Professional Documents
Culture Documents
HL Questions On Electrophilic Addition Reactions
HL Questions On Electrophilic Addition Reactions
Uploaded by
Pranava0 ratings0% found this document useful (0 votes)
309 views1 pagechem questions ib hl
Original Title
HL-Questions-on-Electrophilic-addition-reactions
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentchem questions ib hl
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
309 views1 pageHL Questions On Electrophilic Addition Reactions
HL Questions On Electrophilic Addition Reactions
Uploaded by
Pranavachem questions ib hl
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
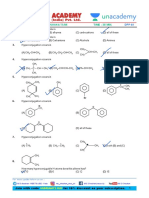
HL Questions on Electrophilic addition reactions
1. Bromine is a non-polar molecule.
(a) Explain how it is able to act as an electrophile when it adds to ethene.
(b) Name the product when bromine is added to ethene in the absence of water.
(c) Explain why the major product obtained is 2-bromethanol if bromine water is
added to ethene.
2. Describe the mechanism for the addition of hydrogen bromide to prop-1-ene.
Your answer should include the use of curly arrows to show the movement of
electron pairs and give the structure and IUPAC name for the major product formed.
3. Deduce the structure and IUPAC name of the major product formed when iodine
chloride, I-Cl, is added to but-1-ene.
4.
2-methylbut-2-ene
Draw the structure of the intermediate carbocation formed when 2-methylbut-2-ene
reacts with hydrogen chloride and state whether the carbocation is primary,
secondary or tertiary.
Dr Geoffrey Neuss, InThinking
©
http://www.thinkib.net/chemistry 1
You might also like
- 531 - Stereochem Practice KeyDocument4 pages531 - Stereochem Practice KeyAli MuhtashamNo ratings yet
- Team Bootcamp - Reaction Summary Sheet PDFDocument30 pagesTeam Bootcamp - Reaction Summary Sheet PDFDagne PovedaNo ratings yet
- CHEM1280 2012 13 Midterm Exam Solution PDFDocument5 pagesCHEM1280 2012 13 Midterm Exam Solution PDFLouisNo ratings yet
- HL Questions On Synthetic Routes: © DR Geoffrey Neuss, InthinkingDocument1 pageHL Questions On Synthetic Routes: © DR Geoffrey Neuss, InthinkingPranavaNo ratings yet
- 02 H.D.A. SN1 and SN2 Reaction 10-08-2021Document2 pages02 H.D.A. SN1 and SN2 Reaction 10-08-2021tejas naigaonkarNo ratings yet
- ThepblockelementsDocument56 pagesThepblockelementsAshutosh Ganesan92% (13)
- Bond Angle Practice SheetDocument3 pagesBond Angle Practice Sheetgaurav100% (1)
- Quantitative and QualitativeDocument15 pagesQuantitative and QualitativesquadralsupremeNo ratings yet
- Kota Worksheet Chemical BondingDocument16 pagesKota Worksheet Chemical BondingYash GargNo ratings yet
- Aldehydes Ketones and Carboxylic AcidsDocument4 pagesAldehydes Ketones and Carboxylic AcidsAnindya AcharyaNo ratings yet
- Chemistry Advanced Level Problem Solving (ALPS-6) - PaperDocument14 pagesChemistry Advanced Level Problem Solving (ALPS-6) - PaperAnanmay ChauhanNo ratings yet
- Critical Reasoning: Drill 1: SolutionsDocument17 pagesCritical Reasoning: Drill 1: SolutionslognNo ratings yet
- Bakliwal Tutorials: Topic: Inductive Effect Part - A: SubjectiveDocument3 pagesBakliwal Tutorials: Topic: Inductive Effect Part - A: SubjectiveRitesh SonawaneNo ratings yet
