Professional Documents
Culture Documents
Week SUMMARY
Week SUMMARY
Uploaded by
Hadith0 ratings0% found this document useful (0 votes)
6 views4 pages1) Cells obtain fatty acids from dietary fats, stored body fats, fats synthesized within organs, and by breaking down their own components. Vertebrates obtain fats from their diet and stored body fat.
2) Dietary fats are broken down by bile salts in the small intestine into fatty acids and mono/diacylglycerols which are absorbed by intestinal cells and repackaged into chylomicrons for transport.
3) Chylomicrons carry fatty acids from intestinal tissue to muscle and fat tissue where the fatty acids are used for energy or stored as body fat. Any remaining chylomicron components are taken up by the liver.
Original Description:
Original Title
3. week SUMMARY
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1) Cells obtain fatty acids from dietary fats, stored body fats, fats synthesized within organs, and by breaking down their own components. Vertebrates obtain fats from their diet and stored body fat.
2) Dietary fats are broken down by bile salts in the small intestine into fatty acids and mono/diacylglycerols which are absorbed by intestinal cells and repackaged into chylomicrons for transport.
3) Chylomicrons carry fatty acids from intestinal tissue to muscle and fat tissue where the fatty acids are used for energy or stored as body fat. Any remaining chylomicron components are taken up by the liver.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
6 views4 pagesWeek SUMMARY
Week SUMMARY
Uploaded by
Hadith1) Cells obtain fatty acids from dietary fats, stored body fats, fats synthesized within organs, and by breaking down their own components. Vertebrates obtain fats from their diet and stored body fat.
2) Dietary fats are broken down by bile salts in the small intestine into fatty acids and mono/diacylglycerols which are absorbed by intestinal cells and repackaged into chylomicrons for transport.
3) Chylomicrons carry fatty acids from intestinal tissue to muscle and fat tissue where the fatty acids are used for energy or stored as body fat. Any remaining chylomicron components are taken up by the liver.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 4
17.
1 Digestion, Mobilization, and Transport of
Fats
Cells can obtain fatty acid fuels from four sources: fats consumed in the diet,
fats stored in cells as lipid droplets, fats synthesized in one organ for export
to another, and fats obtained by autophagy (which degrades the cell’s own
organelles). Some species use all four sources under various circumstances,
others use one or two. Vertebrates, for example, obtain fats in the diet,
mobilize fats stored in specialized tissue (adipose tissue, consisting of cells
called adipocytes), and, in the liver, convert excess dietary carbohydrates to
fats for export to other tissues. During starvation, they can recycle lipids by
autophagy. On average, 40% or more of the daily energy requirement of
humans in highly industrialized countries is supplied by dietary
triacylglycerols (although most nutritional guidelines recommend no more
than 30% of daily caloric intake from fats). Triacylglycerols provide more
than half the energy requirements of some organs, particularly the liver, heart,
and resting skeletal muscle. Stored triacylglycerols are virtually the sole
source of energy in hibernating animals and migrating birds. Protists obtain
fats by consuming organisms lower in the food chain, and some also store
fats as cytosolic lipid droplets. Vascular plants mobilize fats stored in seeds
during germination, but do not otherwise depend on fats for energy.
Dietary Fats Are Absorbed in the Small Intestine
In vertebrates, before ingested triacylglycerols can be absorbed through the
intestinal wall they must be converted from insoluble macroscopic fat
particles to finely dispersed microscopic micelles. This solubilization is
carried out by bile salts, such as taurocholic acid (p. 374), which are
synthesized from cholesterol in the liver, stored in the gallbladder, and
released into the small intestine after ingestion of a fatty meal. Bile salts are
amphipathic compounds that act as biological detergents, converting dietary
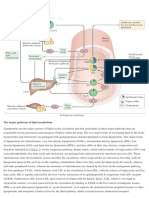
fats into mixed micelles of bile salts and triacylglycerols (Fig. 17-1, step 1 ).
Micelle formation enormously increases the fraction of lipid molecules
accessible to the action of water-soluble lipases in the intestine, and lipase
action converts triacylglycerols to monoacylglycerols (monoglycerides) and
diacylglycerols (diglycerides), free fatty acids, and glycerol (step 2 ). These
products of lipase action diffuse into the epithelial cells lining the intestinal
surface (the intestinal mucosa) (step 3 ), where they are reconverted to
triacylglycerols and packaged with dietary cholesterol and specific proteins
into lipoprotein aggregates called chylomicrons (Fig. 17-2; see also Fig. 17-
1, step 4 ).
