Professional Documents
Culture Documents
CHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)
CHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)
Uploaded by
api-535641126Copyright:
Available Formats
You might also like
- Detailed Lesson Plan in Science 10Document5 pagesDetailed Lesson Plan in Science 10Yeng Santos100% (11)
- Taller Fisica #2Document3 pagesTaller Fisica #2Paula AstudilloNo ratings yet
- Interchangeability PDFDocument16 pagesInterchangeability PDFMaulikNo ratings yet
- CHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)Document6 pagesCHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)api-535713716No ratings yet
- CHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)Document6 pagesCHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)api-535368507No ratings yet
- CHEM 1701 - Lab 7 - Gas Laws by Giulia Barros 100638389 /10 MarksDocument7 pagesCHEM 1701 - Lab 7 - Gas Laws by Giulia Barros 100638389 /10 Marksapi-535498730No ratings yet
- Experiment 4Document12 pagesExperiment 4jamiecfraser08No ratings yet
- General Chemistry 1 Week 5 6Document10 pagesGeneral Chemistry 1 Week 5 6Emmanuel ValenzuelaNo ratings yet
- 11N Rasonable CHEM 01L Experiment 7B Worksheet Gas LawsDocument3 pages11N Rasonable CHEM 01L Experiment 7B Worksheet Gas LawsChristian Jay GallardoNo ratings yet
- Worksheet - Gas LawsDocument4 pagesWorksheet - Gas LawsAshley Kate LupoNo ratings yet
- Gas Law ExpDocument6 pagesGas Law ExpRonet Lopez RodriguezNo ratings yet
- q4 Las2 g10 Science Boyles-LawDocument9 pagesq4 Las2 g10 Science Boyles-Lawtheobarrios14No ratings yet
- Vapro Pressure and Heat Heat of VaporazationDocument5 pagesVapro Pressure and Heat Heat of VaporazationStephen Rey CaldeaNo ratings yet
- Chem301 Lab ManualDocument42 pagesChem301 Lab ManualIreneVeladoNo ratings yet
- Unit 7 Gas Laws Packet 2021Document40 pagesUnit 7 Gas Laws Packet 2021Alberto Laborte Jr.No ratings yet
- Science 10 Las 4-1Document5 pagesScience 10 Las 4-1Michael TuyayNo ratings yet
- For Combined Gas LawDocument44 pagesFor Combined Gas LawApril Bartolome Flores100% (1)
- Canned Lesson 02Document7 pagesCanned Lesson 02Jeramie Mabaet JabagatNo ratings yet
- Key - 8.1 Gas Law Lab PDFDocument6 pagesKey - 8.1 Gas Law Lab PDFzhuzaiNo ratings yet
- WS Practice W GraphsDocument4 pagesWS Practice W GraphsgiyagirlsNo ratings yet
- 4th Quarter Week 1 1Document59 pages4th Quarter Week 1 1Jonathan MayoNo ratings yet
- Pogil Boyles LawDocument4 pagesPogil Boyles LawRussel OtillaNo ratings yet
- 112 Experiment 4Document3 pages112 Experiment 4Abhishek KunduNo ratings yet
- Lab Exercise No. 6: Ideal Gas Law: Department of Mechanical EngineeringDocument3 pagesLab Exercise No. 6: Ideal Gas Law: Department of Mechanical EngineeringJino BalingNo ratings yet
- Q1 - GC1 - Week 7Document6 pagesQ1 - GC1 - Week 7Lani DawisNo ratings yet
- Physical Chemistry Mid Term ExamDocument4 pagesPhysical Chemistry Mid Term ExamMaricar DimasNo ratings yet
- Page 1 of 6 Mechanical Engineering Laboratory I Laboratory ReportDocument6 pagesPage 1 of 6 Mechanical Engineering Laboratory I Laboratory ReportJeremy Balones DadulaNo ratings yet
- KMT ws2Document10 pagesKMT ws2Troy MateoNo ratings yet
- Science Grade 10: Quarter 4 - Behavior of GasesDocument15 pagesScience Grade 10: Quarter 4 - Behavior of GasesalindongaprilmaeNo ratings yet
- Kinetics Expt 4-2011Document7 pagesKinetics Expt 4-2011Wilo JaraNo ratings yet
- 4th Quarter Booklet 20202021 FinalDocument7 pages4th Quarter Booklet 20202021 FinalRAYMUND RODILLONo ratings yet
