Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
24 viewsKinetics PDF
Kinetics PDF
Uploaded by
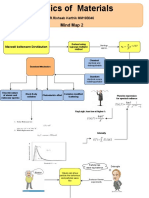
AlizaDoubling the concentration or pressure of particles means doubling the number of particles per unit volume. This leads to more frequent collisions between particles as there are more opportunities for collisions. The increased collision frequency means a higher proportion of particles will collide with the required activation energy for a reaction to occur, resulting in a faster reaction rate. The Maxwell-Boltzmann distribution curve shifts to the right and has a greater area under it, reflecting the increased number of high-energy collisions.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- CML 2017 Marks EH1E PDFDocument7 pagesCML 2017 Marks EH1E PDFAlizaNo ratings yet
- CML 2017 Marks EH3E PDFDocument8 pagesCML 2017 Marks EH3E PDFAlizaNo ratings yet
- Fundamentals of Enzymology The Cell and Molecular Biology of Catalytic ProteinsDocument0 pagesFundamentals of Enzymology The Cell and Molecular Biology of Catalytic Proteinsmonica_elizabeth_35No ratings yet
- CML 2017 Paper EH1E PDFDocument17 pagesCML 2017 Paper EH1E PDFAlizaNo ratings yet
- CatalystsDocument20 pagesCatalystsMargie Reonal LlenoNo ratings yet
- Problem Set ODocument19 pagesProblem Set OnimboNo ratings yet
- Chem2,4-Reaction KineticsDocument3 pagesChem2,4-Reaction KineticsMoeez100% (1)
- 8 Reaction Kinetics: Maxwell Boltzmann DistributionDocument16 pages8 Reaction Kinetics: Maxwell Boltzmann DistributionNoor MuhammadNo ratings yet
- Kinetics - Edexcel A Level ChemistryDocument4 pagesKinetics - Edexcel A Level ChemistryMaddie BaughNo ratings yet
- Light Energy Sound EnergyDocument1 pageLight Energy Sound EnergykenNo ratings yet
- The Periodic Table TrendsDocument2 pagesThe Periodic Table TrendsHannahNo ratings yet
- ChemistryDocument1 pageChemistrycallulafebNo ratings yet
- ChemistryDocument16 pagesChemistryakkay616No ratings yet
- Leii E1Document1 pageLeii E1Viviana CamposNo ratings yet
- Factors Affecting Rate of Reaction Collision TheoryDocument1 pageFactors Affecting Rate of Reaction Collision TheoryMei XiangNo ratings yet
- 6 - 13 FORMS of EnergyDocument30 pages6 - 13 FORMS of EnergyDennis Limosnero MayorNo ratings yet
- Periodicity: ShieldingDocument1 pagePeriodicity: ShieldingjoanaNo ratings yet
- Forms of EnergyDocument13 pagesForms of EnergyVallarasanNo ratings yet
- Edx Ial 词汇手册物理汇总Document27 pagesEdx Ial 词汇手册物理汇总Cai MingleiNo ratings yet
- Mind MapsDocument3 pagesMind Mapsjrolfe477No ratings yet
- % Energy EpDocument1 page% Energy Epgabby fosterNo ratings yet
- Electronic Configuration-1Document15 pagesElectronic Configuration-1Dyutimoy DanNo ratings yet
- MSG - 105 - 139735 - List of AS Level Physics DefinitionsDocument2 pagesMSG - 105 - 139735 - List of AS Level Physics DefinitionsaayushpromasterNo ratings yet
- Mind Map 2: R.Rishaab Karthik MM19B046Document3 pagesMind Map 2: R.Rishaab Karthik MM19B046Hardy SPNo ratings yet
- Transfer of Thermal EnergyDocument1 pageTransfer of Thermal EnergyShakilNo ratings yet
- EnergyDocument1 pageEnergyapi-484459862No ratings yet
- Article Careers360 20230527174852Document25 pagesArticle Careers360 20230527174852naan singhNo ratings yet
- Nuclear Physics PDFDocument17 pagesNuclear Physics PDFsafi mohammadNo ratings yet
