Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
439 viewsEVAN BLIZZARD - 20-21 PhET Simulation - Molecular Geometry
EVAN BLIZZARD - 20-21 PhET Simulation - Molecular Geometry
Uploaded by
EVAN BLIZZARDThis document provides directions for using an online PhET simulation to explore how the number of atoms vs lone pairs of electrons attached to a central atom affects molecular geometry. It includes examples of 8 hypothetical molecules with varying numbers of single, double, and triple bonds as well as lone pairs. It then applies the concepts demonstrated to analyze the molecular geometry of common substances like water, carbon dioxide, boron trifluoride, ammonia, and methane.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Chem Topic 4 Questions + AnswersDocument25 pagesChem Topic 4 Questions + AnswersOscarHigson-Spence50% (2)
- 6.1 Calculation Sheet Shapes of MoleculesDocument2 pages6.1 Calculation Sheet Shapes of MoleculesRoshNo ratings yet
- Molecular GeometryDocument1 pageMolecular Geometrybooty holeNo ratings yet
- Experiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Document6 pagesExperiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Maldini JosnonNo ratings yet
- Practice Test 7Document65 pagesPractice Test 7The LightNo ratings yet
- 4.4 Geometry and Polarity of MoleculesDocument22 pages4.4 Geometry and Polarity of Molecules1972lewonNo ratings yet
- 3.7 Geometry and Dipole MomentDocument9 pages3.7 Geometry and Dipole Momentelbadry mohamedNo ratings yet
- Molecular Geometry: Thinking of Molecules in 3-DimensionsDocument13 pagesMolecular Geometry: Thinking of Molecules in 3-DimensionsFilza AhmadNo ratings yet
- 3-6 Molecular Geometry SlidesDocument8 pages3-6 Molecular Geometry Slidesapi-240915238No ratings yet
- Molecular Geometry - General Chemistry IIDocument5 pagesMolecular Geometry - General Chemistry IINiobe DismasNo ratings yet
- Molecular Geometry and Bonding TheoriesDocument5 pagesMolecular Geometry and Bonding TheoriesPineraserNo ratings yet
- CHM361 - CHAPTER 1 Valence Bond Theory 2Document57 pagesCHM361 - CHAPTER 1 Valence Bond Theory 2EhazNo ratings yet
- Q2W2 - 2 - Molecular Geometry and Polarity of MoleculesDocument35 pagesQ2W2 - 2 - Molecular Geometry and Polarity of MoleculesEl Jie Ancheta EstelaNo ratings yet
- Molecular Geometry 2Document3 pagesMolecular Geometry 23MshopNo ratings yet
- MOLECULAR GEOMETRY NotesDocument4 pagesMOLECULAR GEOMETRY NotesAshley Mae LumanasNo ratings yet
- Geometry of MoleculesDocument8 pagesGeometry of Moleculesjorel marcoNo ratings yet
- Laboratory Activity 3 - Group 10Document6 pagesLaboratory Activity 3 - Group 10Reinier FrancoNo ratings yet
- 3VSEPR Theory 41-48Document8 pages3VSEPR Theory 41-48Raj KishoreNo ratings yet
- Molecular Shapes: Course Outcome 3Document13 pagesMolecular Shapes: Course Outcome 3tin canNo ratings yet
- Chemical Bonding 1Document99 pagesChemical Bonding 1DeviNo ratings yet
- Chem 106 Lab Report 6Document7 pagesChem 106 Lab Report 6IrynaNo ratings yet
- 3-D Shapes of MoleculesDocument14 pages3-D Shapes of MoleculesZhy MalzanNo ratings yet
- Ch-09-Molecular Geometry and Bonding TheoriesDocument104 pagesCh-09-Molecular Geometry and Bonding TheoriesTrọng NguyễnNo ratings yet
