Professional Documents
Culture Documents
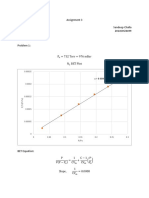
T P H KJ KG S KJ KGK S: Estado 1 Vapor Saturado 7,368°C 6,3 Bar
T P H KJ KG S KJ KGK S: Estado 1 Vapor Saturado 7,368°C 6,3 Bar
Uploaded by
Luis RomeroOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
T P H KJ KG S KJ KGK S: Estado 1 Vapor Saturado 7,368°C 6,3 Bar
T P H KJ KG S KJ KGK S: Estado 1 Vapor Saturado 7,368°C 6,3 Bar
Uploaded by
Luis RomeroCopyright:
Available Formats
Estado 1 VAPOR SATURADO
P S
T 1=¿ 7,368°C 6 0.9186
6,3 S1
P1=¿ 6,3 bar
7 0.9117
KJ
h1 =252,478
Kg
KJ 20 bar
S1=0.9165
KgK
h S
271.43 0.8873
x 0.9165
Estado 2 VAPOR SOBRECALENTADO 281.36 0.9167
P2=¿ 23 bar
KJ KJ
S2=S 1=0.9165 x=281.29
KgK Kg
KJ
h2 =25 4 , 87
Kg
T 2=¿ 78,35°C
24 bar
h S
P h 284.93 0.9133
20 281.27 x 0.9165
23 h2 s
24 286.07 294.75 0.9407
KJ KJ
x=28 4 . 87 x=286.07
Kg Kg
T S T S P T
60 0.8873 80 0.9133 20 69.93
x 0.9165 x 0.9165 23 T2s
70 0.9167 90 0.9407 24 81.16
x=69.93 ° C x=81.16 ° C T 2 s=78,35° C
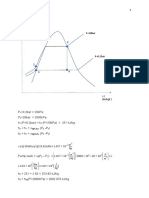
Estado 3 LIQUIDO SATURADO
P S
T 3=¿ 57.41°C 20 0.3895
P3=¿ 23 bar 23 S3
24 0.4241
KJ
h3 =118.622
Kg
KJ
S3=0. 4154
KgK
Estado 4
P Sf
P4 =¿ 6,3 bar
6 0.2019
T 4 s =T 1=7.368 ° C 6,3 Sf
h 4 s=h3 7 0.2231
KJ
S4 s=S3 Sf =0.2082
Kg K
S 4 s−S f
x=
S g−S f
P Sg
X 4 s=0.2925
6 0.9186
6,3 Sg
7 0.9117
KJ
S g=0. 9165
KgK
ṁ h4 ṁ h1
ṁ h4 s−ṁ h1+ Q̇ 1=0
Q̇1=ṁ(h 1−h4 s)
Q̇ 1 KJ
Q̇ 1=3.0294
s
Q̇ 2 ṁ h2 s− ṁh3−Q̇ 2=0
ṁ h3 ṁ h2 Q̇2= ṁ(h2 s−h3 )
KJ
Q̇ 2=3.2734
s
You might also like
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Meneses, Keziah F.: Cop T T T T T T Where: T T T Cop COP 1.4696 1.47Document19 pagesMeneses, Keziah F.: Cop T T T T T T Where: T T T Cop COP 1.4696 1.47Eriane GarciaNo ratings yet
- Excel Pondasi Triaxial TestDocument4 pagesExcel Pondasi Triaxial TestHusnik Maulidya Tungga Dewi100% (1)
- Problem: 9-21 GivenDocument2 pagesProblem: 9-21 Givenmazhar1613No ratings yet
- Formulas and Processes in Fluid DynamicsDocument8 pagesFormulas and Processes in Fluid DynamicsFenrir RozenNo ratings yet
- T P H KJ KG S KJ KGK S: Estado 1 Vapor Saturado 7,368°C 6,3 BarDocument2 pagesT P H KJ KG S KJ KGK S: Estado 1 Vapor Saturado 7,368°C 6,3 BarLuis RomeroNo ratings yet
- Maq TermicasDocument4 pagesMaq TermicasSanti Romero BonillaNo ratings yet
- Module 1 Activity No. 1Document2 pagesModule 1 Activity No. 1PHILIPANTHONY MASILANGNo ratings yet
- Chap 7 8Document4 pagesChap 7 8Hoàng Long Nguyễn BùiNo ratings yet
- Exercicis TermodinàmicaDocument5 pagesExercicis Termodinàmicapochmax18No ratings yet
- Assignment 3Document4 pagesAssignment 3Sandeep ChallaNo ratings yet
- Problema de Ciclo TV MF 1PM21Document16 pagesProblema de Ciclo TV MF 1PM21Leslee Ramirez A.No ratings yet
