Professional Documents
Culture Documents
The Cerebral Circulation: Edward Moss MD FRCA
The Cerebral Circulation: Edward Moss MD FRCA
Uploaded by
Pishulotote LopezOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
The Cerebral Circulation: Edward Moss MD FRCA
The Cerebral Circulation: Edward Moss MD FRCA
Uploaded by
Pishulotote LopezCopyright:
Available Formats
The cerebral circulation
Edward Moss MD FRCA
The cerebral circulation is arguably the most superficial cerebral veins is 2–4 mmHg high-
important in the body because arrest of the er than the intracranial pressure (ICP) to Key points:
circulation for 5 min can cause neuronal ensure venous outflow from the brain. Thus, Neurogenic mechanisms
death. In order to optimise the delivery of ICP is the closest measurable pressure to cere- play a major role in con-
trol of CBF
oxygen and metabolic substrates to the brain, bral venous pressure and the best estimate for
Anaesthetic and intensive
anaesthetists need a clear understanding of the cerebral perfusion pressure (CPP) is mean care interventions may
anatomy of the cerebral circulation and CSF arterial pressure (MAP) minus ICP (CPP = compromise cerebral
pathways, the physiology of the cerebral cir- MAP – ICP). perfusion pressure
culation and the effects of anaesthesia, inten- An adequate cerebral
CSF pathways perfusion pressure is
sive care and other therapeutic interventions
The CSF pathways form a second circulation essential after cerebral
on the cerebral circulation. insults
in the brain and are a major determinant of
Excessive hyperventila-
Anatomy ICP. CSF is mainly produced by active secre-
tion should be avoided
tion, involving Na+/K+-ATPase and carbonic
Mild hypothermia (34°C)
Arterial supply anhydrase, from the choroid plexus in the lat- protects against cerebral
The arterial supply of the brain arises from the eral and third ventricles at a rate of about 0.4 ischaemia
internal carotid arteries (70%) and the verte- ml.min–1. From here, it circulates through the
brobasilar system (30%). The internal carotid fourth ventricle and emerges through the
arteries give rise to the anterior and middle foramina of Luschka and Magendie into the
cerebral arteries on each side and the basilar cerebellar cisterns. It passes over the surface
artery divides into the two posterior cerebral of the cerebral hemispheres and is re-
arteries. The anterior communicating artery absorbed through the arachnoid villi into the
joins the two anterior cerebral arteries and a
posterior communicating artery runs from the
posterior cerebral artery to the internal carotid anterior anterior cerebral arteries
on each side. This anastomosis forms the communicating
Circle of Willis at the base of the brain (Fig. artery
1). The anterior cerebral arteries supply most
middle cerebral
of the medial part of the cerebral hemispheres, carotid
posterior arteries
the middle cerebral arteries supply most of the communicating arteries
lateral sides of the hemispheres and the poste- arteries
Posterior
rior cerebral artery supplies the occipital and cerebral
inferior parts of the temporal lobes. There are arteries
extensive anastomoses between the individual basilar artery
vessels.
Edward Moss
Venous drainage MD FRCA
vertebral arteries Consultant Neuroanaesthesist,
The venous drainage is from the superficial Department of Anaesthesia,
Leeds General Infirmary,
cerebral veins to the dural sinuses which drain Great George Street,
into the jugular bulbs. The pressure in the Fig. 1 Diagram of the Circle of Willis. Leeds LS1 3EX, UK
British Journal of Anaesthesia | CEPD Reviews | Volume 1 Number 3 2001 67
© The Board of Management and Trustees of the British Journal of Anaesthesia 2001
The cerebral circulation
dural sinuses. Under normal circumstances, ICP will remain works through a combination of metabolic (most effective at
stable (normal value 5–15 mmHg), despite changes in the vol- low CPPs) and myogenic (effective at high CPPs) factors.
ume of blood or brain tissue, due to re-absorption of CSF. There may also be some neurogenic control of autoregulation.