FIGURE 17-1 Processing of dietary lipids in vertebrates. Digestion and
absorption of dietary lipids occur in the small intestine, and the fatty acids
released from triacylglycerols are packaged and delivered to muscle and
adipose tissues. The eight steps are discussed in the text.
FIGURE 17-2 Molecular structure of a chylomicron. The surface is a layer
of phospholipids, with head groups facing the aqueous phase. Triacylglycerols
sequestered in the interior (yellow) make up more than 80% of the mass.
Several apolipoproteins that protrude from the surface (B-48, C-II, C-III) act as
signals in the uptake and metabolism of chylomicron contents. The diameter of
chylomicrons ranges from about 100 to 500 nm.
Apolipoproteins are lipid-binding proteins in the blood that are
responsible for the transport of triacylglycerols, phospholipids, cholesterol,
and cholesteryl esters between organs. Apolipoproteins (“apo” means
“detached” or “separate,” designating the protein in its lipid-free form)
combine with lipids to form several classes of lipoprotein particles, spherical
aggregates with hydrophobic lipids at the core and hydrophilic protein side
chains and lipid head groups at the surface. Various combinations of lipid and
protein produce particles of different densities, ranging from chylomicrons
and very-low-density lipoproteins (VLDL) to very-high-density lipoproteins
(VHDL), which can be separated by ultracentrifugation. The structures of
these lipoprotein particles and their roles in lipid transport are detailed in
Chapter 21.
The protein moieties of lipoproteins are recognized by receptors on cell
surfaces. In lipid uptake from the intestine, chylomicrons, which contain
apolipoprotein C-II (apoC-II), move from the intestinal mucosa into the
lymphatic system, and then enter the blood, which carries them to muscle and
adipose tissue (Fig. 17-1, step 5 ). In the capillaries of these tissues, the
extracellular enzyme lipoprotein lipase, activated by apoC-II, hydrolyzes
triacylglycerols to fatty acids and glycerol (step 6 ), which are taken up by
specific transporters in the plasma membranes of cells in the target tissues
(step 7 ). In muscle, the fatty acids are oxidized for energy; in adipose tissue,
they are reesterified for storage as triacylglycerols (step 8 ).
The remnants of chylomicrons, depleted of most of their triacylglycerols
but still containing cholesterol and apolipoproteins, travel in the blood to the
liver, where they are taken up by endocytosis, mediated by receptors for their
apolipoproteins. Triacylglycerols that enter the liver by this route may be
oxidized to provide energy or to provide precursors for the synthesis of
ketone bodies, as described in Section 17.3. When the diet contains more
fatty acids than are needed immediately for fuel or as precursors, the liver
converts them to triacylglycerols, which are packaged with specific
apolipoproteins into VLDLs. These VLDLs are secreted by hepatocytes and
transported in the blood to adipose tissue, where the triacylglycerols are
removed and stored in lipid droplets within adipocytes.
Hormones Trigger Mobilization of Stored
Triacylglycerols
Neutral lipids are stored in adipocytes (and in steroid-synthesizing cells of the
adrenal cortex, ovary, and testis) in the form of lipid droplets, with a core of
triacylglycerols and sterol esters surrounded by a monolayer of
phospholipids. The surface of these droplets is coated with perilipins, a
family of proteins that restrict access to lipid droplets, preventing untimely
lipid mobilization. When hormones signal the need for metabolic energy,
triacylglycerols stored in adipose tissue are mobilized (brought out of
storage) and transported to tissues (skeletal muscle, heart, and renal cortex) in
which fatty acids can be oxidized for energy production. The hormones
epinephrine and glucagon, secreted in response to low blood glucose levels or
a fight-or-flight situation, stimulate the enzyme adenylyl cyclase in the
adipocyte plasma membrane (Fig. 17-3), which produces the intracellular
second messenger cyclic AMP (cAMP; see Fig. 12-4). Cyclic AMP–
dependent protein kinase (PKA) triggers changes that open the lipid droplet
to the action of three cytosolic lipases, which act on tri-, di-, and
monoacylglycerols, releasing fatty acids and glycerol.
