Professional Documents
Culture Documents
25.2: Introduction To Hydrocarbons: Learning Objectives
25.2: Introduction To Hydrocarbons: Learning Objectives
Uploaded by
shasagailCopyright:
Available Formats
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Plant Adaptations WorksheetDocument4 pagesPlant Adaptations WorksheetshasagailNo ratings yet
- Hydrocarbons NotesDocument15 pagesHydrocarbons Notesarjunrkumar2024No ratings yet
- Lecture-10 (Organic Chemistry and Hidrocarbon) - 1Document75 pagesLecture-10 (Organic Chemistry and Hidrocarbon) - 1Permadi BagasNo ratings yet
- Alkenes AlkynesDocument40 pagesAlkenes AlkynesHanindya NugrahaNo ratings yet
- Organic Chem U-2 Functional GroupDocument34 pagesOrganic Chem U-2 Functional Groupsinte beyuNo ratings yet
- Hydrocarbons Class 11 Notes Chemistry Chapter 13 - Learn CBSEDocument1 pageHydrocarbons Class 11 Notes Chemistry Chapter 13 - Learn CBSEjaindhruv850No ratings yet
- Chapter 22: Hydrocarbon Compounds: Lesson 22.2: Unsaturated HydrocarbonsDocument2 pagesChapter 22: Hydrocarbon Compounds: Lesson 22.2: Unsaturated HydrocarbonsKM10 khalidNo ratings yet
- Chemical Bonding & Nomenclature of Hydrocarbons: Physical Science Week 4 HandoutsDocument2 pagesChemical Bonding & Nomenclature of Hydrocarbons: Physical Science Week 4 HandoutsBenj Jamieson DuagNo ratings yet
- Chapter-13 Notes-1Document14 pagesChapter-13 Notes-1Ashok KumarNo ratings yet
- Bonding of Hydrocarbons: Presented by Rina Mae Manceras Benjamin BagayanDocument33 pagesBonding of Hydrocarbons: Presented by Rina Mae Manceras Benjamin BagayanIce BearNo ratings yet
- ALKENEDocument7 pagesALKENEKrystel Ann Demaosa CarballoNo ratings yet
- HydrocarbonsDocument10 pagesHydrocarbonsjoeNo ratings yet
- 2.4 Introduction To Organic ChemistryDocument25 pages2.4 Introduction To Organic ChemistryMin YoonjiNo ratings yet
- Nomenclature of Alkane, Alkene & AlkyneDocument19 pagesNomenclature of Alkane, Alkene & Alkynea6446674No ratings yet
- Gen Chem Organic Chemistry NotesDocument5 pagesGen Chem Organic Chemistry NotesVianneie Dominique BernadasNo ratings yet
- HydrocarbonsDocument43 pagesHydrocarbonsdiyarberwari15No ratings yet
- Organic CompoundsDocument37 pagesOrganic CompoundsAlejandro VillanuevaNo ratings yet
- Carbon and Its CompoundsDocument11 pagesCarbon and Its CompoundsJulia NithdaleNo ratings yet
- Families of Organic CompoundsDocument8 pagesFamilies of Organic CompoundsJessa Mae LangcuyanNo ratings yet
- Q2 - Hydrocarbons and Functional GroupsDocument54 pagesQ2 - Hydrocarbons and Functional GroupsTosee istosee100% (1)
- Daniel 2Document2 pagesDaniel 2Julio Cèsar GarcìaNo ratings yet
- Alkene: University of Zakho College of Basic Education General Science Department 2 Stage, Class ADocument14 pagesAlkene: University of Zakho College of Basic Education General Science Department 2 Stage, Class AasaNo ratings yet
- Organic CompoundsDocument22 pagesOrganic CompoundsMary Rose AguilaNo ratings yet
- Alkane, Alkene, Alkyne PDFDocument17 pagesAlkane, Alkene, Alkyne PDFEra MelaniaNo ratings yet
- General Chemistry: 1 Semester - Petroleum Engineering Koya University 2021 - 2022 Hawar J. Sadiq HawezyDocument41 pagesGeneral Chemistry: 1 Semester - Petroleum Engineering Koya University 2021 - 2022 Hawar J. Sadiq HawezyZana NajatNo ratings yet
