Professional Documents
Culture Documents
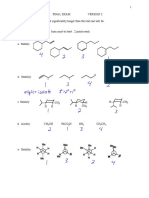
Stereochemistry: Concepts For Chiral Compounds
Stereochemistry: Concepts For Chiral Compounds
Uploaded by
Sankar AdhikariCopyright:
Available Formats
You might also like
- Organic - Class 5 PDFDocument42 pagesOrganic - Class 5 PDFSajan Singh LUCKYNo ratings yet
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Reaction Guide by James Ashenhurst. 1-James AshenhurstDocument76 pagesReaction Guide by James Ashenhurst. 1-James AshenhurstSankar AdhikariNo ratings yet
- Organic Chemistry 1 Summary Sheets - James Ashenhurst - SS (2015)Document20 pagesOrganic Chemistry 1 Summary Sheets - James Ashenhurst - SS (2015)Sankar Adhikari100% (1)
- Organic Chemistry Help! Practice Exam Window For Xula-O1e2Document7 pagesOrganic Chemistry Help! Practice Exam Window For Xula-O1e2Kristia Stephanie BejeranoNo ratings yet
- 9 Draw StructDocument6 pages9 Draw StructkalloliNo ratings yet
- Thin Layer Chromatography in Chiral Separations and Analysis Chromatographic Science SeriesDocument438 pagesThin Layer Chromatography in Chiral Separations and Analysis Chromatographic Science SeriesTrần HiềnNo ratings yet
- Tut 20 - Stereochemistry and Optical ActivityDocument42 pagesTut 20 - Stereochemistry and Optical ActivityNaman KasliwalNo ratings yet
- Stereochemistry TutorialDocument8 pagesStereochemistry TutorialfezilephathiswaNo ratings yet
- CH 5. StereochemistryDocument36 pagesCH 5. StereochemistryAhmed ZakyNo ratings yet
- Organic - Class 6Document29 pagesOrganic - Class 6Sajan Singh LUCKYNo ratings yet
- Organic Chemistry: TopicsDocument8 pagesOrganic Chemistry: TopicsHritwick MannaNo ratings yet
- StereokimiaDocument32 pagesStereokimiaBrenda GloriaNo ratings yet
- Stereochemistry UploadDocument51 pagesStereochemistry Uploadjayaramvardhan2No ratings yet
- NMR Practice 2 Answers Question One (Four Marks) : 3 Diastereotopic Diastereotopic DiastereotopicDocument10 pagesNMR Practice 2 Answers Question One (Four Marks) : 3 Diastereotopic Diastereotopic DiastereotopicGhazwan GhazwanNo ratings yet
- Stereochirality R or SDocument52 pagesStereochirality R or SnifafaniNo ratings yet
- CHM3201 Lab Report S2 2019-2020Document42 pagesCHM3201 Lab Report S2 2019-2020Halimatun MustafaNo ratings yet
- 474 - CHM 222Document68 pages474 - CHM 222Tariq AbdullahiNo ratings yet
- Nuclear Magnetic Resonance (NMR) SpectrosDocument39 pagesNuclear Magnetic Resonance (NMR) SpectrosknkoradiyaNo ratings yet
- 蔡運名Document43 pages蔡運名b101112154No ratings yet
- Organic Chemistry IIDocument13 pagesOrganic Chemistry IIRoberto SIlvaNo ratings yet
- ICT BHB Sem 2 2Document59 pagesICT BHB Sem 2 2Ayushmaan TripathiNo ratings yet
- C1102 Introduction To Organic Chemistry: Lebanese University Faculty of SciencesDocument84 pagesC1102 Introduction To Organic Chemistry: Lebanese University Faculty of SciencesBlack InsomniaNo ratings yet
- Stereochemistry LabDocument4 pagesStereochemistry Labmayra perezNo ratings yet
- Stereochemistry: Chemistry in Three Dimensions (Chiral Compound)Document54 pagesStereochemistry: Chemistry in Three Dimensions (Chiral Compound)yolandNo ratings yet
- NMR SpectrosDocument32 pagesNMR SpectrosIndhuja Preethi BNo ratings yet
- OC - Stereoisomerism - E - CSDocument36 pagesOC - Stereoisomerism - E - CSHARSHIT 12ANo ratings yet
- Chapter 7 SreteochemistryDocument29 pagesChapter 7 SreteochemistryAdzimahNo ratings yet
- Amino Acids Peptides ProteinsDocument112 pagesAmino Acids Peptides ProteinsTeodora MunteanuNo ratings yet
- Nucleophilic Substitution ReactionDocument17 pagesNucleophilic Substitution ReactionRojo JohnNo ratings yet
- Tutorial 2. Stereochemistry PDFDocument5 pagesTutorial 2. Stereochemistry PDFMatthew PokNo ratings yet
- L3 - Chapter3 - StereochemistryDocument61 pagesL3 - Chapter3 - StereochemistryAbishree GunasekhranNo ratings yet
- Stereo ChemDocument12 pagesStereo ChemVanessa AbboudNo ratings yet