- Problema 7.5Document4 pagesProblema 7.5Luisa RodríguezNo ratings yet
- 1st Nungay MergedDocument20 pages1st Nungay MergedGino NungayNo ratings yet
- Toaz - Info Refrigeration Moran Shapiro Solution Manual PRDocument10 pagesToaz - Info Refrigeration Moran Shapiro Solution Manual PREnhIe NkosiNo ratings yet
- Dela Cruz Lesson 5 Exercises Problem 3Document2 pagesDela Cruz Lesson 5 Exercises Problem 3ROBERTJOHN DELACRUZNo ratings yet
- Dela Cruz Lesson 5 Exercises Problem 3Document2 pagesDela Cruz Lesson 5 Exercises Problem 3ROBERTJOHN DELACRUZNo ratings yet
- Heat Pump (Heating) : Experiment No. (2) Mechanical LabDocument16 pagesHeat Pump (Heating) : Experiment No. (2) Mechanical LabDilshad S FaisalNo ratings yet
- Module 4: Activity No. 5: OD 0.0254 M, M KG S, CP 3810 J KG K, T KG S, T W M KDocument2 pagesModule 4: Activity No. 5: OD 0.0254 M, M KG S, CP 3810 J KG K, T KG S, T W M KLeyzer MalumayNo ratings yet
- Homework07 Engineering Thermodynamics MENG 3320Document4 pagesHomework07 Engineering Thermodynamics MENG 3320Andrew AlarconNo ratings yet
- SARMIENTO Exercises Problem 2 Ref and Air ConDocument4 pagesSARMIENTO Exercises Problem 2 Ref and Air ConLeyzer MalumayNo ratings yet
- SARMIENTO Exercises Problem 2 Ref and Air ConDocument4 pagesSARMIENTO Exercises Problem 2 Ref and Air ConLeyzer MalumayNo ratings yet
- Refrigeration Systems Quiz 1Document4 pagesRefrigeration Systems Quiz 1MartiNo ratings yet
- Module 5 Activity No. 2Document4 pagesModule 5 Activity No. 2Seb TayagNo ratings yet
- Lec5-2 PTTDocument2 pagesLec5-2 PTTAmanbay Ali ChalakNo ratings yet
- Sheet (1&2) ThermoDocument17 pagesSheet (1&2) ThermoAhmed A. TaimaNo ratings yet
- Pipe 5-6-7Document4 pagesPipe 5-6-7Elago, Justine M.No ratings yet
- Lesson 3 Exercises Problem 4Document2 pagesLesson 3 Exercises Problem 4Ariel GamboaNo ratings yet
- ch13 PDFDocument6 pagesch13 PDFAkash ThummarNo ratings yet
- kg/cm2 1 Unit Conversion 1000 GW 62.4 F (Radians) 0.477879876 W Footing 12000 G Conc 150Document7 pageskg/cm2 1 Unit Conversion 1000 GW 62.4 F (Radians) 0.477879876 W Footing 12000 G Conc 150Aftub Uz ZamanNo ratings yet
- Q2 SolutionDocument2 pagesQ2 SolutionSam StideNo ratings yet
- Cartajena REVISEDDocument11 pagesCartajena REVISEDJerome Russel PublìcòNo ratings yet
- Uts Daik Celvin Dicky Wahyudi 2031710009Document43 pagesUts Daik Celvin Dicky Wahyudi 2031710009Celvin DickyNo ratings yet
- Bufe Aaron A. 1st Checking LectureDocument18 pagesBufe Aaron A. 1st Checking LectureArabia, Elmo C.No ratings yet
- TABLE 04: Experimental Data For The Determination of The Molar Heat of CombustionDocument6 pagesTABLE 04: Experimental Data For The Determination of The Molar Heat of CombustionRue Elizabeth DaltonNo ratings yet