- Section 1.2Document12 pagesSection 1.2Gmat PrepNo ratings yet
- A. Aliphatic Compound: ExampleDocument4 pagesA. Aliphatic Compound: ExampleAnajane PalattaoNo ratings yet
- Introduction To The World of CarbonDocument26 pagesIntroduction To The World of CarbonJulia krizzea CristobalNo ratings yet
- ChemistryDocument25 pagesChemistryMa. Angelica Claire LuayonNo ratings yet
- Geometrical Isomerism: It is known that a carbon-carbon double bond is made up of one σ bond and one π-bond. The π-bond presents free rotation about the double bond.ThisDocument3 pagesGeometrical Isomerism: It is known that a carbon-carbon double bond is made up of one σ bond and one π-bond. The π-bond presents free rotation about the double bond.ThisKisha KhuranaNo ratings yet
- Organic Chemistry: Alkenes, Alkynes, and Aromatic CompoundsDocument43 pagesOrganic Chemistry: Alkenes, Alkynes, and Aromatic CompoundsDarius Gan100% (1)
- Organic Chemistry HandoutDocument11 pagesOrganic Chemistry HandoutJason Dis-ag Alejandro IsangNo ratings yet
- MIDTERMS CHEM - MazonDocument10 pagesMIDTERMS CHEM - MazonMazon, Dinah Melisse P.No ratings yet
- AlkanesDocument8 pagesAlkanesBenjamen FolarinNo ratings yet
- Hydrocarbons Class 11 Notes Chemistry Chapter 13 - Learn CBSEDocument16 pagesHydrocarbons Class 11 Notes Chemistry Chapter 13 - Learn CBSERishabh Singh RajputNo ratings yet
- ch02.ppt - Representative Carbon CompoundsDocument50 pagesch02.ppt - Representative Carbon CompoundscoltalbNo ratings yet
- UntitledDocument3 pagesUntitledJene Rose DeniegaNo ratings yet
- Organic Module1Document3 pagesOrganic Module1John mark ObiadoNo ratings yet
- Naming Organic Aliphatic CompoundsDocument27 pagesNaming Organic Aliphatic CompoundsAikaterine SmithNo ratings yet
- Organic Chemistry (I)Document5 pagesOrganic Chemistry (I)Tjandrawati NugrahaNo ratings yet
- Bahan Ajar AlkunaDocument28 pagesBahan Ajar Alkunakasma watiNo ratings yet
- Chapter 12Document21 pagesChapter 12AadNo ratings yet
- Ch03 Functional GroupsDocument37 pagesCh03 Functional GroupsSİNEM GÜVENNo ratings yet
- Natural Science PrelimDocument26 pagesNatural Science PrelimMew GulfNo ratings yet
- Activity 2 Nomenclature and StrucureDocument2 pagesActivity 2 Nomenclature and StrucureMANUEL, BUSTY P.No ratings yet
- Chapter 3 Alkenes and Alkynes PowerpointDocument61 pagesChapter 3 Alkenes and Alkynes PowerpointFreya An YbanezNo ratings yet
- Hydrocarbons AliphaticDocument15 pagesHydrocarbons Aliphaticaeroburn26No ratings yet
- Alkenes and AlkynesDocument28 pagesAlkenes and AlkynesDorama AikaNo ratings yet
- Organic Chemistry LecturesDocument32 pagesOrganic Chemistry LecturesAbdulHameedNo ratings yet
- Alkene: This Article Is About The Chemical Compound. For The Material, See - Not To Be Confused With orDocument19 pagesAlkene: This Article Is About The Chemical Compound. For The Material, See - Not To Be Confused With orRAMAKRISHNA PARJANYANo ratings yet
- AlkyneDocument30 pagesAlkyneKiela ArizobalNo ratings yet
- AlkenesDocument5 pagesAlkenesSafa MarwanNo ratings yet
- AlkynesDocument3 pagesAlkynesabdullahNo ratings yet
- Laboratory Activity NO. 2: Structure of HydrocarbonsDocument14 pagesLaboratory Activity NO. 2: Structure of HydrocarbonsLyra Ane IlaganNo ratings yet
- Topic 8 AlkenesDocument17 pagesTopic 8 Alkenesmark regino TuyayNo ratings yet