- Problem Set #4, 5.12 Spring 2003: BR F D H FDocument6 pagesProblem Set #4, 5.12 Spring 2003: BR F D H FKarthikeyanNo ratings yet
- Chapter 5 ProblemsDocument7 pagesChapter 5 Problemsdicoz diphaNo ratings yet
- Matriculation Chemistry (Introduction To Organic Compound) Part 3Document25 pagesMatriculation Chemistry (Introduction To Organic Compound) Part 3ridwanNo ratings yet
- CML 100 Inorganic Part Home Assignment and Solved Problems For Self Study-Part 3Document1 pageCML 100 Inorganic Part Home Assignment and Solved Problems For Self Study-Part 3RhombiNo ratings yet
- CHE-502 (Stereochemistryof Organic Compounds)Document36 pagesCHE-502 (Stereochemistryof Organic Compounds)dasalways4uNo ratings yet
- Lab Report 6 CHEMDocument9 pagesLab Report 6 CHEMaidaNo ratings yet
- PTM-1 ChemsitryDocument6 pagesPTM-1 ChemsitrymohakjainNo ratings yet
- NMR 27 APRIL To 27 May 20Document34 pagesNMR 27 APRIL To 27 May 20Quratul AinNo ratings yet
- NEPHAR 109 Practice Problems - 2 - G1&G2-1Document3 pagesNEPHAR 109 Practice Problems - 2 - G1&G2-1Amirabbas SaffariNo ratings yet
- Essential Organic Chemistry: Isomers and StereochemistryDocument38 pagesEssential Organic Chemistry: Isomers and StereochemistryPaulina Mendoza B.No ratings yet
- RS Configuration DL NomenclatureDocument9 pagesRS Configuration DL NomenclatureDanny юрьевич Usvyat50% (2)
- Exam 1 Review 1 KOTDocument47 pagesExam 1 Review 1 KOTNoranisza MahmudNo ratings yet
- IsomerDocument30 pagesIsomerResy Maya18No ratings yet
- Chapter - 7 Optical Activity and ChiralityDocument15 pagesChapter - 7 Optical Activity and ChiralityManongdo AllanNo ratings yet
- 01 CarbohydratesDocument119 pages01 CarbohydratesDesiree LadabanNo ratings yet
- Stereochemistry and StereoisomerDocument18 pagesStereochemistry and StereoisomerAyNo ratings yet
- Organic Practice Set 12 Chapters 1 11Document9 pagesOrganic Practice Set 12 Chapters 1 11Jastine Ella Maxidel SimporiosNo ratings yet
- CHM 215 Chapter 5Document29 pagesCHM 215 Chapter 5George PiliposyanNo ratings yet
- Org 1 PDFDocument4 pagesOrg 1 PDFTanmay KumarNo ratings yet
- TestFinal 350 v2 AnswersDocument13 pagesTestFinal 350 v2 AnswersJabe KoyNo ratings yet
- ws4 PDFDocument5 pagesws4 PDFmohammedabubakrNo ratings yet
- Carbon ChemistryDocument43 pagesCarbon ChemistryBerslan ArslanNo ratings yet
- 2022 Intro To Org Chem Tutorial Section B AnsDocument17 pages2022 Intro To Org Chem Tutorial Section B AnsLow Jia YingNo ratings yet
- Chem 2016Document4 pagesChem 2016Shubhankar ChakrabortyNo ratings yet
- Chapter 3Document43 pagesChapter 3Jacquelyn GuintoNo ratings yet
- How To Identify Geometrical Isomers by S.K.sinha See Chemistry Animations atDocument4 pagesHow To Identify Geometrical Isomers by S.K.sinha See Chemistry Animations atmyiitchemistry100% (4)
- StereochemistryDocument57 pagesStereochemistrycisna ambarwatiNo ratings yet
- Hyrdogen Storage TechnologiesFrom EverandHyrdogen Storage TechnologiesMehmet SankirNo ratings yet
- Fehling's Solution Is A Chemical Reagent Used To Differentiate BetweenDocument2 pagesFehling's Solution Is A Chemical Reagent Used To Differentiate BetweenSankar AdhikariNo ratings yet
- MCQ On Problems On DiceDocument14 pagesMCQ On Problems On DiceSankar AdhikariNo ratings yet
- Formulas For Clocks Questions - PrepInstaDocument3 pagesFormulas For Clocks Questions - PrepInstaSankar AdhikariNo ratings yet
- DICE - Verbal Reasoning QuestionsDocument13 pagesDICE - Verbal Reasoning QuestionsSankar AdhikariNo ratings yet
- How To Solve Clocks Questions Easily - PrepInstaDocument4 pagesHow To Solve Clocks Questions Easily - PrepInstaSankar AdhikariNo ratings yet
- Math FundasDocument60 pagesMath FundasSankar AdhikariNo ratings yet
- 100 Shortcuts To Quantitative Aptitude Speed MattersDocument102 pages100 Shortcuts To Quantitative Aptitude Speed Mattersbest commentator barack100% (3)
- Organometallics CH 8 NotesDocument11 pagesOrganometallics CH 8 NotesSankar AdhikariNo ratings yet