- SPMRSM Paper 2 2015Document37 pagesSPMRSM Paper 2 2015Natasha BalqisNo ratings yet
- Edexcel Knowledge Mat Topic 8 Energy Forces Doing Work V1 2Document4 pagesEdexcel Knowledge Mat Topic 8 Energy Forces Doing Work V1 2NetkoNo ratings yet
- Physics Definition by Vasumitra GajbhiyeDocument3 pagesPhysics Definition by Vasumitra GajbhiyeHuzaifa ImranNo ratings yet
- 02 First Law of ThermodynamicDocument22 pages02 First Law of ThermodynamicYahya Alhaddi KA201 18No ratings yet
- Nuclear Physics A2Document15 pagesNuclear Physics A2uditi kalraNo ratings yet
- Cascayo, Debelyn S. Beed 3-A (Learning Task 5)Document1 pageCascayo, Debelyn S. Beed 3-A (Learning Task 5)Debelyn CascayoNo ratings yet
- Mass Defect and Binding EnergyDocument1 pageMass Defect and Binding EnergyMichelle nananaNo ratings yet
- Unit 3 ReviewDocument9 pagesUnit 3 ReviewMadi WildeNo ratings yet
- Read To LearnDocument5 pagesRead To LearnosamaNo ratings yet
- 4.4 Chemical KineticsDocument14 pages4.4 Chemical KineticsDamia AziziNo ratings yet
- IB Physics Definitions Needed by The Syllabus of 2009Document2 pagesIB Physics Definitions Needed by The Syllabus of 2009mohsin771100% (1)
- Hand Written Note by DP SIR For Quick RevisionDocument7 pagesHand Written Note by DP SIR For Quick RevisionKishori PatilNo ratings yet
- Mind Map 09-09-2021: Hardhik Pinjala - MM19B043 - September 15, 2021Document2 pagesMind Map 09-09-2021: Hardhik Pinjala - MM19B043 - September 15, 2021Hardy SPNo ratings yet
- Actividad de Sienciea Cuadro 7X7Document1 pageActividad de Sienciea Cuadro 7X7luz gilNo ratings yet
- 3.1 Internal Energy Mind MapDocument1 page3.1 Internal Energy Mind MapAndres LopezNo ratings yet
- Detailed Explanations For TrendsDocument2 pagesDetailed Explanations For TrendsBRITNEY ALEXANDRA VERMINSHUNo ratings yet
- Heat of Fusion: Index Phase Change ConceptsDocument3 pagesHeat of Fusion: Index Phase Change Conceptsscsa31619No ratings yet
- Bond Graph - WikipediaDocument16 pagesBond Graph - WikipediaRanjit KumarNo ratings yet
- سيبلس يبلس يبلسيبلس يبلسيبلDocument8 pagesسيبلس يبلس يبلسيبلس يبلسيبلAsmaa AdelNo ratings yet
- HK Physics DefinitionsDocument3 pagesHK Physics DefinitionsMNRNo ratings yet
- Intro 2 Molecular Modelling & Molecular MechanicsDocument35 pagesIntro 2 Molecular Modelling & Molecular Mechanicsachsanuddin50% (2)
- Particle Interactions - FactRecallDocument2 pagesParticle Interactions - FactRecallHarpritNo ratings yet
- Various Forms of EnergyDocument2 pagesVarious Forms of EnergyRhianna TetlowNo ratings yet
- Statics of ParticlesDocument35 pagesStatics of ParticlesEmmanuel C. Dimpas Jr.No ratings yet
- Note 18-Apr-2024 at 11 - 00 - 26 PMDocument10 pagesNote 18-Apr-2024 at 11 - 00 - 26 PMMurtaza AbbasNo ratings yet
- Metabolism-Note Oct 11, 2023Document7 pagesMetabolism-Note Oct 11, 2023Saumya ThakkerNo ratings yet
- CH 2 Statics of ParticlesDocument35 pagesCH 2 Statics of ParticlesMuhd HafidzNo ratings yet
- Conservation and Dissipation of EnergyDocument2 pagesConservation and Dissipation of EnergyceryssandfordNo ratings yet
- NoteDocument7 pagesNoteRosina KaneNo ratings yet
- Classical Thermo NotesDocument37 pagesClassical Thermo NotesVirendra SheoranNo ratings yet
- Park 1996Document12 pagesPark 1996Muhammad Ali AbroNo ratings yet
- DefinitionsDocument7 pagesDefinitionsdevasanisaiteja2006No ratings yet
