan MoritzNo ratings yet
- Plant Anatomy Assignment 1Document3 pagesPlant Anatomy Assignment 1anon_711152134No ratings yet
- Bio 11 PhotorespirationDocument12 pagesBio 11 PhotorespirationBik WodeNo ratings yet
- 206 L6 Photosynthesis C3Document48 pages206 L6 Photosynthesis C3craigNo ratings yet
- Photosynthesis Dark Reaction KA 2080Document25 pagesPhotosynthesis Dark Reaction KA 2080Subham KalwarNo ratings yet
- Biochemical and Synthetic Biology Approaches To Improve Photosynthetic CO - FixationDocument8 pagesBiochemical and Synthetic Biology Approaches To Improve Photosynthetic CO - FixationLele AsinNo ratings yet
- Simulacion de Procesos Del SBRDocument9 pagesSimulacion de Procesos Del SBRBrian RamosNo ratings yet
- C6 (Photosynthesis)Document16 pagesC6 (Photosynthesis)nurhasinahabrahimNo ratings yet
- Activity10answers PDFDocument6 pagesActivity10answers PDFMuthu LakshmiNo ratings yet
- PhotorespirationDocument16 pagesPhotorespirationMoa ArmyNo ratings yet
- Is Rubisco The Most Important Enzyme in The WorldDocument2 pagesIs Rubisco The Most Important Enzyme in The WorldIqbal WahyuNo ratings yet
- Chapter 3 - Part 8Document8 pagesChapter 3 - Part 8Adibah Qistina QistinaNo ratings yet
- Somerville, PhotorespirationDocument5 pagesSomerville, Photorespirationjoyeeta8No ratings yet
- Photosynthesis: Capturing EnergyDocument26 pagesPhotosynthesis: Capturing EnergyJustineMarieClarisaCruzNo ratings yet
- Fundamentals Fundamentals Fundamentals of Crop Science of Crop Science of Crop ScienceDocument39 pagesFundamentals Fundamentals Fundamentals of Crop Science of Crop Science of Crop ScienceAlthea DoradoNo ratings yet
- Rubisco Activase ConstrainsDocument6 pagesRubisco Activase ConstrainsGabriel CamarenaNo ratings yet
- Photosynthesis:: Variations On The ThemeDocument22 pagesPhotosynthesis:: Variations On The ThemeHaq ChandioNo ratings yet
- Bio 11 c4 and CAMDocument15 pagesBio 11 c4 and CAMBik WodeNo ratings yet
- Lecture 9 - Dark ReactionDocument14 pagesLecture 9 - Dark ReactionAtika ZulfiqarNo ratings yet
- Photosynthesis by Isolated ChloroplastsDocument9 pagesPhotosynthesis by Isolated ChloroplastsFernando JarixNo ratings yet
- '21. Photosynthesis: Give the evidence of the presence of light reaction. 請為光反應提出証明。Document7 pages'21. Photosynthesis: Give the evidence of the presence of light reaction. 請為光反應提出証明。thanks btNo ratings yet
- Algae Technology Report: IndexDocument20 pagesAlgae Technology Report: IndexSteve RacoosinNo ratings yet
- C4 PathwayDocument8 pagesC4 PathwayInnocent mugumeNo ratings yet
- 1st Long Quiz Crop SciDocument15 pages1st Long Quiz Crop SciBulcan 300No ratings yet
- Biology ReviwerDocument7 pagesBiology Reviwerapi-709918261No ratings yet
- Photosynthesis Exam Pack NEW RVDocument15 pagesPhotosynthesis Exam Pack NEW RVkrrish5906No ratings yet
- Photosynthesis Part 2Document2 pagesPhotosynthesis Part 2Hanna Evidente BakalNo ratings yet
- Faizal Akmal Syahputra - 215040201111060 - Resume FistanDocument10 pagesFaizal Akmal Syahputra - 215040201111060 - Resume FistanFaizal AkmalNo ratings yet
- 06photosynthesis 12Document35 pages06photosynthesis 12Anne CuadernoNo ratings yet
- PhotosynthesisDocument26 pagesPhotosynthesisalyaainsyirah04No ratings yet
- Function of PhotosynthesisDocument58 pagesFunction of PhotosynthesisJulian ChristopherNo ratings yet
- The Calvin Cycle: Light Independent ReactionsDocument1 pageThe Calvin Cycle: Light Independent ReactionssintapuspitaNo ratings yet
- A Faster Rubisco With Potential To IncreaseDocument11 pagesA Faster Rubisco With Potential To IncreaseNicolás D.G.LNo ratings yet
- Calvin Cycle (Dark Reactions / Light Independent Reaction)Document47 pagesCalvin Cycle (Dark Reactions / Light Independent Reaction)L'ya Lieslotte100% (2)