- Clock TricksDocument7 pagesClock TricksSankar AdhikariNo ratings yet
- CSIR NET June 2021 Organic ChemistryDocument99 pagesCSIR NET June 2021 Organic ChemistrySankar AdhikariNo ratings yet
- Quenching PyrophoricsDocument3 pagesQuenching PyrophoricsSankar AdhikariNo ratings yet
- Dice, Cube ProblemsDocument13 pagesDice, Cube ProblemsSankar AdhikariNo ratings yet
- Tips & Tricks For Quantitative AptitudeDocument24 pagesTips & Tricks For Quantitative AptitudeSankar Adhikari0% (1)
- Dr. Laurie S. Starkey, Cal Poly Pomona - NMR Spectroscopy: Spin-Spin CouplingDocument1 pageDr. Laurie S. Starkey, Cal Poly Pomona - NMR Spectroscopy: Spin-Spin CouplingSankar AdhikariNo ratings yet
- CSIR NET June 2021 InorganicDocument37 pagesCSIR NET June 2021 InorganicSankar AdhikariNo ratings yet
- 30 of The Most Common Grammatical Errors We All Need To Stop MakingDocument19 pages30 of The Most Common Grammatical Errors We All Need To Stop MakingSankar AdhikariNo ratings yet
- CSIR Chemical Science ORGANICDocument492 pagesCSIR Chemical Science ORGANICSankar AdhikariNo ratings yet
- sp21 234 r10 Extra Problems Organometallics KeyDocument8 pagessp21 234 r10 Extra Problems Organometallics KeySankar AdhikariNo ratings yet
- Introduction To Nuclear Magnetic Resonance (NMR) Spectroscopy Dr. Laurie S. Starkey, Cal Poly PomonaDocument1 pageIntroduction To Nuclear Magnetic Resonance (NMR) Spectroscopy Dr. Laurie S. Starkey, Cal Poly PomonaSankar AdhikariNo ratings yet
- Epoxidation ReviewDocument36 pagesEpoxidation ReviewSankar AdhikariNo ratings yet
- 22-Sharpless Asymmetric Epoxidation ReactionDocument5 pages22-Sharpless Asymmetric Epoxidation ReactionSankar AdhikariNo ratings yet
- Oxidation of Primary Alcohols To Carboxylic Acids - A Guide To Current Common Practice-Gabriel Tojo - Marcos Fernández - Springer (2007)Document123 pagesOxidation of Primary Alcohols To Carboxylic Acids - A Guide To Current Common Practice-Gabriel Tojo - Marcos Fernández - Springer (2007)Sankar AdhikariNo ratings yet
- Stoker CarbohydratesDocument68 pagesStoker CarbohydratesKathryn Caseres-BarreteNo ratings yet
- Chapter 7 - CD and ORD SPECTRADocument22 pagesChapter 7 - CD and ORD SPECTRADemis ZelelewNo ratings yet
- Chapter-1 StereochemistryDocument146 pagesChapter-1 StereochemistryRx Nadeem ChhipaNo ratings yet
- Chapter 10 Haloalkanes and HaloarenesDocument24 pagesChapter 10 Haloalkanes and HaloarenesSuhas GowdaNo ratings yet
- Stereochemistry New L1-L3Document107 pagesStereochemistry New L1-L3Pareen5100% (2)
- Experiments For LBYCHEADocument26 pagesExperiments For LBYCHEABrille OzeniaNo ratings yet
- Chapter 16 OH LaNunDocument7 pagesChapter 16 OH LaNunshehdilanun100% (1)
- Alkyl Halide CompletedDocument26 pagesAlkyl Halide CompletedronNo ratings yet
- Hapter Stereochemistry: Lesson 1Document15 pagesHapter Stereochemistry: Lesson 1Ej FerrerNo ratings yet
- Chapter 2-StereochemistryDocument26 pagesChapter 2-Stereochemistrychalagirma95No ratings yet
- (Lecture 1) Molecular StructureDocument32 pages(Lecture 1) Molecular StructureKasraSrNo ratings yet
- Symmetry ElementsDocument27 pagesSymmetry Elementssekar20120No ratings yet
- Evaluation of Crude Drugs ..Document10 pagesEvaluation of Crude Drugs ..AMINA FAREEZANo ratings yet
- 2423 e 3Document21 pages2423 e 3Abdel Rahman MohamedNo ratings yet
- L5 Isomerism 3Document16 pagesL5 Isomerism 3Cheng FuNo ratings yet
- ExptIS PDFDocument6 pagesExptIS PDFMARIA EUGENIA ZOMETA CHOTONo ratings yet
- Carbohydrates: Organic Vs Inorganic CompoundsDocument16 pagesCarbohydrates: Organic Vs Inorganic CompoundsTsu Wei Chua100% (5)
- Wave Optics Theory MMDocument37 pagesWave Optics Theory MMphultushibls67% (3)
- PolarimetryDocument18 pagesPolarimetryLogavathanaa SamonNo ratings yet
- CLS Aipmt 19 20 XII Che Study Package 4 Level 1 Chapter 10Document36 pagesCLS Aipmt 19 20 XII Che Study Package 4 Level 1 Chapter 10Utkarsh KumarNo ratings yet