- Science 10 Q4 M1Document15 pagesScience 10 Q4 M1Francis Paul PelonesNo ratings yet
- Gas Laws 4th LPDocument17 pagesGas Laws 4th LParlene dioknoNo ratings yet
- Final Lesson Plan For Science 10Document4 pagesFinal Lesson Plan For Science 10Ryan Lloyd Labartine100% (1)
- 121 NLab 6 Molar Mass CO2Document4 pages121 NLab 6 Molar Mass CO2GAVIN KURNIAWANNo ratings yet
- Thermodynamics-II Practical Final Exam & VIVA: InstructionsDocument4 pagesThermodynamics-II Practical Final Exam & VIVA: InstructionsUzair BukhariNo ratings yet
- Learners' Activity Sheet (LAS)Document5 pagesLearners' Activity Sheet (LAS)MARIA THEA CALAGUASNo ratings yet
- 2.1.3-Ans-Gases & Amp Absolute Scale of TempDocument10 pages2.1.3-Ans-Gases & Amp Absolute Scale of TempYara AlaaNo ratings yet
- Measurement of The Gas Constant and Molar Volume of Oxygen GasDocument12 pagesMeasurement of The Gas Constant and Molar Volume of Oxygen GasJennifer Im0% (1)
- Science 10 Q4 LAS Week 1.2Document4 pagesScience 10 Q4 LAS Week 1.2Eainne David DocogNo ratings yet
- Charles' LawDocument5 pagesCharles' LawSuganya BaabuNo ratings yet
- ReportDocument10 pagesReportapi-272632563No ratings yet
- GaslawsexplorationlabDocument5 pagesGaslawsexplorationlabapi-320087626No ratings yet
- Gay-Lussac's Law: Lab 6 - Procedure - Burping BottleDocument3 pagesGay-Lussac's Law: Lab 6 - Procedure - Burping Bottleapi-584842991No ratings yet
- Experiment P2: Bomb Calorimetry: Any Question On This Document ToDocument8 pagesExperiment P2: Bomb Calorimetry: Any Question On This Document TomokilpoNo ratings yet
- Boyle'S Law Module 10Document12 pagesBoyle'S Law Module 10Greth NuevaNo ratings yet
- Sci 10 Q4 Week 1Document6 pagesSci 10 Q4 Week 1Jan romar FloresNo ratings yet
- CHEM301 - Experiment 1 - Manual - P-V and P-T Relationships of Ideal and Real Gases - Fall2021Document9 pagesCHEM301 - Experiment 1 - Manual - P-V and P-T Relationships of Ideal and Real Gases - Fall2021FULL DİAMOND SET HONEYBADGERNo ratings yet
- Gas Laws Test ReviewDocument2 pagesGas Laws Test ReviewXzyle1213No ratings yet
- Lab 5 Enthalpy of VaporizationDocument4 pagesLab 5 Enthalpy of VaporizationFrolian MichaelNo ratings yet
- Science 10 Q4 LAS Week 1.1Document3 pagesScience 10 Q4 LAS Week 1.1Eainne David DocogNo ratings yet
- NRSL GasLawsDocument48 pagesNRSL GasLawsBrenda Aquino100% (1)
- Chem M9 Gas LawsDocument25 pagesChem M9 Gas LawsMa Perpetua Bardelas BaldescoNo ratings yet
- CD III - PALAYAN - HS - Torres, Queencess Ara P.Document3 pagesCD III - PALAYAN - HS - Torres, Queencess Ara P.Queencess Ara TorresNo ratings yet
- Cell RespirationDocument5 pagesCell Respirationapi-256921267No ratings yet
- Microsoft Word - Gas Laws LabDocument4 pagesMicrosoft Word - Gas Laws LabGagandeep SinghNo ratings yet
- Genchem Experiments Gas LawsDocument7 pagesGenchem Experiments Gas LawsMr JNo ratings yet
- Experiment Gas LawsDocument8 pagesExperiment Gas Lawsapi-254428474No ratings yet
- Dalton's Law of Partial Pressures PracticeDocument2 pagesDalton's Law of Partial Pressures PracticemollyNo ratings yet
- Science 10 - Week 27Document3 pagesScience 10 - Week 27Mira VeranoNo ratings yet
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterFrom EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterRating: 5 out of 5 stars5/5 (1)
- 1 Hot Tap MKSR Dec 2018 003Document137 pages1 Hot Tap MKSR Dec 2018 003Billy KurniawanNo ratings yet
- FluidsDocument55 pagesFluidsdanena88No ratings yet
- Thermo II Lecture 21Document16 pagesThermo II Lecture 21Jamil Flt LtNo ratings yet