- 11-STEM Final Lesson (2nd Sem)Document9 pages11-STEM Final Lesson (2nd Sem)Lailah Rose AngkiNo ratings yet
- TopicDocument8 pagesTopicmanu cybercafeNo ratings yet
- Alkenes and Alkynes: Structure and Physical PropertiesDocument16 pagesAlkenes and Alkynes: Structure and Physical PropertiesSaloni JainNo ratings yet
- ALKYNEDocument6 pagesALKYNEPrincess Mae EstabilloNo ratings yet
- Chemistry Note Form 5Document9 pagesChemistry Note Form 5SofiyyahOpieNo ratings yet
- Projectile Motion: By: Ms. Sahara R. Jacob Science 9 TeacherDocument27 pagesProjectile Motion: By: Ms. Sahara R. Jacob Science 9 TeachershasagailNo ratings yet
- Dos and Donts in Making Test QuestionaireDocument10 pagesDos and Donts in Making Test QuestionaireshasagailNo ratings yet
- Group Activity RubricDocument1 pageGroup Activity RubricshasagailNo ratings yet
- Science Project Rubric: CategoryDocument3 pagesScience Project Rubric: CategoryshasagailNo ratings yet
- Scientist Project AssessmentDocument1 pageScientist Project AssessmentshasagailNo ratings yet
- Criterion Very Good 4 Points Good 3 Points Needs Improvement 2 Points Poor 1 Point Scor e 4Document1 pageCriterion Very Good 4 Points Good 3 Points Needs Improvement 2 Points Poor 1 Point Scor e 4shasagailNo ratings yet
- GeneratorsDocument21 pagesGeneratorsshasagailNo ratings yet
- The Electromagnetic SpectrumDocument25 pagesThe Electromagnetic SpectrumshasagailNo ratings yet
- Drag and Drop The Names of The Human BonesDocument1 pageDrag and Drop The Names of The Human BonesshasagailNo ratings yet
- Lab Rubric: Poor Fair Good ExcellentDocument2 pagesLab Rubric: Poor Fair Good ExcellentshasagailNo ratings yet
- WAVES Chapter 9Document8 pagesWAVES Chapter 9shasagailNo ratings yet
- Interactive Science Notebook Scoring Rubric: 6 ExcellentDocument1 pageInteractive Science Notebook Scoring Rubric: 6 ExcellentshasagailNo ratings yet
- Electrromagnetic Spectrum ReviewDocument2 pagesElectrromagnetic Spectrum ReviewshasagailNo ratings yet
- Electromagnetic Waves & The Electromagnetic SpectrumDocument35 pagesElectromagnetic Waves & The Electromagnetic SpectrumshasagailNo ratings yet
- Costing MealsDocument12 pagesCosting MealsshasagailNo ratings yet
- Blood and Circulation LECTUREDocument24 pagesBlood and Circulation LECTUREshasagailNo ratings yet
- Electromagnetic Spectrum ChartDocument1 pageElectromagnetic Spectrum ChartshasagailNo ratings yet
- Boyles Laws Practice WorksheetsDocument1 pageBoyles Laws Practice WorksheetsshasagailNo ratings yet
- Name: - Grade & Se. - Date: - ScoreDocument1 pageName: - Grade & Se. - Date: - ScoreshasagailNo ratings yet
- 3 Organic Chemistry SLDocument91 pages3 Organic Chemistry SLShuaib MohamedNo ratings yet
- Ejercicios QO1-1Document3 pagesEjercicios QO1-1hector juarezNo ratings yet
- AreneDocument37 pagesArene'Aqilah ZulkifliNo ratings yet
- Chapter 13 HydrocarbonsDocument23 pagesChapter 13 HydrocarbonsTreasure SeekerNo ratings yet
- Chem 2412 Lecture 01Document28 pagesChem 2412 Lecture 01Jihee YoonNo ratings yet
- Key - 1157854 - 2023-10-11 10 - 56 - 19 +0000Document19 pagesKey - 1157854 - 2023-10-11 10 - 56 - 19 +0000paaryan2624No ratings yet
- Alcohols Phenols and Ether - DPP - 4Document3 pagesAlcohols Phenols and Ether - DPP - 4Priya RangapureNo ratings yet