- D02IB011EN-E MCP100 150 ManualDocument88 pagesD02IB011EN-E MCP100 150 Manualzitouniianis2No ratings yet
- E4 StereoisomersDocument6 pagesE4 StereoisomersShaun Martel BantuganNo ratings yet
- AssignmentDocument18 pagesAssignmentசிவகுமரன் கார்த்திகாNo ratings yet
- The Volatile OilsDocument14 pagesThe Volatile OilsChatYaka AlvarezNo ratings yet
- P1-Hidrolisis de CarbohidratosDocument7 pagesP1-Hidrolisis de CarbohidratosgOnZo_86100% (1)
- StereoisomerismDocument32 pagesStereoisomerismbruno de jesus fontesNo ratings yet
- Physical Properties of Drug MoleculeDocument57 pagesPhysical Properties of Drug MoleculeNoorul AlamNo ratings yet
- 7 Revesion PDFDocument80 pages7 Revesion PDFMd Sakil AminNo ratings yet
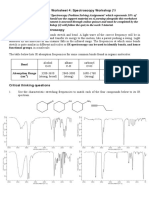
Stereochemistry: Concepts For Chiral Compounds
Stereochemistry: Concepts For Chiral Compounds
Uploaded by
Sankar AdhikariOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Stereochemistry: Concepts For Chiral Compounds
Stereochemistry: Concepts For Chiral Compounds
Uploaded by
Sankar AdhikariCopyright:
Available Formats
lOMoARcPSD|5381057
CHEM 73/8311 CS2 Stereochemistry Conformation Stereoselectivity.docx 2012Jan18 Page1
Stereochemistry
stereoisomers - atomic connections are the same but 3-dimensional structures different
optical activity - rotation of plane polarized light
concepts for chiral compounds
1. stereoisomers not superimposable on mirror image, unique, mirror images are
enantiomers
enantiotopic (http://www.iupac.org/goldbook/E02083.pdf)
“Constitutionally identical atoms or groups in molecules which are related by symmetry elements of
the second kind only (mirror plane, inversion centre or rotation–reflection axis). For example the
two groups c in a grouping Cabcc are enantiotopic. Replacement of one of a pair of enantiotopic
groups forms one of a pair of enantiomers. Analogously, if complexation or addition to one of the
two faces defined by a double bond or other molecular plane gives rise to a chiral species, the two
faces are called enantiotopic.
2. enantiomers have identical physical and chemical properties except with asymmetric
entities
3. racemate - equal mixture of enantiomers, not optically active
4. optical rotation: [ ]lc , = observed rotation, [] = specific rotation, l = pathlength in
decimeters, c = concentration in grams per mL, [] depends on wavelength and temperature
http://www.nsm.buffalo.edu/~jochena/research/opticalactivity.html
5. chiral atom not required
(a) four different groups or electron pairs on atom with tetrahedral geometry, (C, S, P, N)
amines invert as fast as 1011 s-1, slowed down by heteroatom (lone pairs) and small ring
(why?)
(b) restricted rotation of disymmetric planes - biphenyls, allenes, spyro compounds both
sides must be unsymmetrical
H
H3C
H3C H
H CO2Me C C C
Cl H
H CH3
NC CO Me H
N 2 N
O
OMe Me CH3
(c) restricted conformation/rotation - hexahelicene
(d) metallocenes
Br
H H Fe
6. only a racemic mixture of chiral centers can be created by non-chiral reagents???
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
lOMoARcPSD|5381057
CHEM 73/8311 CS2 Stereochemistry Conformation Stereoselectivity.docx 2012Jan18 Page2
OH OH
O 1. PhMgBr
H CH3 H CH3 H CH3
2. H2O Ph Ph
7. Fisher projection: simple 90 degree rotation in the plane not allowed CHO
8. Cahn-Ingold-Prelog: R and S configuration based on atomic number
HO H
9. more than one chiral center - diastereomers can form: stereoisomers that are
not enantiomers- examples H OH
(a) diastereomers are chemically and physically different
(b) 2n isomers possible, sometimes internally racemic (meso), others are dl HO H
pairs H OH
diastereotopic (http://www.iupac.org/goldbook/D01685.pdf) CH2OH
Constitutionally equivalent atoms or groups of a molecule which are not symmetry
related. Replacement of one of two diastereotopic atoms or groups results in the formation of one
of a pair of diastereoisomers. In the example below the two hydrogen atoms of the methylene
group C-3 are diastereotopic.