- Atomic Structure: Examples of Multiple Choice QuestionsDocument4 pagesAtomic Structure: Examples of Multiple Choice Questionsngah lidwineNo ratings yet
- Benzoin CondensationDocument3 pagesBenzoin Condensationprivatesanket710No ratings yet
- This DPP Gives You An Idea About Products Obtained in Elimination Reaction (Including Stereochemistry)Document3 pagesThis DPP Gives You An Idea About Products Obtained in Elimination Reaction (Including Stereochemistry)Aaryan KeshanNo ratings yet
- Ms Chouhan & Team: Organic ChemistryDocument16 pagesMs Chouhan & Team: Organic ChemistryPrithviraj GhoshNo ratings yet
- Ionic EquilibriumDocument39 pagesIonic EquilibriumAnuragPandeyNo ratings yet
- Coordination CompoundsDocument27 pagesCoordination CompoundsIndranilNo ratings yet
- P-Block (Group 13 To 14) NM Solution (-1) Chem PDFDocument31 pagesP-Block (Group 13 To 14) NM Solution (-1) Chem PDFChauhan RonakNo ratings yet
- R IS IR: Iupac & NomenclatureDocument11 pagesR IS IR: Iupac & NomenclatureDhruv KuchhalNo ratings yet
- Model Questions On U-12, 13 & 14Document12 pagesModel Questions On U-12, 13 & 14kadedoxNo ratings yet
- JEE (Main+Advanced) 2023: Chem. Worksheet-5 IupacDocument2 pagesJEE (Main+Advanced) 2023: Chem. Worksheet-5 IupacSoham X ANo ratings yet
- Index: Hydrocarbons (Alkanes, Alkenes & Alkynes)Document31 pagesIndex: Hydrocarbons (Alkanes, Alkenes & Alkynes)Harsh VardhanNo ratings yet
- 11 Chemistry Exemplar Chapter 4Document12 pages11 Chemistry Exemplar Chapter 4adarshNo ratings yet
- Alcohols Ethers and Phenol-03 - Assignments (New)Document26 pagesAlcohols Ethers and Phenol-03 - Assignments (New)Raju SinghNo ratings yet
- Worksheet Chemistry 12Document5 pagesWorksheet Chemistry 12mohit kumarNo ratings yet
- Stereo Chemistry QuestionsDocument19 pagesStereo Chemistry QuestionsfritzNo ratings yet
- Chemistry ProjectDocument17 pagesChemistry ProjectANTARO MASSENNo ratings yet
- Alcohol, Ether & Phenol - QuestionDocument3 pagesAlcohol, Ether & Phenol - Questionbest badmintonNo ratings yet
- BOC Complete 1 To 5 DPPDocument5 pagesBOC Complete 1 To 5 DPPBhawna SharmaNo ratings yet
- PRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNDocument3 pagesPRACTICE SHEET - 03 (Chemistry) : CH - CH - CH CN CN CNABD 17No ratings yet
- 04 IsomerismDocument19 pages04 IsomerismSoham RaneNo ratings yet
- Chemistry Advanced Level Problem Solving (ALPS-5) - SolutionDocument12 pagesChemistry Advanced Level Problem Solving (ALPS-5) - SolutionAnanmay Chauhan100% (1)
- Test1 Goc & Poc Tough by S.K.sinha See Chemistry Animations atDocument3 pagesTest1 Goc & Poc Tough by S.K.sinha See Chemistry Animations atmyiitchemistry100% (1)
- GOC Sheet PDFDocument55 pagesGOC Sheet PDFAayush KharbandaNo ratings yet
- Nomenclature: Chemistry DPP 1 by Garima Verma (Chemistry Faculty) - Referral Code: "Cgvmam"Document4 pagesNomenclature: Chemistry DPP 1 by Garima Verma (Chemistry Faculty) - Referral Code: "Cgvmam"Tanisha SubudhiNo ratings yet
- IsomerismDocument62 pagesIsomerismsubesinghNo ratings yet
- Prince Singh: Physical & Inorganic ChemistryDocument5 pagesPrince Singh: Physical & Inorganic ChemistryJatin Singla100% (1)