When the compensatory mechanisms are exhausted, ICP will Autoregulation is impaired by disease processes, such as head
increase. This is because the skull is a closed box and an injury or cerebrovascular accidents, and by drugs that cause
increase in the volume of one of its contents must be com- cerebral vasodilatation, such as volatile anaesthetic agents or
pensated by a decrease in the volume of another for ICP to glyceryl trinitrate. The limits of autoregulation are shifted to
remain constant. the right in chronic hypertension and are usually restored to
normal when the hypertension is well controlled. Return to
Blood-brain barrier normal limits may not occur if there have been permanent
structural changes in the vessels due to long-standing hyper-
The blood-brain barrier (BBB) prevents any free diffusion of
tension or in the elderly.
blood-borne substances into the brain parenchymal space. It is
due to the endothelial cells lining the vascular wall in the brain Chemical control
being tightly linked with junctional complexes that eliminate
Chemical control includes the influence of changes in hydro-
gaps between cells. Therefore, the endothelial cells of the
gen ion concentrations in the brain due to metabolic activity
brain play a critical role in performing essential biological
and changes in arterial PCO2 and PO2. Changes in PaCO2
functions including transport of micro- and macro-nutrients,
cause alterations in CBF with a linear response in the physio-
receptor-mediated signalling, leukocyte trafficking and
logical range of 30% per kPa (Fig. 2). There is no further
osmoregulation. A number of molecular proteins responsible
increase in CBF above 10.6 kPa due to maximal vasodilata-
for some of these unique properties have now been identified
tion. There is little further reduction in CBF below 3.5 kPa
showing that the brain endothelium is a complex and dynam-
and no further decrease below 2.6 kPa. There is a threshold
ic biological system rather than an inert barrier. These include
response to changes in PaO2 with no change in CBF until 7.5
proteins involved in the formation and assembly of tight junc-
kPa below which there is a dramatic increase in CBF. This is
tions, plasma membrane-embedded proteins responsible for
explained by the shape of the oxyhaemoglobin dissociation
transport of brain energy substrates and nutrients, the multi-
curve because CBF shows a linear response to changes in arte-
drug transporter protein, p-glycoprotein and other drug-reject-
rial oxygen content and a PaO2 of 7.5 kPa corresponds to the
ing proteins that protect the brain from foreign chemicals. The
beginning of the steep part of the curve. Breathing 100% oxy-
BBB acts as a semipermeable membrane and is effectively an
gen causes a small (~10%) reduction in CBF.
osmometer. Therefore, hypotonic fluids, such as Hartmann’s
solution, will cause an increase in brain water.
autoregulation
carbon dioxide
Control of cerebral blood flow (CBF) CBF oxygen
ml /100G /min metabolism
CBF is controlled by four main mechanisms: autoregulation,
chemical, metabolic and neurogenic factors (Fig. 2). The 100
healthy new-born and very preterm respond to physiological
stimuli in the same manner as the mature organism but, as in
the adult, pathological states may impair these responses. The 50
spinal cord also consists of neurones and it has been shown
that spinal cord blood flow is controlled in a similar manner
to CBF.
Autoregulation 50 100 150 mmHg
2.5 5 7.5 10 kPa
Autoregulation keeps cerebral blood flow (CBF) constant CMR
between MAP values of 60–150 mmHg (Fig. 2), or CPP val-
ues of 50–140 mmHg, in normotensive patients and probably Fig. 2 Factors affecting cerebral blood flow.
68 British Journal of Anaesthesia | CEPD Reviews | Volume 1 Number 3 2001
The cerebral circulation
Changes in cerebral metabolic rate latent regulatory mechanism which only becomes important
Changes in cerebral metabolic rate (CMR) will cause changes under conditions of stress.
in CBF. However, under normal circumstances, local CBF Sympathetic nerves protect CBF and blood brain barrier func-
may change but global flow remains constant. This is because, tion during hypertension and hypoxaemia and have trophic
as one region of the brain becomes more active, another effects on cerebral vessels. The role of parasympathetic nerves is
region usually becomes less active and there is diversion of less well defined but they do contribute to cerebrovascular
blood flow from one region to another. However, any factor dilatation in several pathological conditions including
that causes a global increase in CMR will cause an increase in ischaemia/reperfusion. Trigemino-vascular fibres appear to be
CBF. These factors include pyrexia, convulsions and the use involved in cerebrovascular dilatation during post-ischaemia
of analeptic drugs. Factors such as hypothermia, coma and reperfusion, post-seizure hyperaemia, cortical spreading depres-
anaesthesia decrease CMR and CBF (Fig. 2). sion and arterial hypotension. They may have a protective role
against vasospasm in subarachnoid haemorrhage.