Resolution
1. convert enantiomers to diastereomers- derivatize with chiral reagent, diastereomers have
different properties
2. chiral chromatography
3. enantioselective reaction/degradation
4. crystallization
Optical purity
1. % optical purity = 100[]obs/[]max
2. % enantiomeric excess = %R - %S = 100([R] -[S])/([R] + [S])
3. easily determined by NMR using chiral shift reagent or diastereomeric derivatives
Cis-Trans Isomerization (diastereomers)
1. double bonds, E and Z isomers
2. rings - cis and trans substituents (including other rings)
3. single bonds – delocalization in amides
O O
N N
4. bridging rings: endo substituent toward larger ring or other substitutent , exo away
Enantiotopic pairs of groups/atoms – prochiral
1. Enantiotopic ligands- identical groups - replacement of each by a new group gives
enantiomers
2. ethanol, pro-R and pro-S
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
lOMoARcPSD|5381057
CHEM 73/8311 CS2 Stereochemistry Conformation Stereoselectivity.docx 2012Jan18 Page3
H H D H H D
endo
exo
D H H D
exo
H3C OH H3C OH CH3 CH3 CH3
endo H H H
2. diastereotopic ligands - identical groups - replacement of each by a new group gives
diastereomers: terminal alkene? groups near chiral center?
3. prochiral faces - clockwise face is Re and counter clockwise is Si
H H
H3C O O CH3
Re Si
4. stereoselective process - one stereoisomer preferred over another
5. stereospecific - different stereoisomers leads to a different stereoproducts
stereospecific
H3C stereoselective
H3C CH3 CHBr3 H3C Br H CCH CHICH 3 2 3
H H KOC(CH3)3 H KOC(CH3)3 DMSO
H Br
H H3C CH3
H3C H
H3C H CHBr3 H3C Br +
H CH3 H H
H CH3 KOC(CH3)3 H H3C Br
3:1
Asymmetric Synthesis -
1. Asymmetric substrate - induction by chiral center O
M S
(a) Cram's rule - attack of carbonyl rotamer from small side
(b) chiral auxiliary e. g. convert acid to ester with chiral alcohol N
2. asymmetric reagent - sellects prochiral faces or ligands RL
3. asymmetric catalyst
4. polarized light
rotantional barriers
kcal/mol
CH3CH3 2.9
CH3CH2CH3 3.4
CH3NH2 1.98
CH3NHCH3 3.6
CH3OH 1.07
CH3OCH3 2.7
Conformational Analysis
1. conformers - rapid rotation interconvert structures
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
lOMoARcPSD|5381057
CHEM 73/8311 CS2 Stereochemistry Conformation Stereoselectivity.docx 2012Jan18 Page4
2. ethane -2.9 kcal/mol barrier, staggered and eclipsed, average CH interaction is 0.9 kcal/mol
3. butane - gauche - anti = 0.9 kcal/mol, 3.4 and 6 kcal/mol barriers, non bonding interaction of
two methyls = 4 kcal/mol
4. propene - eclipsed 2 kcal < bisected, repulsion of methyl component with bond
carbonyl behaves similarly (propanal?)
eclipsed bisected eclipsed eclipsed
H H H H H H H t-Bu H
H H H
H H H H
H H t-Bu H
G = 2 kcal/mol
5. butadiene - s-trans favored, same for acrolein (propenal) but not bulky ketones
H H
H H H O H O
H H
H H H H
H H H H
H H H H
s-trans s-cis s-trans s-cis
cyclohexane - chair, twist-boat
1. substituents often prefer equitorial position to avoid gauche (1,3 diaxial) interactions with ring
examine relative free energies for substitutents: Me, Et, iPr, tBu, OH, COOMe, HgBr
2. multiple substitutents also avoid each other, transannular interaction (cis 1,3)???
3. rapid chair-chair interconversion - makes racemic mixture of conformations
equitorial - axial equilibrium of OR
monosubstituted cyclohexane O
Me 1.8 kcal/mol
Et 1.8
iPr 2.1 O
tBu >4.5
CN 0.15-0.25
CH=CH2 1.7
OR
CO2H 1.35
OH 0.5 (acetone)
OH 0.9 (methanol) H H H
H H H H
HgBr 0 H H H H H
other rings H
Heffect - lone H H H
1. heteroatoms in 6 membered rings - anomeric
H H H pair H - * H
2. cyclopropane –planar, eclipsed, angle strain H H
3. cyclobutane – butterfly, angle strain
4. cyclopentane - envelope and half chair
molecular mechanics
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
lOMoARcPSD|5381057
CHEM 73/8311 CS2 Stereochemistry Conformation Stereoselectivity.docx 2012Jan18 Page5
computer programs use potential energy functions based on simple molecules
optimize conformations, bond angles, lengths of complex molecules
not applicable to molecules with structures not available in potential energy functions
Strain
1. energy difference between actual structure and hypothetical
molecule
2. (heat of combustion of ring compounds)/(# CH 2 groups)
cyclopropane>cyclobutane>cyclopentane>cyclohexane
3. cyclopropane, banana bond, -analogy
(a) sp3 orbitals cannot point along internuclear axis (60 o vs.