You might also like
- LIPID METABOLISM Notes PDFDocument8 pagesLIPID METABOLISM Notes PDFToga BrandonNo ratings yet
- Triacylglycerols Are Highly Concentrated Energy Stores - Biochemistry - NCBI Bookshelf PDFDocument2 pagesTriacylglycerols Are Highly Concentrated Energy Stores - Biochemistry - NCBI Bookshelf PDFAdreiTheTripleA0% (1)
- Coagulation Cascade For DummiesDocument17 pagesCoagulation Cascade For DummiesHunter D. FahmiNo ratings yet
- Fatty Acid Catabolism PDFDocument25 pagesFatty Acid Catabolism PDFLuceroLalaaNo ratings yet
- Digestion of Lipids. Transport Forms of LipidsDocument91 pagesDigestion of Lipids. Transport Forms of LipidsAhmed FtsNo ratings yet
- Lipids: Chapter 10 (Part III)Document13 pagesLipids: Chapter 10 (Part III)Omer KareemNo ratings yet
- Lipids Digestion and AbsorptionDocument34 pagesLipids Digestion and Absorptionglenn johnstonNo ratings yet
- Biokimia LipidDocument6 pagesBiokimia Lipiddimensi warnaNo ratings yet
- 5.1 Lipids MetabolismDocument31 pages5.1 Lipids MetabolismAbdallah AlasalNo ratings yet
- Lipid Metabolism 1. Lipid Structure and StorageDocument4 pagesLipid Metabolism 1. Lipid Structure and StorageNoorAzizahIsnainiNo ratings yet
- Lipids NotesDocument4 pagesLipids NoteskatNo ratings yet
- Metabolism of FatsDocument13 pagesMetabolism of FatsBaap ko Mat sikhaNo ratings yet
- What Are Lipids?Document16 pagesWhat Are Lipids?Khazel CasimiroNo ratings yet
- Lipid Metabolism - WikipediaDocument31 pagesLipid Metabolism - WikipediaTomiwa AdeshinaNo ratings yet
- Lipoprotein Group 5Document46 pagesLipoprotein Group 5SharNo ratings yet
- Interconversion of Carbohydrate, Protein and FatDocument10 pagesInterconversion of Carbohydrate, Protein and FatKaziMahbubulHaque33% (3)
- Lipid MetabolismDocument15 pagesLipid MetabolismMUTHONI IRERINo ratings yet
- Metabolism of LipidsDocument37 pagesMetabolism of LipidsSafura IjazNo ratings yet
- Metabolism Summary Ch25Document8 pagesMetabolism Summary Ch25Victor EkutaNo ratings yet
- Notes On Lipid ProcessingDocument6 pagesNotes On Lipid ProcessingMicheal BrownNo ratings yet
- AP2 Lecture NU2013 Springclass 38 ChemdigstionnotestopostDocument2 pagesAP2 Lecture NU2013 Springclass 38 Chemdigstionnotestopostjchu2919No ratings yet
- Bile Acids Bile Acids (Bile Salts) Are Polar Derivatives of Cholesterol. They Are Formed in TheDocument9 pagesBile Acids Bile Acids (Bile Salts) Are Polar Derivatives of Cholesterol. They Are Formed in TheayshmoNo ratings yet
- Lipid Metabolism - (V) - Cholesterol and LipoproteinsDocument35 pagesLipid Metabolism - (V) - Cholesterol and Lipoproteinslightning proNo ratings yet
- VojoyDocument7 pagesVojoyFerds SalvatierraNo ratings yet
- Disorders of Lipid Metabolism Full Article Tables FiguresDocument32 pagesDisorders of Lipid Metabolism Full Article Tables Figureshasnawati100% (1)
- Lipids: Overview, Digestion and Absorption LipidsDocument6 pagesLipids: Overview, Digestion and Absorption LipidsCarah Jean Hurtado GillegaoNo ratings yet
- Lec 6 Fed State PDFDocument52 pagesLec 6 Fed State PDFrajeshNo ratings yet
- Lipid MetabolismDocument21 pagesLipid MetabolismTooba GhummanNo ratings yet
- Biochemistry 433Document5 pagesBiochemistry 433Jamila RaniNo ratings yet
- Lipid MetabolismDocument52 pagesLipid MetabolismDr.P.NatarajanNo ratings yet