- 88 RespirationDocument25 pages88 RespirationJohn VukeniNo ratings yet
- Lecture 14 Sample Problem AnswersDocument1 pageLecture 14 Sample Problem Answerssonicdragon100% (1)
- Chapter 6 - Photosynthesis Carbon MetabolismDocument46 pagesChapter 6 - Photosynthesis Carbon Metabolismsayidah nafisahNo ratings yet
- The Calvin CycleDocument8 pagesThe Calvin CycleVaan ChinNo ratings yet
- Outline For PhotosynthesisDocument6 pagesOutline For Photosynthesiswitzy11No ratings yet
- Chapter 17.7 Lecture NotesDocument16 pagesChapter 17.7 Lecture NotesZach MaxwellNo ratings yet
- Photosynthetic Adaptations: C Cycle and CAM: Prepared By: Group 7 (Cabatingan, Ocaya, Cardente, Guillermo, and Andamon)Document8 pagesPhotosynthetic Adaptations: C Cycle and CAM: Prepared By: Group 7 (Cabatingan, Ocaya, Cardente, Guillermo, and Andamon)Una Kaya CabatinganNo ratings yet
- Crop Ecology - Productivity and Management in Agricultural Systems - (2011) Queensland Recomendation-275-295Document21 pagesCrop Ecology - Productivity and Management in Agricultural Systems - (2011) Queensland Recomendation-275-295CarolinaNo ratings yet
- Photosynthesis by Isolated ChloroplastsDocument6 pagesPhotosynthesis by Isolated ChloroplastsFernando JarixNo ratings yet
- EC07 Energy Nutrient RelationsDocument29 pagesEC07 Energy Nutrient Relationsevahhassane2003No ratings yet
- Biological Nutrient RemovalDocument42 pagesBiological Nutrient RemovalGökhan TurhanNo ratings yet
- Plant Life ProcessesDocument53 pagesPlant Life ProcessesGel Mi AmorNo ratings yet
- Reactive Oxygen Species in Plants: Boon Or Bane - Revisiting the Role of ROSFrom EverandReactive Oxygen Species in Plants: Boon Or Bane - Revisiting the Role of ROSDr. Vijay Pratap SinghNo ratings yet
- Medinalis Melanogaster: A. Perhitungan Kerapatan Kerapatan Jenis (I) Jumlah Individu Yang DiamatiDocument6 pagesMedinalis Melanogaster: A. Perhitungan Kerapatan Kerapatan Jenis (I) Jumlah Individu Yang DiamatiHaniar MeyNo ratings yet
- PKN Rangkuman PresentasiDocument2 pagesPKN Rangkuman PresentasiHaniar MeyNo ratings yet
- Ballance A 2000 Howmuchisacleanbeachworth 96 210-213Document4 pagesBallance A 2000 Howmuchisacleanbeachworth 96 210-213Haniar MeyNo ratings yet
- Sea Turtle Survey, Monitoring and Awareness Promotion Programme in Mainland ChinaDocument47 pagesSea Turtle Survey, Monitoring and Awareness Promotion Programme in Mainland ChinaHaniar MeyNo ratings yet
- Iji 2020 3 16Document14 pagesIji 2020 3 16Haniar MeyNo ratings yet
- Pemanfaatan Media Sosial Sebagai Wadah Promosi Produk Rumahan Bagi Perempuan Nelayan Di Desa Ranto Panyang Kecamatan MeureuboDocument11 pagesPemanfaatan Media Sosial Sebagai Wadah Promosi Produk Rumahan Bagi Perempuan Nelayan Di Desa Ranto Panyang Kecamatan MeureuboHaniar MeyNo ratings yet
- Ajib 1 (Q1)Document12 pagesAjib 1 (Q1)Haniar MeyNo ratings yet
- Peta Konsep Air PDFDocument1 pagePeta Konsep Air PDFHaniar MeyNo ratings yet
- Ajib 4 (Q1)Document7 pagesAjib 4 (Q1)Haniar MeyNo ratings yet
- Are We Working Towards Global Research Priorities For Management and Conservation of Sea Turtles?Document46 pagesAre We Working Towards Global Research Priorities For Management and Conservation of Sea Turtles?Haniar MeyNo ratings yet
- Marine Seismic Surveys and Ocean Noise: Time For Coordinated and Prudent PlanningDocument9 pagesMarine Seismic Surveys and Ocean Noise: Time For Coordinated and Prudent PlanningHaniar MeyNo ratings yet
- Survei Kebutuhan Informasi Bidang Kemari D0438bfeDocument10 pagesSurvei Kebutuhan Informasi Bidang Kemari D0438bfeHaniar MeyNo ratings yet