- Differences Between A Thermostatic Expansion Valve and An Electronic Expansion ValveDocument4 pagesDifferences Between A Thermostatic Expansion Valve and An Electronic Expansion Valvexuyen tran100% (1)
- Esp Design Data SheetDocument6 pagesEsp Design Data SheetMohamed Abd El-MoniemNo ratings yet
- Fluid Mechanics (Compre)Document33 pagesFluid Mechanics (Compre)Ruthie GalitNo ratings yet
- Twin Screw Compressor Performance and Its Relationship With RotorDocument7 pagesTwin Screw Compressor Performance and Its Relationship With RotorSaeedAkbarzadehNo ratings yet
- Check Valve c400sDocument3 pagesCheck Valve c400sPatricio Antonio Cortés PeñaNo ratings yet
- OSNA Pumpen Piston PumpsDocument4 pagesOSNA Pumpen Piston Pumpsonur sarıcaNo ratings yet
- Snag ListDocument125 pagesSnag ListNeal JohnsonNo ratings yet
- Unit 2.5, Expansion ValveDocument4 pagesUnit 2.5, Expansion ValveSwarg Vibha100% (1)
- AERO462 - Outline - F2021Document4 pagesAERO462 - Outline - F2021Kyron CallisteNo ratings yet
- Effect of Capillary Tube Shapes On The Performance of Vapour Compression Refrigeration Cycle Using Nano-RefrigerantDocument10 pagesEffect of Capillary Tube Shapes On The Performance of Vapour Compression Refrigeration Cycle Using Nano-RefrigerantIJRASETPublicationsNo ratings yet
- PF Pump Specification: Stanadyne"Document2 pagesPF Pump Specification: Stanadyne"Miguel RojasNo ratings yet
- ViscosityDocument28 pagesViscosityafic219473No ratings yet
- 040Document7 pages040Googool YNo ratings yet
- How Squat, Bank and Bank Cushion Effects Influence Ships in Restricted Waters?Document7 pagesHow Squat, Bank and Bank Cushion Effects Influence Ships in Restricted Waters?shubham100% (1)
- Cfturbo & TCFD: ProjectDocument29 pagesCfturbo & TCFD: ProjectHazim HazimNo ratings yet
- Basic Pump FundamentalDocument99 pagesBasic Pump FundamentalJimit Shah100% (2)
- 072 90 00 EngineAcessoryDocument30 pages072 90 00 EngineAcessoryblackhawkNo ratings yet
- Coming Out of The Ice Age - BASF Durasorb Cryo-HRUDocument11 pagesComing Out of The Ice Age - BASF Durasorb Cryo-HRULiu YangtzeNo ratings yet
- 1 RCF Ammo V RevampDocument7 pages1 RCF Ammo V RevamprvnesariNo ratings yet
- DokwegII - Auxiliary - Maintenance Compressed Air SystemDocument62 pagesDokwegII - Auxiliary - Maintenance Compressed Air SystemCarlin BabuchasNo ratings yet
- Operator S Manual: Non-Cycling Refrigerated Air/Gas Dryers QPNC 75 To QPNC 250Document16 pagesOperator S Manual: Non-Cycling Refrigerated Air/Gas Dryers QPNC 75 To QPNC 250mike leveilleNo ratings yet
- Contoh Soal ViskositasDocument2 pagesContoh Soal ViskositaswsdodoNo ratings yet
- Hydraulic and Surge Analysis in A Pipeline NetworkDocument9 pagesHydraulic and Surge Analysis in A Pipeline Networkrasnowmah2012No ratings yet
- Pressure Vessel Dimension InspectionDocument15 pagesPressure Vessel Dimension Inspectionnaveenbaskaran1989100% (1)
- Log Splitter HydraulicsDocument4 pagesLog Splitter HydraulicsCristian FloricăNo ratings yet
CHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)
CHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)
Uploaded by
api-535641126Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)
CHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)
Uploaded by
api-535641126Copyright:
Available Formats
CHEM 1701 – Lab 7 – Gas Laws
Chemistry I for Pre-Health Sciences (online)
____/33 marks (6% of final grade)
Rationale
The purpose of this lab is to explore the gas laws and their application in a healthcare context.