- The Biochemistry of Digestion, Absorption and Detoxification by Prof. Dr. Hedef D. El-YassinDocument64 pagesThe Biochemistry of Digestion, Absorption and Detoxification by Prof. Dr. Hedef D. El-YassinSayhidoen CepexNo ratings yet
- Abu Bakar Hashaam (3153) LipidsDocument5 pagesAbu Bakar Hashaam (3153) LipidsAsad RazaNo ratings yet
- Biochem 8Document4 pagesBiochem 8Jamila RaniNo ratings yet
- Energy Production 2. Energy Storage 3. Energy UtilizationDocument14 pagesEnergy Production 2. Energy Storage 3. Energy UtilizationLALITH SAI KNo ratings yet
- ClinChem Digestion and Absorption of LipidsDocument3 pagesClinChem Digestion and Absorption of LipidsVincent ReyesNo ratings yet
- 2.LIPIDS DigestionDocument21 pages2.LIPIDS DigestionAlaa Hisham100% (1)
- Lipoprotein MetabolismDocument23 pagesLipoprotein MetabolismDarien LiewNo ratings yet
- Lipid DigestionDocument4 pagesLipid DigestionMUTHONI IRERINo ratings yet
- Chapter 3: Aspects of Fat Digestion and MetabolismDocument8 pagesChapter 3: Aspects of Fat Digestion and MetabolismmccrazywriteNo ratings yet
- Carbohydrate, Protein and Lipid Metabolism-B.NDocument77 pagesCarbohydrate, Protein and Lipid Metabolism-B.NBusy worldNo ratings yet
- Anatomy and Physiology of The LiverDocument8 pagesAnatomy and Physiology of The LiverDianne MacaraigNo ratings yet
- Carbohydrates NotesDocument11 pagesCarbohydrates Notesaliasger786inNo ratings yet
- Lipid MetabolismDocument33 pagesLipid MetabolismDharmveer SharmaNo ratings yet
- BCH 401 Note 2-1Document25 pagesBCH 401 Note 2-1idriscognitoleadsNo ratings yet
- Use of Macromolecules and Micromolecules For Human Body: AssignmentDocument10 pagesUse of Macromolecules and Micromolecules For Human Body: AssignmentSwastu Nurul AzizahNo ratings yet
- Lecture 11. Blood Biochemistry. Erythrocytes Metabolism. HemoglobinDocument27 pagesLecture 11. Blood Biochemistry. Erythrocytes Metabolism. HemoglobinВіталій Михайлович НечипорукNo ratings yet
- Group 2Document17 pagesGroup 2Marvin JeaNo ratings yet
- 1.overview of MetabolismDocument6 pages1.overview of Metabolismقتيبه خالد دحام خلفNo ratings yet
- Lipids Digestion and AbsorptionDocument28 pagesLipids Digestion and AbsorptionEng Matti Ur RehmanNo ratings yet
- LipidsDocument27 pagesLipidsJowe VarnalNo ratings yet
- Lipoprotein - WikipediaDocument38 pagesLipoprotein - WikipediaTejaswiNo ratings yet
- Intergrated MetabolismDocument117 pagesIntergrated MetabolismDerick SemNo ratings yet
- Lipid Metabolism: Sri Suciati Ningsih FK Uhamka 11 Maret 2020Document39 pagesLipid Metabolism: Sri Suciati Ningsih FK Uhamka 11 Maret 2020annisaaislamNo ratings yet
- Lipid MetabolismDocument4 pagesLipid MetabolismNuraisha AbduhadiNo ratings yet
- Metabolisme Kolesterol, Lipoprotein Dan ApolipoproteinDocument32 pagesMetabolisme Kolesterol, Lipoprotein Dan ApolipoproteinAlmira Ulfa Utari NasutionNo ratings yet
- 4.4.3 (Bioc) Lipoproteins: Chylomicrons, VLDL, LDL, and HDL: Biochemistry, TwoDocument22 pages4.4.3 (Bioc) Lipoproteins: Chylomicrons, VLDL, LDL, and HDL: Biochemistry, TwoAyro Business CenterNo ratings yet
- Figure - PMC 2Document1 pageFigure - PMC 2Hareesh ChanderNo ratings yet
- Figure - PMCDocument1 pageFigure - PMCHareesh ChanderNo ratings yet
- Lec34 F08 HandoutDocument9 pagesLec34 F08 HandoutVanessa VillespinNo ratings yet
