Professional Documents
Culture Documents
Eng Sci
Eng Sci
Uploaded by
Azaa anuarOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Eng Sci
Eng Sci
Uploaded by
Azaa anuarCopyright:
Available Formats
TUTORIAL 7
Name Azana liza Anuar
:
Matric Num : 057189 .
Subject :
MTM 1103 Engineering Science
1. List 5 factor affect for the ionization energy Explain for .
each points briefly .
a. Atono mic radius When an atomic radius decreased ionization energy increases
.
. .
b. Nuclear charge If a .
positive charge there is stronger attraction for the
nucleus has a ,
electrons This results in a high amount of ionization energy
. .
C Orbital penetration Since the s orbitals penetrate towards the nucleus more closely than the p
. .
orbitals it's easier to remove electron from p orbitals than from s orbitals So ionization energy
.
.
going to be increase when removing a electron in s orbital .
d. Electron pairing within a sub shell Since repulsion between electrons
-
. in the same orbital is
higher than repulsion between electrons in different orbitals Within a sub shell paired
.
-
,
electrons are easier to remove than unpaired ones .
So ionization energy increase when
removing
an unpaired electron .
e. Shielding or
screening effect of the inner orbitals . Electrons in the outermost orbitals feel
lesser attraction from the nucleus than expected ,
due to presence of electrons in inner
orbitals .
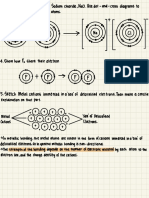
2. What is Valence electrons and sketch the diagram to support your answer .
→ Valence electrons are defines as electrons that are found in the outermost energy level
of atoms .
Nucleus
Valence
electrons
You might also like
- Element Builder SEDocument5 pagesElement Builder SEbarbiee201229% (38)
- Science9.Chapter5.Lesson1 - Ionic BondingDocument51 pagesScience9.Chapter5.Lesson1 - Ionic BondingടHՕՕꝄedкƲȠNo ratings yet
- Ionisation and Energy LevelsDocument23 pagesIonisation and Energy LevelsAzuralianNo ratings yet
- Ionisation EnergiesDocument2 pagesIonisation EnergiesthrowawayemailorangesNo ratings yet
- Semiconductor Electronics Materials, Devices and Simple CircuitsDocument32 pagesSemiconductor Electronics Materials, Devices and Simple CircuitsAyush KushwahaNo ratings yet
- Periodicity Part 1Document1 pagePeriodicity Part 1Green SlimeNo ratings yet
- Caie As Level Chemistry 9701 Theory v1Document30 pagesCaie As Level Chemistry 9701 Theory v1Noora MubarakNo ratings yet
- Priodic Trends Table AnswerDocument1 pagePriodic Trends Table AnswerMr. RomanNo ratings yet
- Semi Conductor: 1. Energy Bands in SolidsDocument8 pagesSemi Conductor: 1. Energy Bands in SolidskhannapuneetNo ratings yet
- SemiconductorDocument6 pagesSemiconductordksingh369No ratings yet
- As 1 PerDocument4 pagesAs 1 PerzoeakatNo ratings yet
- Trends & The Periodic TableDocument34 pagesTrends & The Periodic TableElah PalaganasNo ratings yet
- CH-8 Semi Conductor (Phy +2) PDFDocument41 pagesCH-8 Semi Conductor (Phy +2) PDFAswin PrabuNo ratings yet
- Trends & The Periodic TableDocument34 pagesTrends & The Periodic TableAnita KapadiaNo ratings yet
- Here You Will Get Following Materials:-: All Study Materials in Free of CostDocument10 pagesHere You Will Get Following Materials:-: All Study Materials in Free of CostArpit JainNo ratings yet
- Trends & The Periodic TableDocument34 pagesTrends & The Periodic Tableemo mHAYNo ratings yet
- ELECTRICITYDocument17 pagesELECTRICITYTest Music CopyrightNo ratings yet
- 5DP Ionisation EnergiesDocument17 pages5DP Ionisation EnergiesVaida MatulevičiūtėNo ratings yet
- (New Specification) (Document34 pages(New Specification) (Anuki PereraNo ratings yet
- Lecture 3 Atomic StructureDocument66 pagesLecture 3 Atomic StructureDaniel QuarteyNo ratings yet
- Trends in The Periodic TableDocument41 pagesTrends in The Periodic TablespsarathyNo ratings yet