- Characterization of The Estrous Cycle in Galea Spixii (Wagler, 1831)Document6 pagesCharacterization of The Estrous Cycle in Galea Spixii (Wagler, 1831)Haniar MeyNo ratings yet
- Elden Ussonello Tewart: Questionnaire (Topline Data Removed) From National Poll Regarding The OceanDocument13 pagesElden Ussonello Tewart: Questionnaire (Topline Data Removed) From National Poll Regarding The OceanHaniar MeyNo ratings yet
- Peta Konsep Respirasi Seluler PDFDocument1 pagePeta Konsep Respirasi Seluler PDFHaniar MeyNo ratings yet
- Peta Konsep Nutrisi Mineral PDFDocument1 pagePeta Konsep Nutrisi Mineral PDFHaniar MeyNo ratings yet
- Circumcision Stops Hiv: From An Article by Chris Smith, 2018 (The Naked Scientist)Document9 pagesCircumcision Stops Hiv: From An Article by Chris Smith, 2018 (The Naked Scientist)Haniar MeyNo ratings yet
- Reproductive IsolationDocument2 pagesReproductive IsolationHaniar MeyNo ratings yet
- All Worksheets MYSQLDocument30 pagesAll Worksheets MYSQLKavitha B (CBSE)50% (2)
- BMR CalculatorDocument4 pagesBMR Calculatorharshika tembhurneNo ratings yet
- Mf5102 Computer Integrated Manufacturing Systems 1Document2 pagesMf5102 Computer Integrated Manufacturing Systems 1vijay vNo ratings yet
- Daikin VRV SheetDocument6 pagesDaikin VRV SheetSridhar ReddyNo ratings yet
- Chapter 3 - Forecasting and Demand PlanningDocument24 pagesChapter 3 - Forecasting and Demand PlanningHAN LIEU GIA100% (1)
- Adaptive Partitioning User's Guide: QNX Software Development Platform 6.6Document98 pagesAdaptive Partitioning User's Guide: QNX Software Development Platform 6.6Larken BradynNo ratings yet
- Telephone DirectoryDocument4 pagesTelephone DirectoryDhaniya Karthik33% (3)
- Theodor Wilhelm EngelmannDocument3 pagesTheodor Wilhelm EngelmannJames FranklinNo ratings yet
- Protective Transformers: by N.H, Shton, Revised by E.J.MellorDocument31 pagesProtective Transformers: by N.H, Shton, Revised by E.J.MellorJavier MaldonadoNo ratings yet
- Qin 2005Document8 pagesQin 2005Omkar BordeNo ratings yet
- Installation and Maintenance: Intera AchievaDocument64 pagesInstallation and Maintenance: Intera AchievaАндрей СподобецNo ratings yet
- 1-INRODUCTION TO MICROWAVE ENGINEERING-10-Jul-2019Material - I - 10-Jul-2019 - 1 - IntroductionDocument6 pages1-INRODUCTION TO MICROWAVE ENGINEERING-10-Jul-2019Material - I - 10-Jul-2019 - 1 - Introductionabhignan routhuNo ratings yet
- AWS ClassBook Lesson03Document7 pagesAWS ClassBook Lesson03SAINo ratings yet
- Cooling Unit SpecDocument4 pagesCooling Unit Specmirali74No ratings yet
- Sri Chaitanya IIT Academy., India.: A Right Choice For The Real AspirantDocument10 pagesSri Chaitanya IIT Academy., India.: A Right Choice For The Real AspirantsumanthNo ratings yet
- NRL r14 Thursday Pacific RacingDocument1 pageNRL r14 Thursday Pacific RacingjoanalcarazNo ratings yet
- Computation of Peak Flood Discharge by ShannenDocument3 pagesComputation of Peak Flood Discharge by Shannenraqu.villanueva.cocNo ratings yet
- Longjia Exactly BrugermanualDocument56 pagesLongjia Exactly BrugermanualvladomiljNo ratings yet
- Leica TM6100A Brochure enDocument6 pagesLeica TM6100A Brochure enCarlos CostaNo ratings yet
- Practice Exam 2Document6 pagesPractice Exam 2John SmithNo ratings yet
- Chapter - 44Document5 pagesChapter - 44tito cuadrosNo ratings yet
- Oracle DBADocument15 pagesOracle DBAEric LiNo ratings yet
- Fuzzy Metrics and Statistical Metric SpacesDocument9 pagesFuzzy Metrics and Statistical Metric SpacesbbeeNo ratings yet
- CFD SolidificationMicrostructures PDFDocument18 pagesCFD SolidificationMicrostructures PDFRakesh MahawarNo ratings yet
- Buffer Overflow AttacksDocument2 pagesBuffer Overflow AttacksBADR EDDINE JAMAINo ratings yet
- CA PlantDocument47 pagesCA PlantHarsh KumarNo ratings yet