- Atherosclerosis: Reporter: Collera, Charissa Constantino, Venice Clemena, Adnan Coronel, Romeo Cordova, KarlaDocument73 pagesAtherosclerosis: Reporter: Collera, Charissa Constantino, Venice Clemena, Adnan Coronel, Romeo Cordova, Karlaprecious_bustosNo ratings yet
- Enzyme Activity and Characterization PDFDocument14 pagesEnzyme Activity and Characterization PDFpanrumb06No ratings yet
- Immunoprecipitation-Based Techniques: Purification of Endogenous Proteins Using Agarose BeadsDocument4 pagesImmunoprecipitation-Based Techniques: Purification of Endogenous Proteins Using Agarose BeadsZain BaderNo ratings yet
- Lecture 53 2015 LeaDocument33 pagesLecture 53 2015 LeaYehundinokibaathai FcNo ratings yet
- Nihms 4718Document64 pagesNihms 4718uzzi319 roony26No ratings yet
- TBR Bio2 OptDocument495 pagesTBR Bio2 Optmeyangli88% (25)
- The Three-Dimensional Structure of Proteins: © 2018 Cengage Learning. All Rights ReservedDocument64 pagesThe Three-Dimensional Structure of Proteins: © 2018 Cengage Learning. All Rights Reservedendang dian lestariNo ratings yet
- PathDocument31 pagesPathsandhyaNo ratings yet
- Lipids: BiochemistryDocument13 pagesLipids: BiochemistryMarrell UnajanNo ratings yet
- The Effects of Temperature and PH On The Enzyme Activity of Salivary AmylaseDocument9 pagesThe Effects of Temperature and PH On The Enzyme Activity of Salivary AmylaseCherisse TuazonNo ratings yet
- Lesson 5.2. Other Types of LipidsDocument11 pagesLesson 5.2. Other Types of LipidsCreeper RulezNo ratings yet
- Appendix I: IA IUPAC Nucleotide Ambiguity CodesDocument2 pagesAppendix I: IA IUPAC Nucleotide Ambiguity Codespeeps007No ratings yet
- Plasma ProteinDocument57 pagesPlasma ProteinadulNo ratings yet
- Enzymes ChartDocument8 pagesEnzymes ChartBrandon BarndtNo ratings yet
- 2D Gel Electrophoresis Training: Proteomics and Mass Spectrometry FacilityDocument12 pages2D Gel Electrophoresis Training: Proteomics and Mass Spectrometry FacilityEli HeberNo ratings yet
- Quiz GlycolysisDocument1 pageQuiz GlycolysisCCSASHSJayzeelyn Vanessa CornejoNo ratings yet
- Sources of NADPHDocument41 pagesSources of NADPHPalesa NtsekalleNo ratings yet
- Circardian Clock BookDocument193 pagesCircardian Clock BookSandra DeeNo ratings yet
- Lesson Plan FatDocument11 pagesLesson Plan FatPankaj Jena80% (5)
- BIF401 Final Term Papers Fall 2018Document5 pagesBIF401 Final Term Papers Fall 2018HRrehmanNo ratings yet
- Vitamins & LipidsDocument64 pagesVitamins & LipidsShimmering MoonNo ratings yet
- Factors Affecting Enzyme ActivityDocument7 pagesFactors Affecting Enzyme ActivityDahliza KamatNo ratings yet
- Cytoskeleton 2Document7 pagesCytoskeleton 2Basil Francis AlajidNo ratings yet
- Biol 100 Chapter 8-9Document50 pagesBiol 100 Chapter 8-9Juan CNo ratings yet
- Biochemistry & Natural ProductsDocument10 pagesBiochemistry & Natural Productsjoey XDNo ratings yet
- Complete Product BrochureDocument2 pagesComplete Product BrochureRomeu LaiceNo ratings yet
- Problem Set - Proteins and EnzymesDocument4 pagesProblem Set - Proteins and EnzymesJayvee BillonesNo ratings yet
- Chlosteral LPTestDocument4 pagesChlosteral LPTestJohn StupNo ratings yet
- IPPATH Remove and IPPOOL Add in NodeBDocument15 pagesIPPATH Remove and IPPOOL Add in NodeBasusf6veNo ratings yet
- ProteinsDocument9 pagesProteinsJada HartNo ratings yet