- BiologyDocument3 pagesBiologycarliehtcheungNo ratings yet
- Bond GraphDocument15 pagesBond GraphtafazulijazNo ratings yet
- Heat and Energy: Scope-UnactstandingDocument21 pagesHeat and Energy: Scope-Unactstandingb9835143No ratings yet
- CML 2017 Paper EH3E PDFDocument20 pagesCML 2017 Paper EH3E PDFAlizaNo ratings yet
- CML 2017 Marks EH2E PDFDocument8 pagesCML 2017 Marks EH2E PDFAlizaNo ratings yet
- CML 2017 Paper EH2E PDFDocument19 pagesCML 2017 Paper EH2E PDFAlizaNo ratings yet
- Och2016 17 PDFDocument28 pagesOch2016 17 PDFPayal SharmaNo ratings yet
- Review Packet - Teacher VersionDocument77 pagesReview Packet - Teacher VersionJADFNo ratings yet
- AP Chemistry Free Response by TopicssDocument32 pagesAP Chemistry Free Response by TopicssVincent Zw Liu0% (1)
- GBPUAT SyllabusDocument11 pagesGBPUAT SyllabusMahesh ShahNo ratings yet
- Chemical Kinetics PYQDocument9 pagesChemical Kinetics PYQAmresh MohantyNo ratings yet
- FCH - ChE 331A - JAN - 2019Document2 pagesFCH - ChE 331A - JAN - 2019Abhinav ShuklaNo ratings yet
- Lab 5 - Chemical KineticsDocument3 pagesLab 5 - Chemical KineticsFranck MomoNo ratings yet
- 2017 Y5 T4 Chem Focus - KineticsDocument4 pages2017 Y5 T4 Chem Focus - KineticsxmxmxmxmxmNo ratings yet
- Reaction Technologies: ThreeDocument31 pagesReaction Technologies: ThreeProcess EngineerNo ratings yet
- Advances in Chemical Engineering Vol 11, Volume 11 (Thadvane in Chemicalomas B. Drew) (9780120085118) (Academic Press - 1981)Document469 pagesAdvances in Chemical Engineering Vol 11, Volume 11 (Thadvane in Chemicalomas B. Drew) (9780120085118) (Academic Press - 1981)siavashdm1100% (1)
- Chem U4 EdexcelDocument55 pagesChem U4 EdexcelReez SinhaNo ratings yet
- Catalyst DefinitionDocument17 pagesCatalyst DefinitionTRIBRATA BASKORONo ratings yet
- MM Kinetics No.4 PDFDocument6 pagesMM Kinetics No.4 PDFCarlos MontúfarNo ratings yet
- Chemistry PGDocument33 pagesChemistry PGhrishikeshanandNo ratings yet
- Runaway ReactionsDocument5 pagesRunaway ReactionsDaniel SantosNo ratings yet
- Mark Scheme (Results) January 2008: GCE Chemistry (6242) Paper 1Document11 pagesMark Scheme (Results) January 2008: GCE Chemistry (6242) Paper 1Chong Cheng NamNo ratings yet
- Kinetics For Benzene#ethylene Reaction in Near-Critical RegionsDocument8 pagesKinetics For Benzene#ethylene Reaction in Near-Critical RegionsDulce GradillaNo ratings yet
- Enzymes PresDocument34 pagesEnzymes PresRasmia SalamNo ratings yet
- Chemical Kinetics TestDocument5 pagesChemical Kinetics Testrajneesh kumarNo ratings yet
- Buyon Guo HF Dan Acid Matrix AcidizingDocument22 pagesBuyon Guo HF Dan Acid Matrix AcidizingFenisa SainyakitNo ratings yet
- 201B Work 1 KineticsDocument9 pages201B Work 1 Kineticsahraz93No ratings yet
- Fluid-Solid Non-Catalytic Reaction: Kinetics: Lecture OnDocument15 pagesFluid-Solid Non-Catalytic Reaction: Kinetics: Lecture OnshubhamNo ratings yet
- Vinyl AcetateDocument13 pagesVinyl AcetateRahmahPuspitaSariNo ratings yet
- UAS Tiur Kinkat 2018-2019Document4 pagesUAS Tiur Kinkat 2018-2019Reva MeliyanaNo ratings yet
- Flashcards - Topic 5 Enzymes - CAIE Biology IGCSEDocument25 pagesFlashcards - Topic 5 Enzymes - CAIE Biology IGCSEJane LeaNo ratings yet
- Complied ABC PTest PDFDocument54 pagesComplied ABC PTest PDFanon_281694614100% (1)