109o)
(b) increase p character to decrease interorbital angle, rehybridize to sp 5
(c) orbitals toward H have more s character about sp 2
(d) high p character of orbitals seen in conjugation with bonds
(e) react with electrophilic reagents, Br2 forms 1,3-dibromopropane
H H
H H H H
Br
+ Br2 H
Br
H
H H H H
Angewante Chemie, Int. Ed. 47 (2008) 1107
Journal of Organic Chemistry, 72 (2007) 8597
(f) multiple strained rings: tricyclo[1.1.1.0 1,3]pentane has all bonds directed to one side of
plane
4. medium rings -
(a) cyclohexane - chair has gauche interactions?
(b) 5,7-13 member rings, either trans-annular interaction, large angle strain, or eclipsing
interactions
5. larger rings (>14 members) have no strain
6. cyclopropene - polymerizes at -80 oC, less stable than cyclopropane
7. small rings only have cis double bonds, smallest ring with trans double bond has 8 atoms
Bredt's rule
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
lOMoARcPSD|5381057
CHEM 73/8311 CS2 Stereochemistry Conformation Stereoselectivity.docx 2012Jan18 Page6
double bond does not form at bridgehead unless trans ring has at least 8 atoms
smallest ring with triple bond without strain has 9 carbons
8. steric crowding - restricted rotation move
Baldwin’s Rules
1. exo ring closure - electrons move out of ring
2. endo ring closure - electron move in ring
3. ring closure classified according to geometry
tetrahedral, trigonal, digonal
4. tetrahedral - favored for all exo (endo is rare)
5. trigonal - favored except for 3-5 endo
6. digonal - favored except for 3 and 4 exo
7. rules based on steric requirements of nucleophilic attack
exo endo
N 109 N
180
60
N X N X N C X C X C X
Campbell, Pohlhaus, Min, Ohmatsu,Johnson, JACS 130 (2008) 9180 – 9181
Torsional Effects on Reactivity
1. Small nucleophiles preferentially attack axial to avoid eclipsing of O with equitorial H in TS
2. Large nucleophiles prefer equitorial attack to avoid 1,3 type of interaction
H O Nu O
H axial H equitorial H
H
H O O Nu
H H H
H
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
You might also like
- Organic - Class 5 PDFDocument42 pagesOrganic - Class 5 PDFSajan Singh LUCKYNo ratings yet
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)
- Reaction Guide by James Ashenhurst. 1-James AshenhurstDocument76 pagesReaction Guide by James Ashenhurst. 1-James AshenhurstSankar AdhikariNo ratings yet
- Organic Chemistry 1 Summary Sheets - James Ashenhurst - SS (2015)Document20 pagesOrganic Chemistry 1 Summary Sheets - James Ashenhurst - SS (2015)Sankar Adhikari100% (1)
- Organic Chemistry Help! Practice Exam Window For Xula-O1e2Document7 pagesOrganic Chemistry Help! Practice Exam Window For Xula-O1e2Kristia Stephanie BejeranoNo ratings yet
- 9 Draw StructDocument6 pages9 Draw StructkalloliNo ratings yet
- Thin Layer Chromatography in Chiral Separations and Analysis Chromatographic Science SeriesDocument438 pagesThin Layer Chromatography in Chiral Separations and Analysis Chromatographic Science SeriesTrần HiềnNo ratings yet
- Tut 20 - Stereochemistry and Optical ActivityDocument42 pagesTut 20 - Stereochemistry and Optical ActivityNaman KasliwalNo ratings yet
- Stereochemistry TutorialDocument8 pagesStereochemistry TutorialfezilephathiswaNo ratings yet
- CH 5. StereochemistryDocument36 pagesCH 5. StereochemistryAhmed ZakyNo ratings yet
- Organic - Class 6Document29 pagesOrganic - Class 6Sajan Singh LUCKYNo ratings yet
- Organic Chemistry: TopicsDocument8 pagesOrganic Chemistry: TopicsHritwick MannaNo ratings yet
- StereokimiaDocument32 pagesStereokimiaBrenda GloriaNo ratings yet
- Stereochemistry UploadDocument51 pagesStereochemistry Uploadjayaramvardhan2No ratings yet
- NMR Practice 2 Answers Question One (Four Marks) : 3 Diastereotopic Diastereotopic DiastereotopicDocument10 pagesNMR Practice 2 Answers Question One (Four Marks) : 3 Diastereotopic Diastereotopic DiastereotopicGhazwan GhazwanNo ratings yet
- Stereochirality R or SDocument52 pagesStereochirality R or SnifafaniNo ratings yet