- Chemistry Practical: Experiment # Experiment NameDocument1 pageChemistry Practical: Experiment # Experiment NameSuperior CollegeNo ratings yet
- HydrocarbonDocument11 pagesHydrocarbonAida IshNo ratings yet
- Saponification (Sapo "Soap")Document3 pagesSaponification (Sapo "Soap")Karlo Roberto M. MarianoNo ratings yet
- BAI 4 ĐỒNG VÀ HỢP CHẤTDocument41 pagesBAI 4 ĐỒNG VÀ HỢP CHẤTLinhh ChiiNo ratings yet
- Review Problems Chapter 4 Solutions PDFDocument4 pagesReview Problems Chapter 4 Solutions PDFAntoninoNo ratings yet
- Diktat Naming Inorganic CompoundDocument6 pagesDiktat Naming Inorganic CompoundGeorge AthensNo ratings yet
- Analytical Chemistry AQA AnswersDocument30 pagesAnalytical Chemistry AQA AnswersAhmad BustamiNo ratings yet
- HN Chem Nomenclature Test Review Answer KeyDocument9 pagesHN Chem Nomenclature Test Review Answer KeyAdi ChhNo ratings yet
- General Characteristics of PolysaccharidesDocument3 pagesGeneral Characteristics of PolysaccharidesDick Andrew RodriguezNo ratings yet
- One MinuteDocument6 pagesOne MinuteEdy PurnomoNo ratings yet
- Macromolecules RiceDocument47 pagesMacromolecules Ricepeterr1022No ratings yet
- Lipid WorksheetDocument2 pagesLipid WorksheetMANUELA VENEGAS ESCOVAR0% (1)
- Chemistry Class 10 Chapter 12Document12 pagesChemistry Class 10 Chapter 12Muhammad Owais FayazNo ratings yet
- Las2 Phy SciDocument2 pagesLas2 Phy SciMa WiNo ratings yet
- Potassium Carbonate P0233Document1 pagePotassium Carbonate P0233kadam.arjun1No ratings yet
- Organic Compound - Identification of Functional Group SchemeDocument3 pagesOrganic Compound - Identification of Functional Group SchemeAMBRIN ABDULNo ratings yet
- Chapter 10. Sulphuric Acid: Short QuestionsDocument14 pagesChapter 10. Sulphuric Acid: Short QuestionsAbhay VishwakarmaNo ratings yet
- Alcohol, Ether, Phenol WorksheetDocument22 pagesAlcohol, Ether, Phenol WorksheetSDMNo ratings yet
- Corrosion AstroCosmosDocument11 pagesCorrosion AstroCosmosNattapong PongbootNo ratings yet
- JR - Inter Ipe Chemistry Model Paper 2Document2 pagesJR - Inter Ipe Chemistry Model Paper 2angadibalajithkumarNo ratings yet
- Ammonium 2520sulphate Material 2520balance.Document9 pagesAmmonium 2520sulphate Material 2520balance.AgadmatorNo ratings yet
- STPM Chemistry Definitions Term 3Document4 pagesSTPM Chemistry Definitions Term 3ChooNo ratings yet
- Ions - Ionic BondingDocument35 pagesIons - Ionic BondingAmjadNo ratings yet
25.2: Introduction To Hydrocarbons: Learning Objectives
25.2: Introduction To Hydrocarbons: Learning Objectives
Uploaded by
shasagailOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
25.2: Introduction To Hydrocarbons: Learning Objectives
25.2: Introduction To Hydrocarbons: Learning Objectives
Uploaded by
shasagailCopyright:
Available Formats
25.
2: Introduction to Hydrocarbons
Learning Objectives
To get an overview of hydrocarbons molecules and their four primary classifications
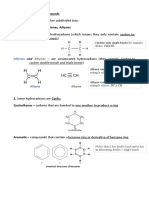
Hydrocarbons are organic compounds that contain only carbon and hydrogen. The four general classes of hydrocarbons are:
alkanes, alkenes, alkynes and arenes. Aromatic compounds derive their names from the fact that many of these compounds in
the early days of discovery were grouped because they were oils with fragrant odors.