- Cre-07 - (2017) - 2Document67 pagesCre-07 - (2017) - 2muhammad shahadat awanNo ratings yet
Kinetics PDF
Kinetics PDF
Uploaded by
Aliza0 ratings0% found this document useful (0 votes)
24 views1 pageDoubling the concentration or pressure of particles means doubling the number of particles per unit volume. This leads to more frequent collisions between particles as there are more opportunities for collisions. The increased collision frequency means a higher proportion of particles will collide with the required activation energy for a reaction to occur, resulting in a faster reaction rate. The Maxwell-Boltzmann distribution curve shifts to the right and has a greater area under it, reflecting the increased number of high-energy collisions.
Original Description:
Original Title
Kinetics.pdf
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentDoubling the concentration or pressure of particles means doubling the number of particles per unit volume. This leads to more frequent collisions between particles as there are more opportunities for collisions. The increased collision frequency means a higher proportion of particles will collide with the required activation energy for a reaction to occur, resulting in a faster reaction rate. The Maxwell-Boltzmann distribution curve shifts to the right and has a greater area under it, reflecting the increased number of high-energy collisions.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
24 views1 pageKinetics PDF
Kinetics PDF
Uploaded by
AlizaDoubling the concentration or pressure of particles means doubling the number of particles per unit volume. This leads to more frequent collisions between particles as there are more opportunities for collisions. The increased collision frequency means a higher proportion of particles will collide with the required activation energy for a reaction to occur, resulting in a faster reaction rate. The Maxwell-Boltzmann distribution curve shifts to the right and has a greater area under it, reflecting the increased number of high-energy collisions.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
Doubling Frequency Total area
concentration/ of successful under the
rate would collisions curve remains
mean double increases the same
Higher the number of Same number
frequency of particles per of particles
successful unit volume
collisions More particles
Emp and mean collide with
Bigger energy both the required
proportion of shift right activation
particles than Collide more energy
the activation frequently
Particles More particles energy
collide more per unit
frequently volume
Wider range To break
Distribution
of energies As the the bonds
shifts to the
than lower right in the temperature
increases the Particles
Greater area temperatures MBD graph must have
under curve particles have
more energy the required
because energy for
there is more a reaction Activation
particles to take
Peak (Emp) energy-
Increasing the place when minimum
stays at the Increasing the
same point
temperature they collide energy needed
but the curve concentration/ for particles
Never meets
the x axis is higher pressure to collide
sucessfully
because there
is no maximum Collision theory
energy for
molecules
Reactions can
Kinetics only occur
when collisions Increases the
Starts at 0 take place
because no
Maxwell rate of reaction