- Module 3: Activity No. 2 Experiment No. 3:: 20 Mton 1.2 T 26 T 8 C 3.05 KJDocument2 pagesModule 3: Activity No. 2 Experiment No. 3:: 20 Mton 1.2 T 26 T 8 C 3.05 KJJB_0929No ratings yet
- Properties PpeDocument8 pagesProperties Ppejanuel borelaNo ratings yet
- Project Sameer HMTDocument9 pagesProject Sameer HMTHamza AliNo ratings yet
- De GUZMAN Module 4 Activity No. 6Document5 pagesDe GUZMAN Module 4 Activity No. 6John Mark AlvesNo ratings yet
- First Law of Thermodynamics: 0 2g + M 2g + M + Q+ W + WDocument3 pagesFirst Law of Thermodynamics: 0 2g + M 2g + M + Q+ W + WDaniel Andres Canro CalderónNo ratings yet
- Qu=Cnc+Qnq+0.5 Γbnγ: Computation Of Ultimate Bearing Capacity: Terzhagi'S EquationDocument3 pagesQu=Cnc+Qnq+0.5 Γbnγ: Computation Of Ultimate Bearing Capacity: Terzhagi'S EquationJeremy Mark SorianoNo ratings yet
- Isentropic ProblemsDocument5 pagesIsentropic ProblemsjecuadranteNo ratings yet
- Design of Footing Dimension: Isolated Rectanguar Footing Irf-1 (L 1.5B)Document10 pagesDesign of Footing Dimension: Isolated Rectanguar Footing Irf-1 (L 1.5B)Amber ArellanoNo ratings yet
- Calorimetry ExperimentDocument8 pagesCalorimetry ExperimentCrystalGamingNo ratings yet
- Analisis Regresi SederhanaDocument3 pagesAnalisis Regresi Sederhanasri nur aulyaNo ratings yet
- Design of Footing Dimension: Isolated Square Footing (Isf)Document34 pagesDesign of Footing Dimension: Isolated Square Footing (Isf)Amber ArellanoNo ratings yet
- Quiz4 by PassDocument3 pagesQuiz4 by PassAllaine VictoriaNo ratings yet
- Design Option 3Document9 pagesDesign Option 3무제untitledNo ratings yet
- Regenerative Rankine CycleDocument14 pagesRegenerative Rankine CycleSamson GabrielNo ratings yet
- Problems On Vapour Compression CycleDocument6 pagesProblems On Vapour Compression CycleTECHNICAL STARNo ratings yet
- Mathcad EjerDocument18 pagesMathcad EjerVictor OcampoNo ratings yet
- Solutions 4 F14Document5 pagesSolutions 4 F14nageshNo ratings yet
- Assignment 3 - Colligative Prop (LEC)Document10 pagesAssignment 3 - Colligative Prop (LEC)Poison PinkNo ratings yet
- Take Home UASDocument10 pagesTake Home UASZariatun Suryani RizkyNo ratings yet
- TONGO - Final ProjectDocument71 pagesTONGO - Final ProjectJhon Nicko TongoNo ratings yet
- Thermodynamics 1 CompressDocument20 pagesThermodynamics 1 Compressjlss jpNo ratings yet
- Phase ChangeDocument2 pagesPhase ChangeA - UNO, France Jhondale B.No ratings yet
- Certeza, Mark Jedrick L. - 4th-Grading-ProjectDocument3 pagesCerteza, Mark Jedrick L. - 4th-Grading-ProjectMark Jedrick CertezaNo ratings yet
- ThermodynamicallyDocument21 pagesThermodynamicallySunde PascuaNo ratings yet
- Module 6 Exercises Problem No. 1Document4 pagesModule 6 Exercises Problem No. 1Ariel GamboaNo ratings yet