- Molecular GeometryDocument50 pagesMolecular GeometryMnhs MomentsNo ratings yet
- Las 7Document3 pagesLas 7Carl DoriaNo ratings yet
- Shapes of Molecules and Ions PDFDocument9 pagesShapes of Molecules and Ions PDFMagenta SparklegemNo ratings yet
- Hybridisation and Bond AngleDocument13 pagesHybridisation and Bond Angleskye sueNo ratings yet
- Lecture B2Document72 pagesLecture B2Nárēsh Yadav GäddēNo ratings yet
- Draw The Lewis Structure and Name The Shape of Each CompoundDocument9 pagesDraw The Lewis Structure and Name The Shape of Each CompoundJuan Frivaldo100% (1)
- Molecular Geometry and PolarityDocument58 pagesMolecular Geometry and Polaritychristiannnoochoa24No ratings yet
- Molecular Geometry (Vsepr Theory) : For Chemistry 1 Grade 12 Quarter 2 / Week 4Document15 pagesMolecular Geometry (Vsepr Theory) : For Chemistry 1 Grade 12 Quarter 2 / Week 4ariinnggg onichaNo ratings yet
- 4.3 Covalent Structures: IB Chemistry SL Mrs. PageDocument41 pages4.3 Covalent Structures: IB Chemistry SL Mrs. Pageapi-546066323No ratings yet
- 13Document7 pages13Anant MadhavNo ratings yet
- 9 VSEPRTheory PPTDocument37 pages9 VSEPRTheory PPTBlessy MartinNo ratings yet
- Bond Angles and VSEPR TheoryDocument3 pagesBond Angles and VSEPR TheoryTaqeeb AbbasNo ratings yet
- Predicting The Shapes of MoleculesDocument3 pagesPredicting The Shapes of Moleculestommy jimmyNo ratings yet
- General Chemistry 1 Qt. 2 Week 4Document12 pagesGeneral Chemistry 1 Qt. 2 Week 4Nina Reca OmisolNo ratings yet
- VSEPR Theory and HybridizationDocument51 pagesVSEPR Theory and Hybridizationerloos236No ratings yet
- UntitledDocument53 pagesUntitledchandrakanth maheshNo ratings yet
- CHM 101 Introductory Chemistry I NewDocument20 pagesCHM 101 Introductory Chemistry I Newekanadefestus007No ratings yet
- Lesson 2.3 VSEPR TheoryDocument53 pagesLesson 2.3 VSEPR Theorymizpehman12No ratings yet
- VSEPR Theory (Molecular Shapes) : A The Central Atom, X An Atom Bonded To A, E A Lone Pair On ADocument2 pagesVSEPR Theory (Molecular Shapes) : A The Central Atom, X An Atom Bonded To A, E A Lone Pair On ArinaazriNo ratings yet
- Q2 Lesson 1Document43 pagesQ2 Lesson 1Sheena AragoNo ratings yet
- PhET VSEPR Simulation (Honors)Document4 pagesPhET VSEPR Simulation (Honors)merryscotNo ratings yet
- Chemis 13Document69 pagesChemis 13hadassahhadidNo ratings yet
- Lecture 02/unit II (Chemical Bonding) VSEPR TheoryDocument4 pagesLecture 02/unit II (Chemical Bonding) VSEPR TheorySkyblueNo ratings yet
- The Chemical BondDocument47 pagesThe Chemical BondopawbunaNo ratings yet
- Molecular GeometryDocument37 pagesMolecular GeometryAllen EspinosaNo ratings yet
- Types of VSEPR TheoryDocument4 pagesTypes of VSEPR TheorySaeed RehmanNo ratings yet
- Shapes of MoleculesDocument17 pagesShapes of Moleculesbasaallen566No ratings yet
- Molecular Geometry VseprDocument25 pagesMolecular Geometry Vseprhidayati helmiNo ratings yet
- Introductory Chemistry Atoms First 5Th Edition Russo Solutions Manual Full Chapter PDFDocument36 pagesIntroductory Chemistry Atoms First 5Th Edition Russo Solutions Manual Full Chapter PDFkimberly.allen777100% (12)
- Introductory Chemistry Atoms First 5th Edition Russo Solutions Manual 1Document27 pagesIntroductory Chemistry Atoms First 5th Edition Russo Solutions Manual 1stefanie100% (55)