Neurogenic factors
Blood rheology
According to traditional teaching, neurogenic factors have lit-
tle influence on control of CBF. However, over recent years, Blood rheology has an effect on CBF and the optimum
it has become clear that they have a major effect. The cerebral haematocrit to balance improvement in flow and oxygen car-
blood vessels have a very rich innervation and the axons of rying capacity is about 30%. Reduction of the haematocrit to
these nerves contain a variety of neurotransmitters. It is diffi- 30% causes an increase in CBF velocity (CBFV) in the mid-
cult to explain their presence if they are not involved in cere- dle cerebral artery of about 20%. The change in CBFV is
brovascular control. In addition, it has become clear that the approximately 2% for each 1% decrease in haematocrit and
metabolic theory of Roy and Sherrington (1890) does not arterial oxygen content. However, this increase in CBF does
completely explain the adjustment of CBF to metabolic needs. not improve oxygen transport or tissue oxygen delivery.
The increases in CBF may be out of proportion to metabolic The cerebral circulation is particularly susceptible to vascu-
demands, may occur without significant change in local lar steal because of the major role that the large arteries play
metabolism and may be much faster than the accumulation of in the regulation of the vascular resistance in the brain.
metabolic end-products. Therefore, it is likely that neurogenic However, focal increases in blood flow in one region of the
stimuli act to produce rapid adjustment of CBF to metabolic brain cause flow-mediated vasodilatation of large arteries
demands and that metabolic and chemical factors are respon- upstream. This phenomenon is particularly well developed in
sible for maintaining the changes. the cerebral circulation.
Sympathetic fibres, which cause vasoconstriction, originate
in the superior cervical ganglion and the stellate ganglion with Ischaemic thresholds
norepinephrine, serotonin and neuropeptide Y as neurotrans- CBF is normally 50 ml 100 g–1 min–1 when the MAP is in the
mitters. Parasympathetic fibres, which cause vasodilatation, autoregulatory range but below an MAP of 60 mmHg, CBF
originate from the sphenopalatine ganglion, the internal passively follows MAP. At approximately 20 mmHg in nor-
carotid mini-ganglion and the otic ganglion with acetyl motensive individuals, the CBF is 20–25 ml 100 g–1 min–1 and
choline, vasoactive intestinal polypeptide and nitric oxide as there is a change in cerebral electrical activity. Electrical
neurotransmitters. The trigemino-vascular fibres arise from activity is lost when the MAP is approximately 15 mmHg
the first division of the trigeminal ganglion and other sensory with a CBF of about 15 ml 100 g–1 min–1 and ionic home-
innervation arises from somato-sensory pathways relaying in ostasis is lost at about 10 mmHg when CBF is 10
the thalamus. The sensory innervation causes vasodilatation ml 100 g–1 min–1. The watershed zones at the periphery of the
and the transmitters include substance P, calcitonin gene relat- supply from the major vessels are particularly vulnerable to
ed peptide, cholecystokinin and neurokinin A. There are also ischaemia, but collaterals do exist and animal studies show
opioid receptors on the cerebral vessels and activation of these normalisation of flow 1 month after occlusion of the middle
receptors modulates the function of the other vasoregulatory cerebral artery due to an increase in the luminal diameter of
mechanisms. It seems that the endogenous opioid system is a collaterals.
British Journal of Anaesthesia | CEPD Reviews | Volume 1 Number 3 2001 69
The cerebral circulation
Table 1 Cerebral circulatory effects of anaesthetic agents movements associated with etomidate which act by increasing
CBF ICP CMRO2 CSF Auto- CO2 CVP. An increase in CVP will increase ICP by two mecha-
production regulation reactivity nisms: (i) a reduction in cerebral venous drainage; and (ii) an
i.v. Anaesthetics
Thiopentone D D D N N N
increase in the pressure in the valveless epidural veins squeez-
Etomidate D D D D N N ing CSF from the vertebral canal into the cranium.
Propofol D D D N I N
Ketamine Ia Ia Ia N N N Anaesthetic agents may also have an effect on the rate of pro-
Midazolam D D D N N N duction of CSF and their effects are summarised in Table 1.
Inhaled agents
Nitrous oxide I I I N N N Bolus doses of the potent short-acting opioids may cause an
Halothane I I D N Impaired N increase in ICP secondary to acute cardiovascular effects, but
Enflurane I I D I Impaired N
Isoflurane I I D N Impaired I this does not occur with infusions. The effects of drugs on the
Sevoflurane I I D N Impaired N cerebral circulation may be modified by co-administration
Desflurane I I N N Impaired N
Xenon I I ? ? ? ? with other agents or by cerebral pathology.