Learning outcomes
The following learning objectives are covered in this lab.
CLO 5: Apply the gas laws to relate the properties of pressure, volume and temperature of gases.
CLO 6: Prepare for and conduct laboratory experiments to investigate scientific questions using
appropriate techniques.
CLO 7: Examine the relationships between chemistry and the health of the human body.
Handing in your lab
When complete, submit your work to the appropriate folder in DC Connect under Assignments.
Required materials:
bottle with a small opening (opening must be smaller than the size of a dime)
dime
large bowl
ice and water
NOTE – You may substitute any of the above items with similar objects that are available to you. There is no
need to purchase any materials for this lab.
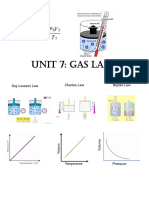
Gas Law Formula Relationship
A gas law that describes the pressure and volume behavior of a gas
Boyle’s law P1V1 = P2V2 sample kept at constant temperature.
V1 V2 A gas law that describes the temperature and volume behavior of a
Charles’s law = gas sample kept at constant pressure.
T1 T2 T is always in K.
Gay-Lusacc’s P1 P2 A gas law that describes the pressure and temperature behavior of a
=
law T1 T2 gas sample kept at constant volume. T is always in K.
CHEM 2701 – Lab 1 – Gas Laws Page 1 of 6
Experiment 1 – The Bottle and the Dime [9 marks]
Background: In this experiment, you will examining the relationship between the pressure and temperature
of a gas under a constant volume. A “closed container” like a bottle with a lid on it has a constant volume. No
air can get in or out.
Procedure & Observations
1. Make an ice bath by filling a large bowl with ice and enough water to cover the ice. Place any bottle in
the ice water bath for 3 minutes as shown in the image to the right.
2. After 3 minutes, remove the bottle from the ice water bath. Working quickly, place a little water around
the rim of the bottle. Then place a dime on the bottle opening so it seals the bottle.
3. Warm the bottle by holding it between your hands for 1 minute.
4. [1 mark] Record your observations in the space below. In 1-2 sentences, explain what happened to the
dime after you warm the bottle up with your hands.
Data & Analysis
1. [1 mark] This demonstration shows the relationship between gas temperature and gas pressure.
Therefore, this experiment demonstrates ________________ law.
2. [2 marks] In 1-2 sentences, describe the effect of temperature on air pressure (i.e. what happens to the
air particles in the ice water bath and when the bottle is warmed up with your hands)
Ice water bath Warming in hands
3. [2 marks] Using the template below, draw how the air particles behave on the molecular level for each
scenario.
Cooled bottle Warm bottle
CHEM 2701 – Lab 1 – Gas Laws Page 2 of 6
4. [3 marks] Fill in the blanks below. Use the words increases, decreases, directly proportional, or
inversely proportional to complete the question.
As temperature ___________________, pressure ___________________.
Therefore, the relationship between the variables is ___________________.
Experiment 2 – Balloon Bonanza [11 marks]
Background: In this experiment, you will observe the effect of temperature on the volume of the balloon under
constant pressure.
Procedure: Watch the video below and answer the questions that follow.