- 3.1.1 Periodicity: Classification of Elements in S, P, D BlocksDocument5 pages3.1.1 Periodicity: Classification of Elements in S, P, D BlocksstudierNo ratings yet
- Caie As Level Chemistry 9701 Theory 63daaa332a33a953847630d5 896Document35 pagesCaie As Level Chemistry 9701 Theory 63daaa332a33a953847630d5 896baysanamir70No ratings yet
- Semiconductor and Devices - Byju'sDocument206 pagesSemiconductor and Devices - Byju'srapellivani789No ratings yet
- Semi Conductor NotesDocument8 pagesSemi Conductor Notesjohhnysins1978No ratings yet
- Factors Affecting The Ionisation EnergyDocument3 pagesFactors Affecting The Ionisation EnergyTi Yee SanNo ratings yet
- GP2 Q3W1Document24 pagesGP2 Q3W1DonggadongNo ratings yet
- Engineering Utilities 1: Negative Charge of Electricity. Positive Charge of Electricity Not Electrically ChargedDocument3 pagesEngineering Utilities 1: Negative Charge of Electricity. Positive Charge of Electricity Not Electrically ChargedE&N CommissionNo ratings yet
- Transes ECEDocument14 pagesTranses ECEI AM CHESCA (IamChesca)No ratings yet
- Chapter One: Introduction To ElectronicsDocument7 pagesChapter One: Introduction To ElectronicsBereket TsegayeNo ratings yet
- Lecture3 2019 MPDocument12 pagesLecture3 2019 MPpumaNo ratings yet
- Ionisation Energy: A Guide For A Level StudentsDocument37 pagesIonisation Energy: A Guide For A Level StudentsLalitha KurumanghatNo ratings yet
- Electrons and HolesDocument14 pagesElectrons and HolesMaxi GarzonNo ratings yet
- Chapter 3 Chemical BondingDocument6 pagesChapter 3 Chemical BondingQutub KhanNo ratings yet
- ElectronicsDocument121 pagesElectronicsDilindri GunasenaNo ratings yet
- Energy Bands in Solids: Dr. Gargi Raina VIT ChennaiDocument18 pagesEnergy Bands in Solids: Dr. Gargi Raina VIT ChennaiAnkit PachouriNo ratings yet
- Trends & The Periodic TableDocument34 pagesTrends & The Periodic TableJoseph ZhangNo ratings yet
- 2 Atomic StructureDocument43 pages2 Atomic StructureRafael ArancibiaNo ratings yet
- ET1006 Chapter 18 Part 1Document42 pagesET1006 Chapter 18 Part 1fastNo ratings yet
- Unit I-PN Junction PDFDocument130 pagesUnit I-PN Junction PDFB VIDWATH . K SRILATHANo ratings yet
- Chapter 7 of MechatronicsDocument15 pagesChapter 7 of MechatronicsSyed Basith MNo ratings yet
- Lecture1 BondingDocument24 pagesLecture1 BondingChantelle KingNo ratings yet
- Chemical Composition of The EarthDocument58 pagesChemical Composition of The EarthPutik Nurul ArasyNo ratings yet
- By. Kurniatuz Zahro 215060300111061Document14 pagesBy. Kurniatuz Zahro 215060300111061KURNIATUZ ZAHRONo ratings yet
- Interatomic Forces: What Kind of Force Holds The Atoms Together in A Solid?Document25 pagesInteratomic Forces: What Kind of Force Holds The Atoms Together in A Solid?Anonymous BW2VsFifi9No ratings yet
- Ionisation EnergyDocument23 pagesIonisation EnergyNurfarhanah KNo ratings yet
- Unless Otherwise Stated, All Images in This File Have Been Reproduced FromDocument18 pagesUnless Otherwise Stated, All Images in This File Have Been Reproduced FromAN NGUYENNo ratings yet
- Aviation Technical TrainingDocument203 pagesAviation Technical TrainingJuliusNo ratings yet
- Energy Bands in Solid 6Document4 pagesEnergy Bands in Solid 6S.M. Abdul Mannan MahdiNo ratings yet
- Chemical Composition of The EarthDocument58 pagesChemical Composition of The EarthPutik Nurul ArasyNo ratings yet
- Lecture 3Document34 pagesLecture 3daniel jerikoNo ratings yet
- Electron Configurations LessonDocument19 pagesElectron Configurations LessonWARREN ESCAPENo ratings yet
- 6 4IonizEnergyDocument1 page6 4IonizEnergyShehbaz YaseenNo ratings yet
- Tech Training Electronics EnglishDocument70 pagesTech Training Electronics Englishkshvsrma100% (1)
- Periodic TrendsDocument35 pagesPeriodic TrendslojeNo ratings yet
- Untitled Notebook PDFDocument28 pagesUntitled Notebook PDF石上 優No ratings yet
- Physisc ReviewerDocument6 pagesPhysisc ReviewerHatisha JailaniNo ratings yet