Opioid analgesics (ventilation controlled)
Morphine N N N N N N Anaesthetic and intensive care interventions
Fentanyl N N N N N N
Alfentanil D D (bolus I) ?D N N N Anaesthetic and intensive care interventions may affect the
Remifentanil D D ?D N N N
Opioid analgesics (spontaneous ventilation) cerebral circulation and increase ICP. Laryngoscopy, intuba-
All opioids I I ?N N N N
Muscle relaxants
tion and extubation cause a sudden increase in MAP that tem-
Suxamethonium I I ?I N N N porarily increases CBF until autoregulation takes effect.
Non-depolarising N N N N N N
Diuretics Hypoxia and hypercarbia both increase CBF (see above).
Mannitol Nc D N D N N Interventions that increase intrathoracic pressure such as
Frusemide N D N D N N
Others IPPV, PEEP, coughing and straining will increase ICP by
Hydralazine I I N N Impaired N increasing central venous pressure as described above.
Glyceryl trinitrate I I N N Impaired N
Sodium nitroprusside I I N N Impaired N Posture may also increase ICP. The prone position will
Nimodipine I I N N N N increase the central venous pressure. Neck rotation can kink
Lignocaine D D Nb N N N
α2-Agonists D D ? ? ? ? the internal jugular veins and obstruct the cerebral venous
Anticholinergics N N N N N N
Anticholinesterases N N N N N N
drainage. The head-down position will also reduce cerebral
venous drainage. Emergence from anaesthesia and sedation is
D, decreased; I, increased; N, no effect; ?, unknown. associated with an increase in cerebral metabolic rate, CBF
a
Effects modified by pretreatment with sedatives.
b
Decreases with large doses. and ICP. Alterations in CPP can affect CBF, particularly if
c
Increases initially.
CO2 reactivity is the slope of the graph relating CBF to changes in
cerebral autoregulation is impaired.
arterial carbon dioxide tension.
Therapeutic considerations
Effects of anaesthesia and intensive care on Hyperventilation
the cerebral circulation Hyperventilation has long been used therapeutically to reduce
ICP in patients with head injury and other intracranial
Anaesthetic agents pathologies, but a recent trial has confirmed that excessive
Anaesthetic agents may increase, decrease or have no effect reduction in PaCO2 can be harmful. It is now generally
on CBF. Agents that cause changes in CBF may do so by accepted that, in head injured patients, hyperventilation
direct effects on the blood vessels, by changing cerebral meta- should be limited to that required to produce a PaCO2 of
bolic rate or by causing respiratory depression and an increase 3.5–4.0 kPa. Similar considerations apply to anaesthesia for
in PaCO2. Changes in CBF cause corresponding changes in operations on the cerebral vasculature because, in some
cerebral blood volume and an increase in ICP. Anaesthetics patients undergoing surgical treatment of ruptured aneurysms,
may increase ICP by other mechanisms, such as the muscle it is impossible to achieve normal cerebral oxygenation whilst
fasciculations caused by suxamethonium, or the involuntary the patient is hypocapnic.
70 British Journal of Anaesthesia | CEPD Reviews | Volume 1 Number 3 2001
The cerebral circulation
Manipulation of CPP Impaired endothelium-dependent relaxation, production of
Manipulation of CPP is used in several clinical situations in endothelium derived constricting factors, including endothe-
an attempt to improve patient outcomes. It is now recom- lin, and impaired activity of potassium channels in the cere-
mended that MAP is artificially raised for a short period after bral blood vessels may all contribute to the vasospasm. At
the restoration of spontaneous circulation following cardiac present, the only effective treatment is to increase CBF by
arrest and it is customary to maintain a CPP of at least 70 hypervolaemic haemodilution with or without induced hyper-
mmHg during the intensive care management of severe head tension. Hypertensive, hypervolaemic haemodilution has
injury. In the presence of normal autoregulation, an increase been termed ‘Triple H’ therapy and, before it is commenced,
in CPP may cause cerebral vasoconstriction and a reduction in it is essential to exclude other causes of neurological deterio-
ICP that further increases cerebral perfusion pressure and ration such as intracranial haematoma, cerebral infarction or
reduces ICP. This has been termed the vasoconstriction cas- hydrocephalus by performing a CT scan. This treatment
cade. Conversely, a reduction in CPP will cause cerebral should be limited to hypervolaemic haemodilution in the pres-
vasodilatation which will further increase ICP and decrease ence of an unclipped aneurysm. If hypertension is used, it
CPP leading to further vasodilatation and a vicious cycle that should initially be limited to a trial period of 6 h. If ineffec-
has been termed the vasodilation cascade. If autoregulation is tive after 6 h, treatment should revert to hypervolaemic
impaired, an increase in MAP will lead to an increase in CBF haemodilution alone because induction of hypertension may
and ICP and possibly cause cerebral oedema. cause cardiac complications.