The classifications for hydrocarbons, defined by IUPAC nomenclature of organic chemistry are as follows:
1. Saturated hydrocarbons (alkanes) are the simplest of the hydrocarbon species. They are composed entirely of single bonds
and are saturated with hydrogen. The general formula for saturated hydrocarbons is C H n 2n+2(assuming non-cyclic
structures). Saturated hydrocarbons are the basis of petroleum fuels and are found as either linear or branched species.The
simplest alkanes have their C atoms bonded in a straight chain; these are called normal alkanes. They are named according
to the number of C atoms in the chain. The smallest alkane is methane:
2. Unsaturated hydrocarbons have one or more double or triple bonds between carbon atoms. Those with double bond are
called alkenes and those with one double bond have the formula C H (assuming non-cyclic structures). Those
n 2n
containing triple bonds are called alkynes, with general formula C H
n . The smallest alkene—ethene—has two C atoms
2n−2
and is also known by its common name ethylene:
The smallest alkyne is ethyne, which is also known as acetylene:
3. Cycloalkanes are hydrocarbons containing one or more carbon rings to which hydrogen atoms are attached. The general
formula for a saturated hydrocarbon containing one ring is C H .
n 2n
4. Aromatic hydrocarbons, also known as arenes, are hydrocarbons that have at least one aromatic ring. Aromatic compounds
contain the benzene unit. Benzene itself is composed of six C atoms in a ring, with alternating single and double C–C
bonds:
Because of differences in molecular structure, the empirical formula remains different between hydrocarbons; in linear, or
"straight-run" alkanes, alkenes and alkynes, the amount of bonded hydrogen lessens in alkenes and alkynes due to the "self-
bonding" or catenation of carbon preventing entire saturation of the hydrocarbon by the formation of double or triple bonds.
The inherent ability of hydrocarbons to bond to themselves is known as catenation, and allows hydrocarbon to form more
complex molecules, such as cyclohexane, and in rarer cases, arenes such as benzene. This ability comes from the fact that the
11/11/2020 25.2.1 https://chem.libretexts.org/@go/page/91377
bond character between carbon atoms is entirely non-polar, in that the distribution of electrons between the two elements is
somewhat even due to the same electronegativity values of the elements (~0.30).
Contributors and Attributions
Wikipedia
11/11/2020 25.2.2 https://chem.libretexts.org/@go/page/91377
You might also like
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Plant Adaptations WorksheetDocument4 pagesPlant Adaptations WorksheetshasagailNo ratings yet
- Hydrocarbons NotesDocument15 pagesHydrocarbons Notesarjunrkumar2024No ratings yet
- Lecture-10 (Organic Chemistry and Hidrocarbon) - 1Document75 pagesLecture-10 (Organic Chemistry and Hidrocarbon) - 1Permadi BagasNo ratings yet
- Alkenes AlkynesDocument40 pagesAlkenes AlkynesHanindya NugrahaNo ratings yet
- Organic Chem U-2 Functional GroupDocument34 pagesOrganic Chem U-2 Functional Groupsinte beyuNo ratings yet
- Hydrocarbons Class 11 Notes Chemistry Chapter 13 - Learn CBSEDocument1 pageHydrocarbons Class 11 Notes Chemistry Chapter 13 - Learn CBSEjaindhruv850No ratings yet
- Chapter 22: Hydrocarbon Compounds: Lesson 22.2: Unsaturated HydrocarbonsDocument2 pagesChapter 22: Hydrocarbon Compounds: Lesson 22.2: Unsaturated HydrocarbonsKM10 khalidNo ratings yet
- Chemical Bonding & Nomenclature of Hydrocarbons: Physical Science Week 4 HandoutsDocument2 pagesChemical Bonding & Nomenclature of Hydrocarbons: Physical Science Week 4 HandoutsBenj Jamieson DuagNo ratings yet