molecules Boltzmann by offering
have no distribution Adding a an alternative
energy Shows the catalyst route with
spread of a lower
energies that Increasing activation
molecules surface area energy
have at a Activation
particular Most probable energy is
temperature energy is Incrases lower so
the peak the rate of more particles
reactions will have Higher
that energy frequency
A few particles of sucessful
Area under
have low collisions
the curve Emp Causes
energy
represents collisions to
because
the number occur more
collisions
of particles frequently
can slow Not the
them down. mean energy Make it smaller Activation
energy
To the right
of the Emp
You might also like
- CML 2017 Marks EH1E PDFDocument7 pagesCML 2017 Marks EH1E PDFAlizaNo ratings yet
- CML 2017 Marks EH3E PDFDocument8 pagesCML 2017 Marks EH3E PDFAlizaNo ratings yet
- Fundamentals of Enzymology The Cell and Molecular Biology of Catalytic ProteinsDocument0 pagesFundamentals of Enzymology The Cell and Molecular Biology of Catalytic Proteinsmonica_elizabeth_35No ratings yet
- CML 2017 Paper EH1E PDFDocument17 pagesCML 2017 Paper EH1E PDFAlizaNo ratings yet
- CatalystsDocument20 pagesCatalystsMargie Reonal LlenoNo ratings yet
- Problem Set ODocument19 pagesProblem Set OnimboNo ratings yet
- Chem2,4-Reaction KineticsDocument3 pagesChem2,4-Reaction KineticsMoeez100% (1)
- 8 Reaction Kinetics: Maxwell Boltzmann DistributionDocument16 pages8 Reaction Kinetics: Maxwell Boltzmann DistributionNoor MuhammadNo ratings yet
- Kinetics - Edexcel A Level ChemistryDocument4 pagesKinetics - Edexcel A Level ChemistryMaddie BaughNo ratings yet
- Light Energy Sound EnergyDocument1 pageLight Energy Sound EnergykenNo ratings yet
- The Periodic Table TrendsDocument2 pagesThe Periodic Table TrendsHannahNo ratings yet
- ChemistryDocument1 pageChemistrycallulafebNo ratings yet
- ChemistryDocument16 pagesChemistryakkay616No ratings yet
- Leii E1Document1 pageLeii E1Viviana CamposNo ratings yet
- Factors Affecting Rate of Reaction Collision TheoryDocument1 pageFactors Affecting Rate of Reaction Collision TheoryMei XiangNo ratings yet
- 6 - 13 FORMS of EnergyDocument30 pages6 - 13 FORMS of EnergyDennis Limosnero MayorNo ratings yet
- Periodicity: ShieldingDocument1 pagePeriodicity: ShieldingjoanaNo ratings yet
- Forms of EnergyDocument13 pagesForms of EnergyVallarasanNo ratings yet
- Edx Ial 词汇手册物理汇总Document27 pagesEdx Ial 词汇手册物理汇总Cai MingleiNo ratings yet
- Mind MapsDocument3 pagesMind Mapsjrolfe477No ratings yet
- % Energy EpDocument1 page% Energy Epgabby fosterNo ratings yet
- Electronic Configuration-1Document15 pagesElectronic Configuration-1Dyutimoy DanNo ratings yet
- MSG - 105 - 139735 - List of AS Level Physics DefinitionsDocument2 pagesMSG - 105 - 139735 - List of AS Level Physics DefinitionsaayushpromasterNo ratings yet
- Mind Map 2: R.Rishaab Karthik MM19B046Document3 pagesMind Map 2: R.Rishaab Karthik MM19B046Hardy SPNo ratings yet
- Transfer of Thermal EnergyDocument1 pageTransfer of Thermal EnergyShakilNo ratings yet
- EnergyDocument1 pageEnergyapi-484459862No ratings yet
- Article Careers360 20230527174852Document25 pagesArticle Careers360 20230527174852naan singhNo ratings yet
- Nuclear Physics PDFDocument17 pagesNuclear Physics PDFsafi mohammadNo ratings yet
- SPMRSM Paper 2 2015Document37 pagesSPMRSM Paper 2 2015Natasha BalqisNo ratings yet