Hypothermia
Key references
Hypothermia reduces cerebral electrical activity and depress-
Black S, Michenfelder JD. Cerebral blood flow and metabolism. In:
es the metabolic processes required to maintain the integrity Cucchiara RF, Black S, Michenfelder JD. (eds) Clinical Neuroanaesthesia.
of the neurones. Animal work has shown that a reduction of New York: Churchill Livingstone, 1998; 1–40
body temperature by as little as 2 or 3°C has some cerebral Dearden NM, Fale AD. Medical management of head injury and neuro-
protection. In: Moss E, Ellis FR. (eds) Baillières Best Practice and Research,
protective effects. Consequently, mild hypothermia (34°C) is Clinical Anaesthesiology, Neuroanaesthesia, vol. 13. London: Baillière
commonly used during clipping of cerebral aneurysms and Tindall, 1999; 659–73
there has been renewed interest in its use in the intensive care Drewes LR. What is the blood-brain barrier? A molecular perspective.
management of severe head injuries. Cerebral vascular biology. Adv Exp Med Biol 1999; 474: 111–22
Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of
Cerebral vasospasm endothelium and potassium channels. Physiol Rev 1998; 78: 53–97
Fitch W. Physiology of the cerebral circulation. In: Moss E, Ellis FR. (eds)
Cerebral vasospasm is a frequent complication following Baillières Best Practice and Research, Clinical Anaesthesiology, Neuro-
aneurysmal subarachnoid haemorrhage thought to be caused anaesthesia, vol. 13. London: Baillière Tindall, 1999; 487–98
by the breakdown products of blood in the subarachnoid Roy CS, Sherrington CS. On the regulation of the blood supply of the brain.
space. It is most frequent 3–10 days after the haemorrhage J Physiol 1890; 11: 85–108
and it is more severe with a larger blood load in the CSF. See multiple choice questions 38–42.
British Journal of Anaesthesia | CEPD Reviews | Volume 1 Number 3 2001 71
You might also like
- Arc Guideline 11 1 Introduction To and Principles of in Hospital Resuscitation Feb 2019Document22 pagesArc Guideline 11 1 Introduction To and Principles of in Hospital Resuscitation Feb 2019hernandez2812No ratings yet
- Case Study 8: Coronary Artery DiseaseDocument10 pagesCase Study 8: Coronary Artery Diseaseintrovoyz041100% (3)
- Case Studies in Clinical Cardiac Electrophysiology (PDFDrive)Document432 pagesCase Studies in Clinical Cardiac Electrophysiology (PDFDrive)aafagih100% (1)
- 12 LeadDocument7 pages12 Leadjerry3gowenNo ratings yet
- NUR3111 Past Paper Care PlanDocument14 pagesNUR3111 Past Paper Care PlanliNo ratings yet
- Fisiología CerebralDocument6 pagesFisiología CerebralLupillo Losil LopezNo ratings yet
- Cerebral MtabolismDocument6 pagesCerebral Mtabolismdpeka dpekaNo ratings yet
- Cerebral Circulation 1 Anatomy 2021 BjaeDocument6 pagesCerebral Circulation 1 Anatomy 2021 BjaeCarlos AguaguiñaNo ratings yet
- Cerebral Circulation & Auto-RegulationDocument38 pagesCerebral Circulation & Auto-Regulationsalah200No ratings yet
- Cerebral Pressure Autoregulation in Brain Injury and DisorderseA Review On Monitoring, Management, and Future DirectionsDocument14 pagesCerebral Pressure Autoregulation in Brain Injury and DisorderseA Review On Monitoring, Management, and Future DirectionsAnonymous QLadTClydkNo ratings yet
- Common Neurologic Disorders: Krista M. GarnerDocument12 pagesCommon Neurologic Disorders: Krista M. GarnerTeda G.No ratings yet