- Chapter-13 Notes-1Document14 pagesChapter-13 Notes-1Ashok KumarNo ratings yet
- Bonding of Hydrocarbons: Presented by Rina Mae Manceras Benjamin BagayanDocument33 pagesBonding of Hydrocarbons: Presented by Rina Mae Manceras Benjamin BagayanIce BearNo ratings yet
- ALKENEDocument7 pagesALKENEKrystel Ann Demaosa CarballoNo ratings yet
- HydrocarbonsDocument10 pagesHydrocarbonsjoeNo ratings yet
- 2.4 Introduction To Organic ChemistryDocument25 pages2.4 Introduction To Organic ChemistryMin YoonjiNo ratings yet
- Nomenclature of Alkane, Alkene & AlkyneDocument19 pagesNomenclature of Alkane, Alkene & Alkynea6446674No ratings yet
- Gen Chem Organic Chemistry NotesDocument5 pagesGen Chem Organic Chemistry NotesVianneie Dominique BernadasNo ratings yet
- HydrocarbonsDocument43 pagesHydrocarbonsdiyarberwari15No ratings yet
- Organic CompoundsDocument37 pagesOrganic CompoundsAlejandro VillanuevaNo ratings yet
- Carbon and Its CompoundsDocument11 pagesCarbon and Its CompoundsJulia NithdaleNo ratings yet
- Families of Organic CompoundsDocument8 pagesFamilies of Organic CompoundsJessa Mae LangcuyanNo ratings yet
- Q2 - Hydrocarbons and Functional GroupsDocument54 pagesQ2 - Hydrocarbons and Functional GroupsTosee istosee100% (1)
- Daniel 2Document2 pagesDaniel 2Julio Cèsar GarcìaNo ratings yet
- Alkene: University of Zakho College of Basic Education General Science Department 2 Stage, Class ADocument14 pagesAlkene: University of Zakho College of Basic Education General Science Department 2 Stage, Class AasaNo ratings yet
- Organic CompoundsDocument22 pagesOrganic CompoundsMary Rose AguilaNo ratings yet
- Alkane, Alkene, Alkyne PDFDocument17 pagesAlkane, Alkene, Alkyne PDFEra MelaniaNo ratings yet
- General Chemistry: 1 Semester - Petroleum Engineering Koya University 2021 - 2022 Hawar J. Sadiq HawezyDocument41 pagesGeneral Chemistry: 1 Semester - Petroleum Engineering Koya University 2021 - 2022 Hawar J. Sadiq HawezyZana NajatNo ratings yet
- Section 1.2Document12 pagesSection 1.2Gmat PrepNo ratings yet
- A. Aliphatic Compound: ExampleDocument4 pagesA. Aliphatic Compound: ExampleAnajane PalattaoNo ratings yet
- Introduction To The World of CarbonDocument26 pagesIntroduction To The World of CarbonJulia krizzea CristobalNo ratings yet
- ChemistryDocument25 pagesChemistryMa. Angelica Claire LuayonNo ratings yet
- Geometrical Isomerism: It is known that a carbon-carbon double bond is made up of one σ bond and one π-bond. The π-bond presents free rotation about the double bond.ThisDocument3 pagesGeometrical Isomerism: It is known that a carbon-carbon double bond is made up of one σ bond and one π-bond. The π-bond presents free rotation about the double bond.ThisKisha KhuranaNo ratings yet
- Organic Chemistry: Alkenes, Alkynes, and Aromatic CompoundsDocument43 pagesOrganic Chemistry: Alkenes, Alkynes, and Aromatic CompoundsDarius Gan100% (1)
- Organic Chemistry HandoutDocument11 pagesOrganic Chemistry HandoutJason Dis-ag Alejandro IsangNo ratings yet
- MIDTERMS CHEM - MazonDocument10 pagesMIDTERMS CHEM - MazonMazon, Dinah Melisse P.No ratings yet
- AlkanesDocument8 pagesAlkanesBenjamen FolarinNo ratings yet
- Hydrocarbons Class 11 Notes Chemistry Chapter 13 - Learn CBSEDocument16 pagesHydrocarbons Class 11 Notes Chemistry Chapter 13 - Learn CBSERishabh Singh RajputNo ratings yet
- ch02.ppt - Representative Carbon CompoundsDocument50 pagesch02.ppt - Representative Carbon CompoundscoltalbNo ratings yet
- UntitledDocument3 pagesUntitledJene Rose DeniegaNo ratings yet
- Organic Module1Document3 pagesOrganic Module1John mark ObiadoNo ratings yet