- Basic ElectronicsDocument114 pagesBasic ElectronicsfawNo ratings yet
- CH 7Document2 pagesCH 7Heather SiuNo ratings yet
- c1 Homogeneous Semiconductor - Revision (Compatibility Mode)Document35 pagesc1 Homogeneous Semiconductor - Revision (Compatibility Mode)Mu'izz KaharNo ratings yet
- Lecture 13 PDFDocument1 pageLecture 13 PDFAzaa anuarNo ratings yet
- Tutorial 2 (Eng Science)Document1 pageTutorial 2 (Eng Science)Azaa anuarNo ratings yet
- Eng Sci (T3) 4 PDFDocument1 pageEng Sci (T3) 4 PDFAzaa anuarNo ratings yet
- Resultant The Acting Together Triangle Apply: Component E FR Using TriangleDocument1 pageResultant The Acting Together Triangle Apply: Component E FR Using TriangleAzaa anuarNo ratings yet
- Eng Sci 3Document1 pageEng Sci 3Azaa anuarNo ratings yet
- Eng Sci 2Document1 pageEng Sci 2Azaa anuarNo ratings yet
- Molecular Mechanics Force Fields: Basic PremiseDocument23 pagesMolecular Mechanics Force Fields: Basic PremisekdsarodeNo ratings yet
- The Black Hole Information ParadoxDocument45 pagesThe Black Hole Information Paradoxursml12No ratings yet
- Semiconductor Physics: Syllabus Code: BSC-PHY-103GDocument39 pagesSemiconductor Physics: Syllabus Code: BSC-PHY-103GSusheel Gupta100% (1)
- Generation and Application of Attosecond Pulse TrainsDocument24 pagesGeneration and Application of Attosecond Pulse TrainsvozaheerNo ratings yet
- GP Thompson ExperimentDocument8 pagesGP Thompson ExperimentHemanth GoliNo ratings yet
- Electrical Engineering SyllabusDocument8 pagesElectrical Engineering SyllabusvineetNo ratings yet
- Callate y CalculaDocument3 pagesCallate y Calculaadi63No ratings yet
- Quantum Wells, Supperlattices and Bandgap EngineeringDocument21 pagesQuantum Wells, Supperlattices and Bandgap Engineering4ACONo ratings yet
- How Are Electrons Arranged Around The NucleusDocument3 pagesHow Are Electrons Arranged Around The NucleussukhnayakNo ratings yet
- SusicDocument19 pagesSusicALVARO DIEGO MACHACA CONDORINo ratings yet
- ISM Chapter 04Document28 pagesISM Chapter 04戴瑋志No ratings yet
- Wave PacketDocument7 pagesWave Packetimran hossainNo ratings yet
- Concept Building Classes (CBC)Document4 pagesConcept Building Classes (CBC)Ravindar PurohitNo ratings yet
- Atomic Structure PDFDocument19 pagesAtomic Structure PDFggk2013100% (3)
- The Non-Local Universe The New Physics and Matters of The MindDocument252 pagesThe Non-Local Universe The New Physics and Matters of The MindTony Al100% (1)
- Quantum Simulators: Rajesh Sathya KumarDocument8 pagesQuantum Simulators: Rajesh Sathya KumarRajesh SathyakumarNo ratings yet
- RCHK DP Physics: Topics 7,13: Atomic, Nuclear & Quantum Physics (HL)Document50 pagesRCHK DP Physics: Topics 7,13: Atomic, Nuclear & Quantum Physics (HL)Denning KWANNo ratings yet
- Atomic ModelsDocument18 pagesAtomic Modelssurvanity wilsonNo ratings yet
- Electronic SpectrosDocument22 pagesElectronic SpectrosBandita DattaNo ratings yet
- 1D LuttingerDocument9 pages1D LuttingerTonmoy DharNo ratings yet
- Lecture 21 22199Document7 pagesLecture 21 22199Iris GardnerNo ratings yet
- Chemistry Assignment HelpDocument3 pagesChemistry Assignment HelptutorhelpdeskNo ratings yet
- Quantum Neural Networks: Concepts, Applications, and ChallengesDocument4 pagesQuantum Neural Networks: Concepts, Applications, and Challengesqazokm78987No ratings yet
- Quantum 'Spooky Action at A Distance' Travels at Least 10,000 Times Faster Than LightDocument2 pagesQuantum 'Spooky Action at A Distance' Travels at Least 10,000 Times Faster Than LightquantumrealmNo ratings yet
- Student - Element BuilderDocument5 pagesStudent - Element BuilderChimamanda NWERRIH100% (1)
- Tao Et Al. (2010)Document5 pagesTao Et Al. (2010)mateus_rocha0% (1)
- Tunneling EffectDocument16 pagesTunneling Effectshabaresh MbNo ratings yet
- The Rise of The Quantum InternetDocument37 pagesThe Rise of The Quantum InternetPuput Adi SaputroNo ratings yet