- Edexcel Knowledge Mat Topic 8 Energy Forces Doing Work V1 2Document4 pagesEdexcel Knowledge Mat Topic 8 Energy Forces Doing Work V1 2NetkoNo ratings yet
- Physics Definition by Vasumitra GajbhiyeDocument3 pagesPhysics Definition by Vasumitra GajbhiyeHuzaifa ImranNo ratings yet
- 02 First Law of ThermodynamicDocument22 pages02 First Law of ThermodynamicYahya Alhaddi KA201 18No ratings yet
- Nuclear Physics A2Document15 pagesNuclear Physics A2uditi kalraNo ratings yet
- Cascayo, Debelyn S. Beed 3-A (Learning Task 5)Document1 pageCascayo, Debelyn S. Beed 3-A (Learning Task 5)Debelyn CascayoNo ratings yet
- Mass Defect and Binding EnergyDocument1 pageMass Defect and Binding EnergyMichelle nananaNo ratings yet
- Unit 3 ReviewDocument9 pagesUnit 3 ReviewMadi WildeNo ratings yet
- Read To LearnDocument5 pagesRead To LearnosamaNo ratings yet
- 4.4 Chemical KineticsDocument14 pages4.4 Chemical KineticsDamia AziziNo ratings yet
- IB Physics Definitions Needed by The Syllabus of 2009Document2 pagesIB Physics Definitions Needed by The Syllabus of 2009mohsin771100% (1)
- Hand Written Note by DP SIR For Quick RevisionDocument7 pagesHand Written Note by DP SIR For Quick RevisionKishori PatilNo ratings yet
- Mind Map 09-09-2021: Hardhik Pinjala - MM19B043 - September 15, 2021Document2 pagesMind Map 09-09-2021: Hardhik Pinjala - MM19B043 - September 15, 2021Hardy SPNo ratings yet
- Actividad de Sienciea Cuadro 7X7Document1 pageActividad de Sienciea Cuadro 7X7luz gilNo ratings yet
- 3.1 Internal Energy Mind MapDocument1 page3.1 Internal Energy Mind MapAndres LopezNo ratings yet
- Detailed Explanations For TrendsDocument2 pagesDetailed Explanations For TrendsBRITNEY ALEXANDRA VERMINSHUNo ratings yet
- Heat of Fusion: Index Phase Change ConceptsDocument3 pagesHeat of Fusion: Index Phase Change Conceptsscsa31619No ratings yet
- Bond Graph - WikipediaDocument16 pagesBond Graph - WikipediaRanjit KumarNo ratings yet
- سيبلس يبلس يبلسيبلس يبلسيبلDocument8 pagesسيبلس يبلس يبلسيبلس يبلسيبلAsmaa AdelNo ratings yet
- HK Physics DefinitionsDocument3 pagesHK Physics DefinitionsMNRNo ratings yet
- Intro 2 Molecular Modelling & Molecular MechanicsDocument35 pagesIntro 2 Molecular Modelling & Molecular Mechanicsachsanuddin50% (2)
- Particle Interactions - FactRecallDocument2 pagesParticle Interactions - FactRecallHarpritNo ratings yet
- Various Forms of EnergyDocument2 pagesVarious Forms of EnergyRhianna TetlowNo ratings yet
- Statics of ParticlesDocument35 pagesStatics of ParticlesEmmanuel C. Dimpas Jr.No ratings yet
- Note 18-Apr-2024 at 11 - 00 - 26 PMDocument10 pagesNote 18-Apr-2024 at 11 - 00 - 26 PMMurtaza AbbasNo ratings yet
- Metabolism-Note Oct 11, 2023Document7 pagesMetabolism-Note Oct 11, 2023Saumya ThakkerNo ratings yet
- CH 2 Statics of ParticlesDocument35 pagesCH 2 Statics of ParticlesMuhd HafidzNo ratings yet
- Conservation and Dissipation of EnergyDocument2 pagesConservation and Dissipation of EnergyceryssandfordNo ratings yet
- NoteDocument7 pagesNoteRosina KaneNo ratings yet
- Classical Thermo NotesDocument37 pagesClassical Thermo NotesVirendra SheoranNo ratings yet
- Park 1996Document12 pagesPark 1996Muhammad Ali AbroNo ratings yet
- DefinitionsDocument7 pagesDefinitionsdevasanisaiteja2006No ratings yet
- BiologyDocument3 pagesBiologycarliehtcheungNo ratings yet