- Molecular Geometry: Molecular Shape Can Be Predicted by Using The Valence-Shell ElectronDocument11 pagesMolecular Geometry: Molecular Shape Can Be Predicted by Using The Valence-Shell ElectronSherin TeeNo ratings yet
- Chemistry Notes Gr.11Document33 pagesChemistry Notes Gr.11iyermagsNo ratings yet
- Vsepr TheoryDocument65 pagesVsepr TheoryNeliswa DlaminiNo ratings yet
- Experiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Document6 pagesExperiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Maldini JosnonNo ratings yet
- Chapter 9 - Part 1 - 4 Pages Per SlideDocument24 pagesChapter 9 - Part 1 - 4 Pages Per SlideAly HashamNo ratings yet
- CMY 117 For VSEPR and Molecular GeometryDocument8 pagesCMY 117 For VSEPR and Molecular GeometryJack WilliamsNo ratings yet
- Vesper TheoryDocument28 pagesVesper TheoryAmin GNo ratings yet
- Chemistry Atoms First 2nd Edition Burdge Test Bank 1Document154 pagesChemistry Atoms First 2nd Edition Burdge Test Bank 1lillian100% (38)
- Notes On Bonding and StructureDocument5 pagesNotes On Bonding and Structurefletcherberryheath2006No ratings yet
- Lecture 1.2 Organic Chemistry - MKDocument32 pagesLecture 1.2 Organic Chemistry - MKqurrelNo ratings yet
- 2b. Shapes of MoleculesDocument78 pages2b. Shapes of MoleculesKareem MckenzieNo ratings yet
- CUP IBChemistry c03 It BondingDocument59 pagesCUP IBChemistry c03 It BondingAdnan ChowdhuryNo ratings yet
- Module 2 Chemical Bonding and The Shapes of MoleculesDocument30 pagesModule 2 Chemical Bonding and The Shapes of MoleculesJulius Gutierrez EngbinoNo ratings yet
- ChemChapter8 Ladringan PDFDocument7 pagesChemChapter8 Ladringan PDFTn F'dzNo ratings yet
- CH 110 Course Outline 2019-2020 - Updated On 19 12 2020-2Document12 pagesCH 110 Course Outline 2019-2020 - Updated On 19 12 2020-2HarrisonNo ratings yet
- 59d743bce4b0a57ac4f4f590 PDFDocument24 pages59d743bce4b0a57ac4f4f590 PDFAkshat SharmaNo ratings yet
- 3.0 Chemical BondingDocument27 pages3.0 Chemical BondingTafadzwa MachongweNo ratings yet
- CHEM14 - (5) The Chemical Bond 2Document81 pagesCHEM14 - (5) The Chemical Bond 2Kariza AbuNo ratings yet
- Lecture 9 - Molecular Geometry and Bonding TheoriesDocument32 pagesLecture 9 - Molecular Geometry and Bonding Theoriesapi-19824406No ratings yet
- #25 Revision Sheet Chemical BondingDocument6 pages#25 Revision Sheet Chemical BondingShivam Kumar PathakNo ratings yet
- A Level Aqa Chemistry Unit 1 NotesDocument52 pagesA Level Aqa Chemistry Unit 1 Noteskhansoniaaa67% (3)
- HybridizationDocument21 pagesHybridizationpinehas nguluNo ratings yet
- Chemical Bonding - by WWW - Learnengineering.inDocument30 pagesChemical Bonding - by WWW - Learnengineering.inPrakhar MishraaNo ratings yet
- C Per Negative Aayega: AllenDocument7 pagesC Per Negative Aayega: AllenDurgeshTiwariNo ratings yet
- 2013 VseprDocument57 pages2013 Vseprapi-266061131No ratings yet
- CMY 117 For VSEPR and Molecular GeometryDocument8 pagesCMY 117 For VSEPR and Molecular GeometryJack WilliamsNo ratings yet
- C-116 (20-22) Chemical Bonding-6Document13 pagesC-116 (20-22) Chemical Bonding-633-Siddharth NairNo ratings yet
- Ap One Pagers Combined PDFDocument10 pagesAp One Pagers Combined PDFJack KirbyNo ratings yet