Video [stop at 3:36]: https://youtu.be/NplVuTrr59U
Data & Analysis
1. [1 mark] This demonstration shows the relationship between gas temperature and gas volume. Therefore,
this experiment demonstrates _____________ law.
2. [1 mark] In 1-2 sentences, record your observations in the space below of what happened to the balloon
while in the beaker of hot water?
CHEM 2701 – Lab 1 – Gas Laws Page 3 of 6
3. [3 marks] Use the template below to draw how the air particles in the balloon behave on the molecular
level at each temperature.
Room temperature balloon Balloon in hot water Balloon in freezer
4. [2 marks] Look up the boiling point of water in oC. Convert this value to Kelvin. Show your work and
include the link to the reference used in your research.
5. [1 mark] You are in a party store buying a birthday balloon. Using the observations from this experiment,
predict what would happen to the volume of the balloon when you walk it to your car in the winter when it is
cold outside.
6. [3 marks] Fill in the blanks below. Use the words increases, decreases, directly proportional, or inversely
proportional to complete the question.
As temperature ________________, volume _______________. Therefore, the relationship between
temperature and gas volume is _______________.
CHEM 2701 – Lab 1 – Gas Laws Page 4 of 6
Health Connection Questions [13 marks]
Respiration is driven by Boyle’s law. The process is shown in the diagram below.
Inspiration Expiration
diaphragm moves down diaphragm moves up
1. [4 marks] Use the diagram to fill in the blanks below. Include proper units.
a. What is the external pressure in each case? _____________
b. What is the internal pressure during inspiration? _____________
c. What is the internal pressure during expiration? _____________
d. What is the difference between the internal and external pressure in each case? ___________
2. [4 marks] Use the words up, down, increases, decreases, in or out to fill in the blanks below.
a. Inspiration causes the diaphragm to move ________. This is shown in the image on the left. When
this happens, the size of the thoracic cage ____________________ and internal pressure
____________________. As a result, and air moves ______.
b. Expiration causes the diaphragm to move ________. This is shown in the image on the right.
When this happens, the size of the thoracic cage ____________________ and internal pressure
____________________. As a result, air moves _____.
CHEM 2701 – Lab 1 – Gas Laws Page 5 of 6
3. You are working as a paramedic and arrive at a 911 call to find a patient unconscious with labored
breathing. One intervention you decide is to administer oxygen immediately. It has been a busy
night though and you are concerned you may need to use the oxygen tank again on the next call.
The oxygen in the tank is under high pressure. The oxygen tank in the ambulance has a physical
volume of 4.7 L and an internal pressure of 13,700 kPa. The external pressure (the pressure 4.7 L
outside of the cylinder) is atmospheric pressure, 101.3 kPa.
a. Use the information above to identify the variables P1, V1 and P2.
Internal conditions External conditions
P1 = P2 =
V1 = V2 = ???
b. [3 marks] Using the data in part a, solve for the volume of the oxygen tank at atmospheric
pressure. Show your work including the gas law equation and substituting the variables into the
equation. Include the correct units and significant digits.
c. [2 marks] If the average basal rate of oxygen consumption for an adult is 15 L/hour, then
calculate how many hours of oxygen use will you get from the 4.7 L tank? Round your answer to
1 decimal place.