- CHM3201 Lab Report S2 2019-2020Document42 pagesCHM3201 Lab Report S2 2019-2020Halimatun MustafaNo ratings yet
- 474 - CHM 222Document68 pages474 - CHM 222Tariq AbdullahiNo ratings yet
- Nuclear Magnetic Resonance (NMR) SpectrosDocument39 pagesNuclear Magnetic Resonance (NMR) SpectrosknkoradiyaNo ratings yet
- 蔡運名Document43 pages蔡運名b101112154No ratings yet
- Organic Chemistry IIDocument13 pagesOrganic Chemistry IIRoberto SIlvaNo ratings yet
- ICT BHB Sem 2 2Document59 pagesICT BHB Sem 2 2Ayushmaan TripathiNo ratings yet
- C1102 Introduction To Organic Chemistry: Lebanese University Faculty of SciencesDocument84 pagesC1102 Introduction To Organic Chemistry: Lebanese University Faculty of SciencesBlack InsomniaNo ratings yet
- Stereochemistry LabDocument4 pagesStereochemistry Labmayra perezNo ratings yet
- Stereochemistry: Chemistry in Three Dimensions (Chiral Compound)Document54 pagesStereochemistry: Chemistry in Three Dimensions (Chiral Compound)yolandNo ratings yet
- NMR SpectrosDocument32 pagesNMR SpectrosIndhuja Preethi BNo ratings yet
- OC - Stereoisomerism - E - CSDocument36 pagesOC - Stereoisomerism - E - CSHARSHIT 12ANo ratings yet
- Chapter 7 SreteochemistryDocument29 pagesChapter 7 SreteochemistryAdzimahNo ratings yet
- Amino Acids Peptides ProteinsDocument112 pagesAmino Acids Peptides ProteinsTeodora MunteanuNo ratings yet
- Nucleophilic Substitution ReactionDocument17 pagesNucleophilic Substitution ReactionRojo JohnNo ratings yet
- Tutorial 2. Stereochemistry PDFDocument5 pagesTutorial 2. Stereochemistry PDFMatthew PokNo ratings yet
- L3 - Chapter3 - StereochemistryDocument61 pagesL3 - Chapter3 - StereochemistryAbishree GunasekhranNo ratings yet
- Stereo ChemDocument12 pagesStereo ChemVanessa AbboudNo ratings yet
- Problem Set #4, 5.12 Spring 2003: BR F D H FDocument6 pagesProblem Set #4, 5.12 Spring 2003: BR F D H FKarthikeyanNo ratings yet
- Chapter 5 ProblemsDocument7 pagesChapter 5 Problemsdicoz diphaNo ratings yet
- Matriculation Chemistry (Introduction To Organic Compound) Part 3Document25 pagesMatriculation Chemistry (Introduction To Organic Compound) Part 3ridwanNo ratings yet
- CML 100 Inorganic Part Home Assignment and Solved Problems For Self Study-Part 3Document1 pageCML 100 Inorganic Part Home Assignment and Solved Problems For Self Study-Part 3RhombiNo ratings yet
- CHE-502 (Stereochemistryof Organic Compounds)Document36 pagesCHE-502 (Stereochemistryof Organic Compounds)dasalways4uNo ratings yet
- Lab Report 6 CHEMDocument9 pagesLab Report 6 CHEMaidaNo ratings yet
- PTM-1 ChemsitryDocument6 pagesPTM-1 ChemsitrymohakjainNo ratings yet
- NMR 27 APRIL To 27 May 20Document34 pagesNMR 27 APRIL To 27 May 20Quratul AinNo ratings yet
- NEPHAR 109 Practice Problems - 2 - G1&G2-1Document3 pagesNEPHAR 109 Practice Problems - 2 - G1&G2-1Amirabbas SaffariNo ratings yet
- Essential Organic Chemistry: Isomers and StereochemistryDocument38 pagesEssential Organic Chemistry: Isomers and StereochemistryPaulina Mendoza B.No ratings yet
- RS Configuration DL NomenclatureDocument9 pagesRS Configuration DL NomenclatureDanny юрьевич Usvyat50% (2)
- Exam 1 Review 1 KOTDocument47 pagesExam 1 Review 1 KOTNoranisza MahmudNo ratings yet
- IsomerDocument30 pagesIsomerResy Maya18No ratings yet
- Chapter - 7 Optical Activity and ChiralityDocument15 pagesChapter - 7 Optical Activity and ChiralityManongdo AllanNo ratings yet
- 01 CarbohydratesDocument119 pages01 CarbohydratesDesiree LadabanNo ratings yet
- Stereochemistry and StereoisomerDocument18 pagesStereochemistry and StereoisomerAyNo ratings yet
- Organic Practice Set 12 Chapters 1 11Document9 pagesOrganic Practice Set 12 Chapters 1 11Jastine Ella Maxidel SimporiosNo ratings yet
- CHM 215 Chapter 5Document29 pagesCHM 215 Chapter 5George PiliposyanNo ratings yet
- Org 1 PDFDocument4 pagesOrg 1 PDFTanmay KumarNo ratings yet
- TestFinal 350 v2 AnswersDocument13 pagesTestFinal 350 v2 AnswersJabe KoyNo ratings yet
- ws4 PDFDocument5 pagesws4 PDFmohammedabubakrNo ratings yet
- Carbon ChemistryDocument43 pagesCarbon ChemistryBerslan ArslanNo ratings yet