- Naming Organic Aliphatic CompoundsDocument27 pagesNaming Organic Aliphatic CompoundsAikaterine SmithNo ratings yet
- Organic Chemistry (I)Document5 pagesOrganic Chemistry (I)Tjandrawati NugrahaNo ratings yet
- Bahan Ajar AlkunaDocument28 pagesBahan Ajar Alkunakasma watiNo ratings yet
- Chapter 12Document21 pagesChapter 12AadNo ratings yet
- Ch03 Functional GroupsDocument37 pagesCh03 Functional GroupsSİNEM GÜVENNo ratings yet
- Natural Science PrelimDocument26 pagesNatural Science PrelimMew GulfNo ratings yet
- Activity 2 Nomenclature and StrucureDocument2 pagesActivity 2 Nomenclature and StrucureMANUEL, BUSTY P.No ratings yet
- Chapter 3 Alkenes and Alkynes PowerpointDocument61 pagesChapter 3 Alkenes and Alkynes PowerpointFreya An YbanezNo ratings yet
- Hydrocarbons AliphaticDocument15 pagesHydrocarbons Aliphaticaeroburn26No ratings yet
- Alkenes and AlkynesDocument28 pagesAlkenes and AlkynesDorama AikaNo ratings yet
- Organic Chemistry LecturesDocument32 pagesOrganic Chemistry LecturesAbdulHameedNo ratings yet
- Alkene: This Article Is About The Chemical Compound. For The Material, See - Not To Be Confused With orDocument19 pagesAlkene: This Article Is About The Chemical Compound. For The Material, See - Not To Be Confused With orRAMAKRISHNA PARJANYANo ratings yet
- AlkyneDocument30 pagesAlkyneKiela ArizobalNo ratings yet
- AlkenesDocument5 pagesAlkenesSafa MarwanNo ratings yet
- AlkynesDocument3 pagesAlkynesabdullahNo ratings yet
- Laboratory Activity NO. 2: Structure of HydrocarbonsDocument14 pagesLaboratory Activity NO. 2: Structure of HydrocarbonsLyra Ane IlaganNo ratings yet
- Topic 8 AlkenesDocument17 pagesTopic 8 Alkenesmark regino TuyayNo ratings yet
- 11-STEM Final Lesson (2nd Sem)Document9 pages11-STEM Final Lesson (2nd Sem)Lailah Rose AngkiNo ratings yet
- TopicDocument8 pagesTopicmanu cybercafeNo ratings yet
- Alkenes and Alkynes: Structure and Physical PropertiesDocument16 pagesAlkenes and Alkynes: Structure and Physical PropertiesSaloni JainNo ratings yet
- ALKYNEDocument6 pagesALKYNEPrincess Mae EstabilloNo ratings yet
- Chemistry Note Form 5Document9 pagesChemistry Note Form 5SofiyyahOpieNo ratings yet
- Projectile Motion: By: Ms. Sahara R. Jacob Science 9 TeacherDocument27 pagesProjectile Motion: By: Ms. Sahara R. Jacob Science 9 TeachershasagailNo ratings yet
- Dos and Donts in Making Test QuestionaireDocument10 pagesDos and Donts in Making Test QuestionaireshasagailNo ratings yet
- Group Activity RubricDocument1 pageGroup Activity RubricshasagailNo ratings yet
- Science Project Rubric: CategoryDocument3 pagesScience Project Rubric: CategoryshasagailNo ratings yet
- Scientist Project AssessmentDocument1 pageScientist Project AssessmentshasagailNo ratings yet
- Criterion Very Good 4 Points Good 3 Points Needs Improvement 2 Points Poor 1 Point Scor e 4Document1 pageCriterion Very Good 4 Points Good 3 Points Needs Improvement 2 Points Poor 1 Point Scor e 4shasagailNo ratings yet
- GeneratorsDocument21 pagesGeneratorsshasagailNo ratings yet
- The Electromagnetic SpectrumDocument25 pagesThe Electromagnetic SpectrumshasagailNo ratings yet
- Drag and Drop The Names of The Human BonesDocument1 pageDrag and Drop The Names of The Human BonesshasagailNo ratings yet
- Lab Rubric: Poor Fair Good ExcellentDocument2 pagesLab Rubric: Poor Fair Good ExcellentshasagailNo ratings yet
- WAVES Chapter 9Document8 pagesWAVES Chapter 9shasagailNo ratings yet
- Interactive Science Notebook Scoring Rubric: 6 ExcellentDocument1 pageInteractive Science Notebook Scoring Rubric: 6 ExcellentshasagailNo ratings yet
- Electrromagnetic Spectrum ReviewDocument2 pagesElectrromagnetic Spectrum ReviewshasagailNo ratings yet