- C-113 (20-22) Chemical Bonding-3Document22 pagesC-113 (20-22) Chemical Bonding-333-Siddharth NairNo ratings yet
- Vesper TheoryDocument28 pagesVesper TheoryAmin GNo ratings yet
- Chem281 - Chapter 3: Covalent Bonding Bonding TheoriesDocument57 pagesChem281 - Chapter 3: Covalent Bonding Bonding TheoriesNuansak3No ratings yet
- 1.assignment Chemical BondingDocument18 pages1.assignment Chemical BondingAishley MatharooNo ratings yet
- Chapter 3Document48 pagesChapter 3Abdullah HasanNo ratings yet
- (2102) Lecture Notes Chemical Bonding eDocument69 pages(2102) Lecture Notes Chemical Bonding erennyabhaskaran_4560No ratings yet
- CHAPTER 1 - Covalent Bonding and Shapes of MoleculesDocument10 pagesCHAPTER 1 - Covalent Bonding and Shapes of MoleculeslorrainebarandonNo ratings yet
- 4.0 CHEMICAL BONDING - NOTES & TUTORIAL Q's..Document72 pages4.0 CHEMICAL BONDING - NOTES & TUTORIAL Q's..Dee -AdilaNo ratings yet
EVAN BLIZZARD - 20-21 PhET Simulation - Molecular Geometry
EVAN BLIZZARD - 20-21 PhET Simulation - Molecular Geometry
Uploaded by
EVAN BLIZZARD0 ratings0% found this document useful (0 votes)
439 views7 pagesThis document provides directions for using an online PhET simulation to explore how the number of atoms vs lone pairs of electrons attached to a central atom affects molecular geometry. It includes examples of 8 hypothetical molecules with varying numbers of single, double, and triple bonds as well as lone pairs. It then applies the concepts demonstrated to analyze the molecular geometry of common substances like water, carbon dioxide, boron trifluoride, ammonia, and methane.
Original Description:
Original Title
EVAN BLIZZARD - 20-21 PhET Simulation- Molecular Geometry
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThis document provides directions for using an online PhET simulation to explore how the number of atoms vs lone pairs of electrons attached to a central atom affects molecular geometry. It includes examples of 8 hypothetical molecules with varying numbers of single, double, and triple bonds as well as lone pairs. It then applies the concepts demonstrated to analyze the molecular geometry of common substances like water, carbon dioxide, boron trifluoride, ammonia, and methane.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
439 views7 pagesEVAN BLIZZARD - 20-21 PhET Simulation - Molecular Geometry
EVAN BLIZZARD - 20-21 PhET Simulation - Molecular Geometry
Uploaded by
EVAN BLIZZARDThis document provides directions for using an online PhET simulation to explore how the number of atoms vs lone pairs of electrons attached to a central atom affects molecular geometry. It includes examples of 8 hypothetical molecules with varying numbers of single, double, and triple bonds as well as lone pairs. It then applies the concepts demonstrated to analyze the molecular geometry of common substances like water, carbon dioxide, boron trifluoride, ammonia, and methane.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 7
Molecular Geometry PhET Simulation
Directions: use the online PhET simulation to explore how atoms vs lone pairs of electrons affect the shape of molecules.
Click here for the link to the PhET/ Online simulation.