CHEM 2701 – Lab 1 – Gas Laws Page 6 of 6
You might also like
- Detailed Lesson Plan in Science 10Document5 pagesDetailed Lesson Plan in Science 10Yeng Santos100% (11)
- Taller Fisica #2Document3 pagesTaller Fisica #2Paula AstudilloNo ratings yet
- Interchangeability PDFDocument16 pagesInterchangeability PDFMaulikNo ratings yet
- CHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)Document6 pagesCHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)api-535713716No ratings yet
- CHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)Document6 pagesCHEM 1701 - Lab 7 - Gas Laws: Chemistry I For Pre-Health Sciences (Online)api-535368507No ratings yet
- CHEM 1701 - Lab 7 - Gas Laws by Giulia Barros 100638389 /10 MarksDocument7 pagesCHEM 1701 - Lab 7 - Gas Laws by Giulia Barros 100638389 /10 Marksapi-535498730No ratings yet
- Experiment 4Document12 pagesExperiment 4jamiecfraser08No ratings yet
- General Chemistry 1 Week 5 6Document10 pagesGeneral Chemistry 1 Week 5 6Emmanuel ValenzuelaNo ratings yet
- 11N Rasonable CHEM 01L Experiment 7B Worksheet Gas LawsDocument3 pages11N Rasonable CHEM 01L Experiment 7B Worksheet Gas LawsChristian Jay GallardoNo ratings yet
- Worksheet - Gas LawsDocument4 pagesWorksheet - Gas LawsAshley Kate LupoNo ratings yet
- Gas Law ExpDocument6 pagesGas Law ExpRonet Lopez RodriguezNo ratings yet
- q4 Las2 g10 Science Boyles-LawDocument9 pagesq4 Las2 g10 Science Boyles-Lawtheobarrios14No ratings yet
- Vapro Pressure and Heat Heat of VaporazationDocument5 pagesVapro Pressure and Heat Heat of VaporazationStephen Rey CaldeaNo ratings yet
- Chem301 Lab ManualDocument42 pagesChem301 Lab ManualIreneVeladoNo ratings yet
- Unit 7 Gas Laws Packet 2021Document40 pagesUnit 7 Gas Laws Packet 2021Alberto Laborte Jr.No ratings yet
- Science 10 Las 4-1Document5 pagesScience 10 Las 4-1Michael TuyayNo ratings yet
- For Combined Gas LawDocument44 pagesFor Combined Gas LawApril Bartolome Flores100% (1)
- Canned Lesson 02Document7 pagesCanned Lesson 02Jeramie Mabaet JabagatNo ratings yet
- Key - 8.1 Gas Law Lab PDFDocument6 pagesKey - 8.1 Gas Law Lab PDFzhuzaiNo ratings yet
- WS Practice W GraphsDocument4 pagesWS Practice W GraphsgiyagirlsNo ratings yet
- 4th Quarter Week 1 1Document59 pages4th Quarter Week 1 1Jonathan MayoNo ratings yet
- Pogil Boyles LawDocument4 pagesPogil Boyles LawRussel OtillaNo ratings yet
- 112 Experiment 4Document3 pages112 Experiment 4Abhishek KunduNo ratings yet
- Lab Exercise No. 6: Ideal Gas Law: Department of Mechanical EngineeringDocument3 pagesLab Exercise No. 6: Ideal Gas Law: Department of Mechanical EngineeringJino BalingNo ratings yet
- Q1 - GC1 - Week 7Document6 pagesQ1 - GC1 - Week 7Lani DawisNo ratings yet
- Physical Chemistry Mid Term ExamDocument4 pagesPhysical Chemistry Mid Term ExamMaricar DimasNo ratings yet
- Page 1 of 6 Mechanical Engineering Laboratory I Laboratory ReportDocument6 pagesPage 1 of 6 Mechanical Engineering Laboratory I Laboratory ReportJeremy Balones DadulaNo ratings yet
- KMT ws2Document10 pagesKMT ws2Troy MateoNo ratings yet
- Science Grade 10: Quarter 4 - Behavior of GasesDocument15 pagesScience Grade 10: Quarter 4 - Behavior of GasesalindongaprilmaeNo ratings yet
- Kinetics Expt 4-2011Document7 pagesKinetics Expt 4-2011Wilo JaraNo ratings yet