- Regulasi Aliran Darah Cerebral Dan Aneurisma CerebralDocument12 pagesRegulasi Aliran Darah Cerebral Dan Aneurisma CerebralchandradwtrNo ratings yet
- Intracraneanal PressureDocument8 pagesIntracraneanal PressurearturoNo ratings yet
- WrightSinclair-CSFandLPpracticalreview 2012JNeuroDocument19 pagesWrightSinclair-CSFandLPpracticalreview 2012JNeuroIoana CucuNo ratings yet
- CC, BBB, CM - FinalDocument6 pagesCC, BBB, CM - FinalpurletpunkNo ratings yet
- Monro Kellie Doctrine/ PrincipleDocument5 pagesMonro Kellie Doctrine/ PrincipleAngelu Gabrielle CastroNo ratings yet
- Blood Supply of The Brain and Spinal CordDocument10 pagesBlood Supply of The Brain and Spinal CordJennima PaulsamyNo ratings yet
- Cerebrovascular AccidentDocument6 pagesCerebrovascular AccidentRodel CamposoNo ratings yet
- Anatomy and Physiology: Spinal CordDocument4 pagesAnatomy and Physiology: Spinal CordOliver C. PagkalinawanNo ratings yet
- Arterial Blood Supply of The BrainDocument27 pagesArterial Blood Supply of The BrainFirdaus SeptiawanNo ratings yet
- Cerebral Blood Flow and Pathophysiology: The Vascular SystemDocument3 pagesCerebral Blood Flow and Pathophysiology: The Vascular SystemJerome ReyNo ratings yet
- Cerebral Blood FlowDocument51 pagesCerebral Blood FlowAiswarya Wilson100% (1)
- Peggy Mason Chapter 8 Blood Suppply and Blood Brain BarrierDocument24 pagesPeggy Mason Chapter 8 Blood Suppply and Blood Brain BarrierLydia RousseauNo ratings yet
- Blood Supply of The Brain and Spinal CordDocument10 pagesBlood Supply of The Brain and Spinal CordJennima PaulsamyNo ratings yet
- Neurologic Conditions 4 StrokeDocument4 pagesNeurologic Conditions 4 StrokeEdward De LeonNo ratings yet
- Cerebrovasular DiseasesDocument65 pagesCerebrovasular DiseasesIfabiyi OlaniyiNo ratings yet
- Circulation and The Central Nervous SystemDocument6 pagesCirculation and The Central Nervous SystemRhoda CatamoraNo ratings yet
- Session 11 Cerebral Blood FlowDocument28 pagesSession 11 Cerebral Blood FlowParth ShahNo ratings yet
- Cerebrospinal Fluid and Lumbar Puncture: A Practical ReviewDocument16 pagesCerebrospinal Fluid and Lumbar Puncture: A Practical ReviewVictoria AlejandraNo ratings yet
- Pi Is 1472029919302590Document2 pagesPi Is 1472029919302590Praneeth KumarNo ratings yet
- Middle Cerebral Artery - PhysiopediaDocument1 pageMiddle Cerebral Artery - Physiopedia6gydhn8ydhNo ratings yet
- Cerebral CirculationDocument25 pagesCerebral Circulation20AR018 HARIHARA SUBRAMANIANNo ratings yet
- HEAD INJURIES and ICP SCI STROKEDocument20 pagesHEAD INJURIES and ICP SCI STROKENicole Anne ValerioNo ratings yet
- Detección de Isquemia Focal en HSADocument9 pagesDetección de Isquemia Focal en HSAWaldemar PiñaNo ratings yet
- Cerebral Circulation Anatomy and PhysiologyDocument5 pagesCerebral Circulation Anatomy and PhysiologyMayrjun Lo JacosalemNo ratings yet
- Liquido CerebroespinalDocument28 pagesLiquido Cerebroespinalnataly yañezNo ratings yet
- Chapter 14 The Brain Cranial Nerves2Document21 pagesChapter 14 The Brain Cranial Nerves2Aysha AishaNo ratings yet
- Mechanisms Involved in Regulation of Systemic Blood PressureDocument6 pagesMechanisms Involved in Regulation of Systemic Blood PressureMumtaz MaulanaNo ratings yet
- Selim Mahmoud Abdel-Hakim: by Professor DRDocument27 pagesSelim Mahmoud Abdel-Hakim: by Professor DRAhmed TarekNo ratings yet
- Cerebral Blood Flow and Intracranial Pressure Update 24 2 2Document6 pagesCerebral Blood Flow and Intracranial Pressure Update 24 2 2Anna ListianaNo ratings yet