Part 1: Warming up and Understanding Molecular Shapes
Molecule 1 Screenshot of Model
Attach to the central atom:
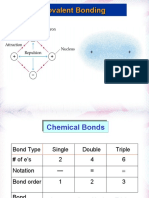
● 2 single bonds
AXE notation AX2
Molecular Geometry Linear
Electron Geometry Linear
Bond Angle 180 degrees
Molecule 2 Screenshot of Model
Attach to the central atom:
1. 3 single bonds
AXE notation AX3
Molecular Geometry Trigonal planar
Electron Geometry Trigonal planar
Bond Angle 120 degrees
Molecule 3 Screenshot of Model
Attach to the central atom:
● 4 single bonds
AXE notation AX4
Molecular Geometry Bent
Electron Geometry Bent
Bond Angle 109.5 degrees
Molecule 4 Screenshot of Model
Attach to the central atom:
● 3 single bonds
● 1 lone pair of electrons
AXE notation AX3E
Molecular Geometry Trigonal pyramidal
Electron Geometry Tetrahedral
Bond Angle 109.5 degrees
Molecule 5 Screenshot of Model
Attach to the central atom:
● 2 single bonds
● 2 lone pairs of electrons
AXE notation AX2E2
Molecular Geometry Bent
Electron Geometry Tetrahedral
Bond Angle 109.5 degrees
Molecule 6 Screenshot of Model
Attach to the central atom:
● 2 single bonds
● 1 double bond
AXE notation AX3
Molecular Geometry Trigonal planar
Electron Geometry Trigonal planar
Bond Angle 120 degrees
Molecule 7 Screenshot of Model
Attach to the central atom:
● 1 single bonds
● 1 triple bond
AXE notation AX2
Molecular Geometry Linear
Electron Geometry Linear
Bond Angle 180 degrees
Molecule 8 Screenshot of Model
Attach to the central atom:
● 2 double bonds
AXE notation AX2
Molecular Geometry Linear
Electron Geometry Linear
Bond Angle 180 degrees
Part 2: Applying this Simulation to Real Molecules
Water, H2O Screenshot of Model
AXE notation AX2E2
Molecular Geometry Bent
Electron Geometry Tetrahedral
Bond Angle 104.5 degrees
Carbon Dioxide, CO2 Screenshot of Model
AXE notation AX2
Molecular Geometry Linear
Electron Geometry Linear
Bond Angle 180 degrees
Boron Trifluoride, BF3 Screenshot of Model
AXE notation AX3
Molecular Geometry Trigonal planar
Electron Geometry Trigonal planar
Bond Angle 120 degrees
Ammonia, NH3 Screenshot of Model
AXE notation AX3E
Molecular Geometry Trigonal pyramidal
Electron Geometry Tetrahedral
Bond Angle 107.8 degrees
Methane, CH4 Screenshot of Model
AXE notation AX4
Molecular Geometry tetrahedral
Electron Geometry tetrahedral
Bond Angle 109.5 degrees
You might also like
- Chem Topic 4 Questions + AnswersDocument25 pagesChem Topic 4 Questions + AnswersOscarHigson-Spence50% (2)
- 6.1 Calculation Sheet Shapes of MoleculesDocument2 pages6.1 Calculation Sheet Shapes of MoleculesRoshNo ratings yet
- Molecular GeometryDocument1 pageMolecular Geometrybooty holeNo ratings yet
- Experiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Document6 pagesExperiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Maldini JosnonNo ratings yet
- Practice Test 7Document65 pagesPractice Test 7The LightNo ratings yet
- 4.4 Geometry and Polarity of MoleculesDocument22 pages4.4 Geometry and Polarity of Molecules1972lewonNo ratings yet
- 3.7 Geometry and Dipole MomentDocument9 pages3.7 Geometry and Dipole Momentelbadry mohamedNo ratings yet