- 4th Quarter Booklet 20202021 FinalDocument7 pages4th Quarter Booklet 20202021 FinalRAYMUND RODILLONo ratings yet
- Science 10 Q4 M1Document15 pagesScience 10 Q4 M1Francis Paul PelonesNo ratings yet
- Gas Laws 4th LPDocument17 pagesGas Laws 4th LParlene dioknoNo ratings yet
- Final Lesson Plan For Science 10Document4 pagesFinal Lesson Plan For Science 10Ryan Lloyd Labartine100% (1)
- 121 NLab 6 Molar Mass CO2Document4 pages121 NLab 6 Molar Mass CO2GAVIN KURNIAWANNo ratings yet
- Thermodynamics-II Practical Final Exam & VIVA: InstructionsDocument4 pagesThermodynamics-II Practical Final Exam & VIVA: InstructionsUzair BukhariNo ratings yet
- Learners' Activity Sheet (LAS)Document5 pagesLearners' Activity Sheet (LAS)MARIA THEA CALAGUASNo ratings yet
- 2.1.3-Ans-Gases & Amp Absolute Scale of TempDocument10 pages2.1.3-Ans-Gases & Amp Absolute Scale of TempYara AlaaNo ratings yet
- Measurement of The Gas Constant and Molar Volume of Oxygen GasDocument12 pagesMeasurement of The Gas Constant and Molar Volume of Oxygen GasJennifer Im0% (1)
- Science 10 Q4 LAS Week 1.2Document4 pagesScience 10 Q4 LAS Week 1.2Eainne David DocogNo ratings yet
- Charles' LawDocument5 pagesCharles' LawSuganya BaabuNo ratings yet
- ReportDocument10 pagesReportapi-272632563No ratings yet
- GaslawsexplorationlabDocument5 pagesGaslawsexplorationlabapi-320087626No ratings yet
- Gay-Lussac's Law: Lab 6 - Procedure - Burping BottleDocument3 pagesGay-Lussac's Law: Lab 6 - Procedure - Burping Bottleapi-584842991No ratings yet
- Experiment P2: Bomb Calorimetry: Any Question On This Document ToDocument8 pagesExperiment P2: Bomb Calorimetry: Any Question On This Document TomokilpoNo ratings yet
- Boyle'S Law Module 10Document12 pagesBoyle'S Law Module 10Greth NuevaNo ratings yet
- Sci 10 Q4 Week 1Document6 pagesSci 10 Q4 Week 1Jan romar FloresNo ratings yet
- CHEM301 - Experiment 1 - Manual - P-V and P-T Relationships of Ideal and Real Gases - Fall2021Document9 pagesCHEM301 - Experiment 1 - Manual - P-V and P-T Relationships of Ideal and Real Gases - Fall2021FULL DİAMOND SET HONEYBADGERNo ratings yet
- Gas Laws Test ReviewDocument2 pagesGas Laws Test ReviewXzyle1213No ratings yet
- Lab 5 Enthalpy of VaporizationDocument4 pagesLab 5 Enthalpy of VaporizationFrolian MichaelNo ratings yet
- Science 10 Q4 LAS Week 1.1Document3 pagesScience 10 Q4 LAS Week 1.1Eainne David DocogNo ratings yet
- NRSL GasLawsDocument48 pagesNRSL GasLawsBrenda Aquino100% (1)
- Chem M9 Gas LawsDocument25 pagesChem M9 Gas LawsMa Perpetua Bardelas BaldescoNo ratings yet
- CD III - PALAYAN - HS - Torres, Queencess Ara P.Document3 pagesCD III - PALAYAN - HS - Torres, Queencess Ara P.Queencess Ara TorresNo ratings yet
- Cell RespirationDocument5 pagesCell Respirationapi-256921267No ratings yet
- Microsoft Word - Gas Laws LabDocument4 pagesMicrosoft Word - Gas Laws LabGagandeep SinghNo ratings yet
- Genchem Experiments Gas LawsDocument7 pagesGenchem Experiments Gas LawsMr JNo ratings yet
- Experiment Gas LawsDocument8 pagesExperiment Gas Lawsapi-254428474No ratings yet
- Dalton's Law of Partial Pressures PracticeDocument2 pagesDalton's Law of Partial Pressures PracticemollyNo ratings yet
- Science 10 - Week 27Document3 pagesScience 10 - Week 27Mira VeranoNo ratings yet