- Bond GraphDocument15 pagesBond GraphtafazulijazNo ratings yet
- Heat and Energy: Scope-UnactstandingDocument21 pagesHeat and Energy: Scope-Unactstandingb9835143No ratings yet
- CML 2017 Paper EH3E PDFDocument20 pagesCML 2017 Paper EH3E PDFAlizaNo ratings yet
- CML 2017 Marks EH2E PDFDocument8 pagesCML 2017 Marks EH2E PDFAlizaNo ratings yet
- CML 2017 Paper EH2E PDFDocument19 pagesCML 2017 Paper EH2E PDFAlizaNo ratings yet
- Och2016 17 PDFDocument28 pagesOch2016 17 PDFPayal SharmaNo ratings yet
- Review Packet - Teacher VersionDocument77 pagesReview Packet - Teacher VersionJADFNo ratings yet
- AP Chemistry Free Response by TopicssDocument32 pagesAP Chemistry Free Response by TopicssVincent Zw Liu0% (1)
- GBPUAT SyllabusDocument11 pagesGBPUAT SyllabusMahesh ShahNo ratings yet
- Chemical Kinetics PYQDocument9 pagesChemical Kinetics PYQAmresh MohantyNo ratings yet
- FCH - ChE 331A - JAN - 2019Document2 pagesFCH - ChE 331A - JAN - 2019Abhinav ShuklaNo ratings yet
- Lab 5 - Chemical KineticsDocument3 pagesLab 5 - Chemical KineticsFranck MomoNo ratings yet
- 2017 Y5 T4 Chem Focus - KineticsDocument4 pages2017 Y5 T4 Chem Focus - KineticsxmxmxmxmxmNo ratings yet
- Reaction Technologies: ThreeDocument31 pagesReaction Technologies: ThreeProcess EngineerNo ratings yet
- Advances in Chemical Engineering Vol 11, Volume 11 (Thadvane in Chemicalomas B. Drew) (9780120085118) (Academic Press - 1981)Document469 pagesAdvances in Chemical Engineering Vol 11, Volume 11 (Thadvane in Chemicalomas B. Drew) (9780120085118) (Academic Press - 1981)siavashdm1100% (1)
- Chem U4 EdexcelDocument55 pagesChem U4 EdexcelReez SinhaNo ratings yet
- Catalyst DefinitionDocument17 pagesCatalyst DefinitionTRIBRATA BASKORONo ratings yet
- MM Kinetics No.4 PDFDocument6 pagesMM Kinetics No.4 PDFCarlos MontúfarNo ratings yet
- Chemistry PGDocument33 pagesChemistry PGhrishikeshanandNo ratings yet
- Runaway ReactionsDocument5 pagesRunaway ReactionsDaniel SantosNo ratings yet
- Mark Scheme (Results) January 2008: GCE Chemistry (6242) Paper 1Document11 pagesMark Scheme (Results) January 2008: GCE Chemistry (6242) Paper 1Chong Cheng NamNo ratings yet
- Kinetics For Benzene#ethylene Reaction in Near-Critical RegionsDocument8 pagesKinetics For Benzene#ethylene Reaction in Near-Critical RegionsDulce GradillaNo ratings yet
- Enzymes PresDocument34 pagesEnzymes PresRasmia SalamNo ratings yet
- Chemical Kinetics TestDocument5 pagesChemical Kinetics Testrajneesh kumarNo ratings yet
- Buyon Guo HF Dan Acid Matrix AcidizingDocument22 pagesBuyon Guo HF Dan Acid Matrix AcidizingFenisa SainyakitNo ratings yet
- 201B Work 1 KineticsDocument9 pages201B Work 1 Kineticsahraz93No ratings yet
- Fluid-Solid Non-Catalytic Reaction: Kinetics: Lecture OnDocument15 pagesFluid-Solid Non-Catalytic Reaction: Kinetics: Lecture OnshubhamNo ratings yet
- Vinyl AcetateDocument13 pagesVinyl AcetateRahmahPuspitaSariNo ratings yet
- UAS Tiur Kinkat 2018-2019Document4 pagesUAS Tiur Kinkat 2018-2019Reva MeliyanaNo ratings yet
- Flashcards - Topic 5 Enzymes - CAIE Biology IGCSEDocument25 pagesFlashcards - Topic 5 Enzymes - CAIE Biology IGCSEJane LeaNo ratings yet
- Complied ABC PTest PDFDocument54 pagesComplied ABC PTest PDFanon_281694614100% (1)
- Cre-07 - (2017) - 2Document67 pagesCre-07 - (2017) - 2muhammad shahadat awanNo ratings yet