- Molecular Geometry: Thinking of Molecules in 3-DimensionsDocument13 pagesMolecular Geometry: Thinking of Molecules in 3-DimensionsFilza AhmadNo ratings yet
- 3-6 Molecular Geometry SlidesDocument8 pages3-6 Molecular Geometry Slidesapi-240915238No ratings yet
- Molecular Geometry - General Chemistry IIDocument5 pagesMolecular Geometry - General Chemistry IINiobe DismasNo ratings yet
- Molecular Geometry and Bonding TheoriesDocument5 pagesMolecular Geometry and Bonding TheoriesPineraserNo ratings yet
- CHM361 - CHAPTER 1 Valence Bond Theory 2Document57 pagesCHM361 - CHAPTER 1 Valence Bond Theory 2EhazNo ratings yet
- Q2W2 - 2 - Molecular Geometry and Polarity of MoleculesDocument35 pagesQ2W2 - 2 - Molecular Geometry and Polarity of MoleculesEl Jie Ancheta EstelaNo ratings yet
- Molecular Geometry 2Document3 pagesMolecular Geometry 23MshopNo ratings yet
- MOLECULAR GEOMETRY NotesDocument4 pagesMOLECULAR GEOMETRY NotesAshley Mae LumanasNo ratings yet
- Geometry of MoleculesDocument8 pagesGeometry of Moleculesjorel marcoNo ratings yet
- Laboratory Activity 3 - Group 10Document6 pagesLaboratory Activity 3 - Group 10Reinier FrancoNo ratings yet
- 3VSEPR Theory 41-48Document8 pages3VSEPR Theory 41-48Raj KishoreNo ratings yet
- Molecular Shapes: Course Outcome 3Document13 pagesMolecular Shapes: Course Outcome 3tin canNo ratings yet
- Chemical Bonding 1Document99 pagesChemical Bonding 1DeviNo ratings yet
- Chem 106 Lab Report 6Document7 pagesChem 106 Lab Report 6IrynaNo ratings yet
- 3-D Shapes of MoleculesDocument14 pages3-D Shapes of MoleculesZhy MalzanNo ratings yet
- Ch-09-Molecular Geometry and Bonding TheoriesDocument104 pagesCh-09-Molecular Geometry and Bonding TheoriesTrọng NguyễnNo ratings yet
- Molecular GeometryDocument50 pagesMolecular GeometryMnhs MomentsNo ratings yet
- Las 7Document3 pagesLas 7Carl DoriaNo ratings yet
- Shapes of Molecules and Ions PDFDocument9 pagesShapes of Molecules and Ions PDFMagenta SparklegemNo ratings yet
- Hybridisation and Bond AngleDocument13 pagesHybridisation and Bond Angleskye sueNo ratings yet
- Lecture B2Document72 pagesLecture B2Nárēsh Yadav GäddēNo ratings yet
- Draw The Lewis Structure and Name The Shape of Each CompoundDocument9 pagesDraw The Lewis Structure and Name The Shape of Each CompoundJuan Frivaldo100% (1)
- Molecular Geometry and PolarityDocument58 pagesMolecular Geometry and Polaritychristiannnoochoa24No ratings yet
- Molecular Geometry (Vsepr Theory) : For Chemistry 1 Grade 12 Quarter 2 / Week 4Document15 pagesMolecular Geometry (Vsepr Theory) : For Chemistry 1 Grade 12 Quarter 2 / Week 4ariinnggg onichaNo ratings yet
- 4.3 Covalent Structures: IB Chemistry SL Mrs. PageDocument41 pages4.3 Covalent Structures: IB Chemistry SL Mrs. Pageapi-546066323No ratings yet
- 13Document7 pages13Anant MadhavNo ratings yet
- 9 VSEPRTheory PPTDocument37 pages9 VSEPRTheory PPTBlessy MartinNo ratings yet
- Bond Angles and VSEPR TheoryDocument3 pagesBond Angles and VSEPR TheoryTaqeeb AbbasNo ratings yet