- Electromagnetic Waves & The Electromagnetic SpectrumDocument35 pagesElectromagnetic Waves & The Electromagnetic SpectrumshasagailNo ratings yet
- Costing MealsDocument12 pagesCosting MealsshasagailNo ratings yet
- Blood and Circulation LECTUREDocument24 pagesBlood and Circulation LECTUREshasagailNo ratings yet
- Electromagnetic Spectrum ChartDocument1 pageElectromagnetic Spectrum ChartshasagailNo ratings yet
- Boyles Laws Practice WorksheetsDocument1 pageBoyles Laws Practice WorksheetsshasagailNo ratings yet
- Name: - Grade & Se. - Date: - ScoreDocument1 pageName: - Grade & Se. - Date: - ScoreshasagailNo ratings yet
- 3 Organic Chemistry SLDocument91 pages3 Organic Chemistry SLShuaib MohamedNo ratings yet
- Ejercicios QO1-1Document3 pagesEjercicios QO1-1hector juarezNo ratings yet
- AreneDocument37 pagesArene'Aqilah ZulkifliNo ratings yet
- Chapter 13 HydrocarbonsDocument23 pagesChapter 13 HydrocarbonsTreasure SeekerNo ratings yet
- Chem 2412 Lecture 01Document28 pagesChem 2412 Lecture 01Jihee YoonNo ratings yet
- Key - 1157854 - 2023-10-11 10 - 56 - 19 +0000Document19 pagesKey - 1157854 - 2023-10-11 10 - 56 - 19 +0000paaryan2624No ratings yet
- Alcohols Phenols and Ether - DPP - 4Document3 pagesAlcohols Phenols and Ether - DPP - 4Priya RangapureNo ratings yet
- Chemistry Practical: Experiment # Experiment NameDocument1 pageChemistry Practical: Experiment # Experiment NameSuperior CollegeNo ratings yet
- HydrocarbonDocument11 pagesHydrocarbonAida IshNo ratings yet
- Saponification (Sapo "Soap")Document3 pagesSaponification (Sapo "Soap")Karlo Roberto M. MarianoNo ratings yet
- BAI 4 ĐỒNG VÀ HỢP CHẤTDocument41 pagesBAI 4 ĐỒNG VÀ HỢP CHẤTLinhh ChiiNo ratings yet
- Review Problems Chapter 4 Solutions PDFDocument4 pagesReview Problems Chapter 4 Solutions PDFAntoninoNo ratings yet
- Diktat Naming Inorganic CompoundDocument6 pagesDiktat Naming Inorganic CompoundGeorge AthensNo ratings yet
- Analytical Chemistry AQA AnswersDocument30 pagesAnalytical Chemistry AQA AnswersAhmad BustamiNo ratings yet
- HN Chem Nomenclature Test Review Answer KeyDocument9 pagesHN Chem Nomenclature Test Review Answer KeyAdi ChhNo ratings yet
- General Characteristics of PolysaccharidesDocument3 pagesGeneral Characteristics of PolysaccharidesDick Andrew RodriguezNo ratings yet
- One MinuteDocument6 pagesOne MinuteEdy PurnomoNo ratings yet
- Macromolecules RiceDocument47 pagesMacromolecules Ricepeterr1022No ratings yet
- Lipid WorksheetDocument2 pagesLipid WorksheetMANUELA VENEGAS ESCOVAR0% (1)
- Chemistry Class 10 Chapter 12Document12 pagesChemistry Class 10 Chapter 12Muhammad Owais FayazNo ratings yet
- Las2 Phy SciDocument2 pagesLas2 Phy SciMa WiNo ratings yet
- Potassium Carbonate P0233Document1 pagePotassium Carbonate P0233kadam.arjun1No ratings yet
- Organic Compound - Identification of Functional Group SchemeDocument3 pagesOrganic Compound - Identification of Functional Group SchemeAMBRIN ABDULNo ratings yet
- Chapter 10. Sulphuric Acid: Short QuestionsDocument14 pagesChapter 10. Sulphuric Acid: Short QuestionsAbhay VishwakarmaNo ratings yet
- Alcohol, Ether, Phenol WorksheetDocument22 pagesAlcohol, Ether, Phenol WorksheetSDMNo ratings yet
- Corrosion AstroCosmosDocument11 pagesCorrosion AstroCosmosNattapong PongbootNo ratings yet
- JR - Inter Ipe Chemistry Model Paper 2Document2 pagesJR - Inter Ipe Chemistry Model Paper 2angadibalajithkumarNo ratings yet
- Ammonium 2520sulphate Material 2520balance.Document9 pagesAmmonium 2520sulphate Material 2520balance.AgadmatorNo ratings yet
- STPM Chemistry Definitions Term 3Document4 pagesSTPM Chemistry Definitions Term 3ChooNo ratings yet
- Ions - Ionic BondingDocument35 pagesIons - Ionic BondingAmjadNo ratings yet