- Raj7 PDFDocument22 pagesRaj7 PDFAvdhoot GautamNo ratings yet
- Anic Chemistry Carbonyl CompoundsDocument6 pagesAnic Chemistry Carbonyl Compoundseamcetmaterials100% (1)
- DPP - 01 - Reaction MechanismDocument5 pagesDPP - 01 - Reaction Mechanismbaibhav singhNo ratings yet
- Aromaticity Assingment PDFDocument10 pagesAromaticity Assingment PDFGaurav YadavNo ratings yet
- 13 05 13 Chemistry Electrochemistry Assignment 3Document7 pages13 05 13 Chemistry Electrochemistry Assignment 3Gadde Gopala KrishnaNo ratings yet
- DPP 2Document2 pagesDPP 2ashutosh99878No ratings yet
- Reaction Mechanism Jeemain - GuruDocument12 pagesReaction Mechanism Jeemain - Guruprexa indiaNo ratings yet
- Chemical BondingDocument36 pagesChemical BondingMohitNo ratings yet
- 11.alcohol, Phenol & Ethers Colour Booklet PDFDocument59 pages11.alcohol, Phenol & Ethers Colour Booklet PDFMridu BhandariNo ratings yet
- Important Questions For CBSE Class 12 Chemistry The P-Block ElementsDocument41 pagesImportant Questions For CBSE Class 12 Chemistry The P-Block ElementsyndtfndtgndNo ratings yet
- Unacademy - IOCXII MegaDPP 23withoutDocument2 pagesUnacademy - IOCXII MegaDPP 23withoutAaryan KeshanNo ratings yet
- CLS Aipmt-19-20 XIII Che Study-Package-3 Level-1 Chapter-15 PDFDocument40 pagesCLS Aipmt-19-20 XIII Che Study-Package-3 Level-1 Chapter-15 PDFThavasimariselvam N100% (1)
- Mock Test 5 Paper 1 Q. PaperDocument16 pagesMock Test 5 Paper 1 Q. PaperRare RootNo ratings yet
- SN DPPDocument34 pagesSN DPPtyagiabhishek145No ratings yet
- (Career Endeavour) GATE Chemistry Test Series (B-Ok - Xyz)Document61 pages(Career Endeavour) GATE Chemistry Test Series (B-Ok - Xyz)stormNo ratings yet
- Solutions - Practice Sheet - Sarthak KCET PDFDocument6 pagesSolutions - Practice Sheet - Sarthak KCET PDFAkanksh KNo ratings yet
- Reaction Mechanism PDFDocument14 pagesReaction Mechanism PDFSreeragNo ratings yet
- Alcohols WsDocument5 pagesAlcohols WsVedanta DesikNo ratings yet
- C - 2Y - Dilute Solution and Colligative Properties - Assignment 1Document5 pagesC - 2Y - Dilute Solution and Colligative Properties - Assignment 1Phani PadmasriNo ratings yet
- Questions Chapter 1-10 PDFDocument107 pagesQuestions Chapter 1-10 PDFrashidNo ratings yet
- Practice Questions AlkenesDocument9 pagesPractice Questions Alkenesibrahim ahmedNo ratings yet
- XII - Second Unit Test - CHEMISTRYDocument3 pagesXII - Second Unit Test - CHEMISTRYrshobana78No ratings yet
- Calculations - Acid and BasesDocument6 pagesCalculations - Acid and BasesPranavaNo ratings yet
- Applying Machine Learning in Liver Disease & Transplantation: A Comprehensive ReviewDocument24 pagesApplying Machine Learning in Liver Disease & Transplantation: A Comprehensive ReviewPranavaNo ratings yet
- SL & HL Questions On The Mole and Avogadro'S Constant: © DR Geoffrey Neuss, InthinkingDocument1 pageSL & HL Questions On The Mole and Avogadro'S Constant: © DR Geoffrey Neuss, InthinkingPranavaNo ratings yet
- DP Year 1 Subject: Biology: Worksheet: Revision 1 Date: 3.12.2020Document2 pagesDP Year 1 Subject: Biology: Worksheet: Revision 1 Date: 3.12.2020PranavaNo ratings yet