- Increased Intracranial Pressure AnyphyDocument8 pagesIncreased Intracranial Pressure AnyphyClemence Morales FloresNo ratings yet
- Self Study On Cerebral Circulation: Medical Surgical NursingDocument19 pagesSelf Study On Cerebral Circulation: Medical Surgical NursingGargi MPNo ratings yet
- Anatomy and Physiology of Intrinsic Cardiac Autonomic Nervous SystemDocument5 pagesAnatomy and Physiology of Intrinsic Cardiac Autonomic Nervous SystemSori B. Requena C.No ratings yet
- Brain Attack Needing ResucitationDocument11 pagesBrain Attack Needing ResucitationCony MaccagnanNo ratings yet
- Physiology of The Cerebrospinal FluidDocument6 pagesPhysiology of The Cerebrospinal FluidShereen Al-ObinayNo ratings yet
- Blood Pressure Regulation HandoutDocument10 pagesBlood Pressure Regulation Handoutsac50900100% (2)
- Cerebral EdemaDocument15 pagesCerebral EdemaMae FlagerNo ratings yet
- Baroreceptor Modulation of The Cardiovascular System, Pain, Consciousness, and CognitionDocument51 pagesBaroreceptor Modulation of The Cardiovascular System, Pain, Consciousness, and CognitionPatoNo ratings yet
- Triad Virchow Basic of Cerebral InjuryDocument16 pagesTriad Virchow Basic of Cerebral Injuryariani putri devantiNo ratings yet
- Brain ICHDocument19 pagesBrain ICHAditya KurniantoNo ratings yet
- Neurology LecturesDocument348 pagesNeurology LecturesRaj DeepakNo ratings yet
- Cerebrovascular DiseasesDocument41 pagesCerebrovascular DiseasesIbtissame BadadNo ratings yet
- Cerebral Spinal Fluid & The MeningesDocument25 pagesCerebral Spinal Fluid & The MeningeschintyamontangNo ratings yet
- Management of Increased Intracranial Pressure in The Critically Ill Child With An Acute Neurological Injury PDFDocument20 pagesManagement of Increased Intracranial Pressure in The Critically Ill Child With An Acute Neurological Injury PDFydtrgnNo ratings yet
- Art. Koroner Penerapan Praktis High Frequency Oscillatory VentilationpdfDocument4 pagesArt. Koroner Penerapan Praktis High Frequency Oscillatory VentilationpdfTri RachmadijantoNo ratings yet
- CvaDocument11 pagesCvaNilo Carmelo LumanogNo ratings yet
- Methodic Materials Ischemic StrokeDocument22 pagesMethodic Materials Ischemic StrokeKapil PancholiNo ratings yet
- Block 4Document68 pagesBlock 4Priya SinghNo ratings yet
- Chapter 40-OxygenationDocument14 pagesChapter 40-OxygenationHaji RajiNo ratings yet
- Cerebral Vein and Dural Sinus ThrombosisDocument32 pagesCerebral Vein and Dural Sinus ThrombosisdwiNo ratings yet
- Arterial Supply To The Brain - Carotid - Vertebral - TeachMeAnatomyDocument4 pagesArterial Supply To The Brain - Carotid - Vertebral - TeachMeAnatomyraihaana turawaNo ratings yet
- Lymphatic Drainage of The Neuraxis in Chronic Fatigue SyndromeDocument7 pagesLymphatic Drainage of The Neuraxis in Chronic Fatigue SyndromeAndrei CalabriaNo ratings yet
- Explain The Physiological Mechanism That Maintain Normal Intracranial PressureDocument25 pagesExplain The Physiological Mechanism That Maintain Normal Intracranial PressureRAFNo ratings yet
- Blood SupplyDocument4 pagesBlood Supplyyash sharmaNo ratings yet
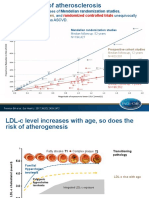
- Comparison of The Efficacy of Rosuvastatin Versus Atorvastatin, Simvastatin, and Pravastatin in Achieving Lipid Goals: Results From The STELLAR TrialDocument11 pagesComparison of The Efficacy of Rosuvastatin Versus Atorvastatin, Simvastatin, and Pravastatin in Achieving Lipid Goals: Results From The STELLAR Trialamit khanNo ratings yet