- Predicting The Shapes of MoleculesDocument3 pagesPredicting The Shapes of Moleculestommy jimmyNo ratings yet
- General Chemistry 1 Qt. 2 Week 4Document12 pagesGeneral Chemistry 1 Qt. 2 Week 4Nina Reca OmisolNo ratings yet
- VSEPR Theory and HybridizationDocument51 pagesVSEPR Theory and Hybridizationerloos236No ratings yet
- UntitledDocument53 pagesUntitledchandrakanth maheshNo ratings yet
- CHM 101 Introductory Chemistry I NewDocument20 pagesCHM 101 Introductory Chemistry I Newekanadefestus007No ratings yet
- Lesson 2.3 VSEPR TheoryDocument53 pagesLesson 2.3 VSEPR Theorymizpehman12No ratings yet
- VSEPR Theory (Molecular Shapes) : A The Central Atom, X An Atom Bonded To A, E A Lone Pair On ADocument2 pagesVSEPR Theory (Molecular Shapes) : A The Central Atom, X An Atom Bonded To A, E A Lone Pair On ArinaazriNo ratings yet
- Q2 Lesson 1Document43 pagesQ2 Lesson 1Sheena AragoNo ratings yet
- PhET VSEPR Simulation (Honors)Document4 pagesPhET VSEPR Simulation (Honors)merryscotNo ratings yet
- Chemis 13Document69 pagesChemis 13hadassahhadidNo ratings yet
- Lecture 02/unit II (Chemical Bonding) VSEPR TheoryDocument4 pagesLecture 02/unit II (Chemical Bonding) VSEPR TheorySkyblueNo ratings yet
- The Chemical BondDocument47 pagesThe Chemical BondopawbunaNo ratings yet
- Molecular GeometryDocument37 pagesMolecular GeometryAllen EspinosaNo ratings yet
- Types of VSEPR TheoryDocument4 pagesTypes of VSEPR TheorySaeed RehmanNo ratings yet
- Shapes of MoleculesDocument17 pagesShapes of Moleculesbasaallen566No ratings yet
- Molecular Geometry VseprDocument25 pagesMolecular Geometry Vseprhidayati helmiNo ratings yet
- Introductory Chemistry Atoms First 5Th Edition Russo Solutions Manual Full Chapter PDFDocument36 pagesIntroductory Chemistry Atoms First 5Th Edition Russo Solutions Manual Full Chapter PDFkimberly.allen777100% (12)
- Introductory Chemistry Atoms First 5th Edition Russo Solutions Manual 1Document27 pagesIntroductory Chemistry Atoms First 5th Edition Russo Solutions Manual 1stefanie100% (55)
- Molecular Geometry: Molecular Shape Can Be Predicted by Using The Valence-Shell ElectronDocument11 pagesMolecular Geometry: Molecular Shape Can Be Predicted by Using The Valence-Shell ElectronSherin TeeNo ratings yet
- Chemistry Notes Gr.11Document33 pagesChemistry Notes Gr.11iyermagsNo ratings yet
- Vsepr TheoryDocument65 pagesVsepr TheoryNeliswa DlaminiNo ratings yet
- Experiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Document6 pagesExperiment 7 Molecular Geometry 7.1 Objectives: SKU3073 Chemistry Semester 1 2020/2021Maldini JosnonNo ratings yet
- Chapter 9 - Part 1 - 4 Pages Per SlideDocument24 pagesChapter 9 - Part 1 - 4 Pages Per SlideAly HashamNo ratings yet
- CMY 117 For VSEPR and Molecular GeometryDocument8 pagesCMY 117 For VSEPR and Molecular GeometryJack WilliamsNo ratings yet
- Vesper TheoryDocument28 pagesVesper TheoryAmin GNo ratings yet
- Chemistry Atoms First 2nd Edition Burdge Test Bank 1Document154 pagesChemistry Atoms First 2nd Edition Burdge Test Bank 1lillian100% (38)
- Notes On Bonding and StructureDocument5 pagesNotes On Bonding and Structurefletcherberryheath2006No ratings yet