- Practice Makes Perfect in Chemistry: The Physical Behavior of MatterFrom EverandPractice Makes Perfect in Chemistry: The Physical Behavior of MatterRating: 5 out of 5 stars5/5 (1)
- 1 Hot Tap MKSR Dec 2018 003Document137 pages1 Hot Tap MKSR Dec 2018 003Billy KurniawanNo ratings yet
- FluidsDocument55 pagesFluidsdanena88No ratings yet
- Thermo II Lecture 21Document16 pagesThermo II Lecture 21Jamil Flt LtNo ratings yet
- Differences Between A Thermostatic Expansion Valve and An Electronic Expansion ValveDocument4 pagesDifferences Between A Thermostatic Expansion Valve and An Electronic Expansion Valvexuyen tran100% (1)
- Esp Design Data SheetDocument6 pagesEsp Design Data SheetMohamed Abd El-MoniemNo ratings yet
- Fluid Mechanics (Compre)Document33 pagesFluid Mechanics (Compre)Ruthie GalitNo ratings yet
- Twin Screw Compressor Performance and Its Relationship With RotorDocument7 pagesTwin Screw Compressor Performance and Its Relationship With RotorSaeedAkbarzadehNo ratings yet
- Check Valve c400sDocument3 pagesCheck Valve c400sPatricio Antonio Cortés PeñaNo ratings yet
- OSNA Pumpen Piston PumpsDocument4 pagesOSNA Pumpen Piston Pumpsonur sarıcaNo ratings yet
- Snag ListDocument125 pagesSnag ListNeal JohnsonNo ratings yet
- Unit 2.5, Expansion ValveDocument4 pagesUnit 2.5, Expansion ValveSwarg Vibha100% (1)
- AERO462 - Outline - F2021Document4 pagesAERO462 - Outline - F2021Kyron CallisteNo ratings yet
- Effect of Capillary Tube Shapes On The Performance of Vapour Compression Refrigeration Cycle Using Nano-RefrigerantDocument10 pagesEffect of Capillary Tube Shapes On The Performance of Vapour Compression Refrigeration Cycle Using Nano-RefrigerantIJRASETPublicationsNo ratings yet
- PF Pump Specification: Stanadyne"Document2 pagesPF Pump Specification: Stanadyne"Miguel RojasNo ratings yet
- ViscosityDocument28 pagesViscosityafic219473No ratings yet
- 040Document7 pages040Googool YNo ratings yet
- How Squat, Bank and Bank Cushion Effects Influence Ships in Restricted Waters?Document7 pagesHow Squat, Bank and Bank Cushion Effects Influence Ships in Restricted Waters?shubham100% (1)
- Cfturbo & TCFD: ProjectDocument29 pagesCfturbo & TCFD: ProjectHazim HazimNo ratings yet
- Basic Pump FundamentalDocument99 pagesBasic Pump FundamentalJimit Shah100% (2)
- 072 90 00 EngineAcessoryDocument30 pages072 90 00 EngineAcessoryblackhawkNo ratings yet
- Coming Out of The Ice Age - BASF Durasorb Cryo-HRUDocument11 pagesComing Out of The Ice Age - BASF Durasorb Cryo-HRULiu YangtzeNo ratings yet
- 1 RCF Ammo V RevampDocument7 pages1 RCF Ammo V RevamprvnesariNo ratings yet
- DokwegII - Auxiliary - Maintenance Compressed Air SystemDocument62 pagesDokwegII - Auxiliary - Maintenance Compressed Air SystemCarlin BabuchasNo ratings yet
- Operator S Manual: Non-Cycling Refrigerated Air/Gas Dryers QPNC 75 To QPNC 250Document16 pagesOperator S Manual: Non-Cycling Refrigerated Air/Gas Dryers QPNC 75 To QPNC 250mike leveilleNo ratings yet
- Contoh Soal ViskositasDocument2 pagesContoh Soal ViskositaswsdodoNo ratings yet
- Hydraulic and Surge Analysis in A Pipeline NetworkDocument9 pagesHydraulic and Surge Analysis in A Pipeline Networkrasnowmah2012No ratings yet
- Pressure Vessel Dimension InspectionDocument15 pagesPressure Vessel Dimension Inspectionnaveenbaskaran1989100% (1)
- Log Splitter HydraulicsDocument4 pagesLog Splitter HydraulicsCristian FloricăNo ratings yet