- Complete Physical Exam AbbreviationsDocument4 pagesComplete Physical Exam Abbreviationseal772328No ratings yet
- CardiomyopathyDocument98 pagesCardiomyopathyZellanien hdNo ratings yet
- Key Lessons On LDL C and CV RiskDocument17 pagesKey Lessons On LDL C and CV RiskSaad KhanNo ratings yet
- Jadwal Sympo Edit1Document3 pagesJadwal Sympo Edit1reyNo ratings yet
- Hospital Visit Case Study 1 PDFDocument3 pagesHospital Visit Case Study 1 PDFShaiel Fabiana Redondo QuinteroNo ratings yet
- Cardiopulmonary ResuscitationDocument82 pagesCardiopulmonary ResuscitationPraveen RadhakrishnanNo ratings yet
- Perioperative Management of Patients Receiving Anticoagulants - UpToDate PDFDocument52 pagesPerioperative Management of Patients Receiving Anticoagulants - UpToDate PDFGustavo MartinezNo ratings yet
- Rivaroxaban in Patients With Atrial Fibrillation and A Bioprosthetic Mitral ValveDocument11 pagesRivaroxaban in Patients With Atrial Fibrillation and A Bioprosthetic Mitral ValveJuan JoseNo ratings yet
- 1985-Cardiac Adaptations To Chronic ExerciseDocument28 pages1985-Cardiac Adaptations To Chronic ExerciseJavier Mora BaizNo ratings yet
- Region City Province LevelDocument49 pagesRegion City Province LevelGlomarie Alyssa RacelisNo ratings yet
- Mitral Stenosis: Dr. Mohammed Asrafur Rahman MBBS, Bcs (H), MD Resident (Internal Medicine) (P-A) Chattogram Medical CollegeDocument16 pagesMitral Stenosis: Dr. Mohammed Asrafur Rahman MBBS, Bcs (H), MD Resident (Internal Medicine) (P-A) Chattogram Medical CollegeAsrafur RahmanNo ratings yet
- Bradycardia AlgorithmDocument1 pageBradycardia AlgorithmGideon BahuleNo ratings yet
- Ebstein's AnomalyDocument7 pagesEbstein's AnomalyRJMNo ratings yet
- Nifedipine-Drug Study 2BSN3Document3 pagesNifedipine-Drug Study 2BSN3Nichole DancelNo ratings yet
- Treatment of Acute Decompensated Heart Failure - Components of TherapyDocument22 pagesTreatment of Acute Decompensated Heart Failure - Components of TherapyYahaira Borquez RiosNo ratings yet
- Propaq MD Defibrillator Product InformationDocument270 pagesPropaq MD Defibrillator Product InformationPoh Yu JianNo ratings yet
- Unclear Outcomes of Heart Rate Variability Following A Concussion: A Systematic ReviewDocument15 pagesUnclear Outcomes of Heart Rate Variability Following A Concussion: A Systematic ReviewMahdi HosseiniNo ratings yet
- WAKO Medical Certificate Kickboxer 2021-v2Document2 pagesWAKO Medical Certificate Kickboxer 2021-v2Bruno AuguštinNo ratings yet
- Heart Failure Guidelines For India Update 2017Document6 pagesHeart Failure Guidelines For India Update 2017Aditya SutarNo ratings yet
- RECOVER Parte 7 Algoritmos y Tabla.Document31 pagesRECOVER Parte 7 Algoritmos y Tabla.Camila Andrea PeñaNo ratings yet
- Basic PrincipleDocument92 pagesBasic PrincipleAde SinagaNo ratings yet
- ER TallyDocument1 pageER TallyMarvinNo ratings yet
- Manual Cardiovascular SystemDocument5 pagesManual Cardiovascular SystemVynz Morales CosepNo ratings yet
- Efektivitas Pemberian Konseling Tentang Diet Dash Terhadap Asupan Natrium, Kalium, Kalsium, Magnesium, Aktivitas Fisik, Dan Tekanan Darah Pasien HipertensiDocument13 pagesEfektivitas Pemberian Konseling Tentang Diet Dash Terhadap Asupan Natrium, Kalium, Kalsium, Magnesium, Aktivitas Fisik, Dan Tekanan Darah Pasien Hipertensiadel fudelNo ratings yet