- Lecture 1.2 Organic Chemistry - MKDocument32 pagesLecture 1.2 Organic Chemistry - MKqurrelNo ratings yet
- 2b. Shapes of MoleculesDocument78 pages2b. Shapes of MoleculesKareem MckenzieNo ratings yet
- CUP IBChemistry c03 It BondingDocument59 pagesCUP IBChemistry c03 It BondingAdnan ChowdhuryNo ratings yet
- Module 2 Chemical Bonding and The Shapes of MoleculesDocument30 pagesModule 2 Chemical Bonding and The Shapes of MoleculesJulius Gutierrez EngbinoNo ratings yet
- ChemChapter8 Ladringan PDFDocument7 pagesChemChapter8 Ladringan PDFTn F'dzNo ratings yet
- CH 110 Course Outline 2019-2020 - Updated On 19 12 2020-2Document12 pagesCH 110 Course Outline 2019-2020 - Updated On 19 12 2020-2HarrisonNo ratings yet
- 59d743bce4b0a57ac4f4f590 PDFDocument24 pages59d743bce4b0a57ac4f4f590 PDFAkshat SharmaNo ratings yet
- 3.0 Chemical BondingDocument27 pages3.0 Chemical BondingTafadzwa MachongweNo ratings yet
- CHEM14 - (5) The Chemical Bond 2Document81 pagesCHEM14 - (5) The Chemical Bond 2Kariza AbuNo ratings yet
- Lecture 9 - Molecular Geometry and Bonding TheoriesDocument32 pagesLecture 9 - Molecular Geometry and Bonding Theoriesapi-19824406No ratings yet
- #25 Revision Sheet Chemical BondingDocument6 pages#25 Revision Sheet Chemical BondingShivam Kumar PathakNo ratings yet
- A Level Aqa Chemistry Unit 1 NotesDocument52 pagesA Level Aqa Chemistry Unit 1 Noteskhansoniaaa67% (3)
- HybridizationDocument21 pagesHybridizationpinehas nguluNo ratings yet
- Chemical Bonding - by WWW - Learnengineering.inDocument30 pagesChemical Bonding - by WWW - Learnengineering.inPrakhar MishraaNo ratings yet
- C Per Negative Aayega: AllenDocument7 pagesC Per Negative Aayega: AllenDurgeshTiwariNo ratings yet
- 2013 VseprDocument57 pages2013 Vseprapi-266061131No ratings yet
- CMY 117 For VSEPR and Molecular GeometryDocument8 pagesCMY 117 For VSEPR and Molecular GeometryJack WilliamsNo ratings yet
- C-116 (20-22) Chemical Bonding-6Document13 pagesC-116 (20-22) Chemical Bonding-633-Siddharth NairNo ratings yet
- Ap One Pagers Combined PDFDocument10 pagesAp One Pagers Combined PDFJack KirbyNo ratings yet
- C-113 (20-22) Chemical Bonding-3Document22 pagesC-113 (20-22) Chemical Bonding-333-Siddharth NairNo ratings yet
- Vesper TheoryDocument28 pagesVesper TheoryAmin GNo ratings yet
- Chem281 - Chapter 3: Covalent Bonding Bonding TheoriesDocument57 pagesChem281 - Chapter 3: Covalent Bonding Bonding TheoriesNuansak3No ratings yet
- 1.assignment Chemical BondingDocument18 pages1.assignment Chemical BondingAishley MatharooNo ratings yet
- Chapter 3Document48 pagesChapter 3Abdullah HasanNo ratings yet
- (2102) Lecture Notes Chemical Bonding eDocument69 pages(2102) Lecture Notes Chemical Bonding erennyabhaskaran_4560No ratings yet
- CHAPTER 1 - Covalent Bonding and Shapes of MoleculesDocument10 pagesCHAPTER 1 - Covalent Bonding and Shapes of MoleculeslorrainebarandonNo ratings yet
- 4.0 CHEMICAL BONDING - NOTES & TUTORIAL Q's..Document72 pages4.0 CHEMICAL BONDING - NOTES & TUTORIAL Q's..Dee -AdilaNo ratings yet