- 2022 Intro To Org Chem Tutorial Section B AnsDocument17 pages2022 Intro To Org Chem Tutorial Section B AnsLow Jia YingNo ratings yet
- Chem 2016Document4 pagesChem 2016Shubhankar ChakrabortyNo ratings yet
- Chapter 3Document43 pagesChapter 3Jacquelyn GuintoNo ratings yet
- How To Identify Geometrical Isomers by S.K.sinha See Chemistry Animations atDocument4 pagesHow To Identify Geometrical Isomers by S.K.sinha See Chemistry Animations atmyiitchemistry100% (4)
- StereochemistryDocument57 pagesStereochemistrycisna ambarwatiNo ratings yet
- Hyrdogen Storage TechnologiesFrom EverandHyrdogen Storage TechnologiesMehmet SankirNo ratings yet
- Fehling's Solution Is A Chemical Reagent Used To Differentiate BetweenDocument2 pagesFehling's Solution Is A Chemical Reagent Used To Differentiate BetweenSankar AdhikariNo ratings yet
- MCQ On Problems On DiceDocument14 pagesMCQ On Problems On DiceSankar AdhikariNo ratings yet
- Formulas For Clocks Questions - PrepInstaDocument3 pagesFormulas For Clocks Questions - PrepInstaSankar AdhikariNo ratings yet
- DICE - Verbal Reasoning QuestionsDocument13 pagesDICE - Verbal Reasoning QuestionsSankar AdhikariNo ratings yet
- How To Solve Clocks Questions Easily - PrepInstaDocument4 pagesHow To Solve Clocks Questions Easily - PrepInstaSankar AdhikariNo ratings yet
- Math FundasDocument60 pagesMath FundasSankar AdhikariNo ratings yet
- 100 Shortcuts To Quantitative Aptitude Speed MattersDocument102 pages100 Shortcuts To Quantitative Aptitude Speed Mattersbest commentator barack100% (3)
- Organometallics CH 8 NotesDocument11 pagesOrganometallics CH 8 NotesSankar AdhikariNo ratings yet
- Clock TricksDocument7 pagesClock TricksSankar AdhikariNo ratings yet
- CSIR NET June 2021 Organic ChemistryDocument99 pagesCSIR NET June 2021 Organic ChemistrySankar AdhikariNo ratings yet
- Quenching PyrophoricsDocument3 pagesQuenching PyrophoricsSankar AdhikariNo ratings yet
- Dice, Cube ProblemsDocument13 pagesDice, Cube ProblemsSankar AdhikariNo ratings yet
- Tips & Tricks For Quantitative AptitudeDocument24 pagesTips & Tricks For Quantitative AptitudeSankar Adhikari0% (1)
- Dr. Laurie S. Starkey, Cal Poly Pomona - NMR Spectroscopy: Spin-Spin CouplingDocument1 pageDr. Laurie S. Starkey, Cal Poly Pomona - NMR Spectroscopy: Spin-Spin CouplingSankar AdhikariNo ratings yet
- CSIR NET June 2021 InorganicDocument37 pagesCSIR NET June 2021 InorganicSankar AdhikariNo ratings yet
- 30 of The Most Common Grammatical Errors We All Need To Stop MakingDocument19 pages30 of The Most Common Grammatical Errors We All Need To Stop MakingSankar AdhikariNo ratings yet
- CSIR Chemical Science ORGANICDocument492 pagesCSIR Chemical Science ORGANICSankar AdhikariNo ratings yet
- sp21 234 r10 Extra Problems Organometallics KeyDocument8 pagessp21 234 r10 Extra Problems Organometallics KeySankar AdhikariNo ratings yet
- Introduction To Nuclear Magnetic Resonance (NMR) Spectroscopy Dr. Laurie S. Starkey, Cal Poly PomonaDocument1 pageIntroduction To Nuclear Magnetic Resonance (NMR) Spectroscopy Dr. Laurie S. Starkey, Cal Poly PomonaSankar AdhikariNo ratings yet
- Epoxidation ReviewDocument36 pagesEpoxidation ReviewSankar AdhikariNo ratings yet
- 22-Sharpless Asymmetric Epoxidation ReactionDocument5 pages22-Sharpless Asymmetric Epoxidation ReactionSankar AdhikariNo ratings yet
- Oxidation of Primary Alcohols To Carboxylic Acids - A Guide To Current Common Practice-Gabriel Tojo - Marcos Fernández - Springer (2007)Document123 pagesOxidation of Primary Alcohols To Carboxylic Acids - A Guide To Current Common Practice-Gabriel Tojo - Marcos Fernández - Springer (2007)Sankar AdhikariNo ratings yet
- Stoker CarbohydratesDocument68 pagesStoker CarbohydratesKathryn Caseres-BarreteNo ratings yet
- Chapter 7 - CD and ORD SPECTRADocument22 pagesChapter 7 - CD and ORD SPECTRADemis ZelelewNo ratings yet
- Chapter-1 StereochemistryDocument146 pagesChapter-1 StereochemistryRx Nadeem ChhipaNo ratings yet
- Chapter 10 Haloalkanes and HaloarenesDocument24 pagesChapter 10 Haloalkanes and HaloarenesSuhas GowdaNo ratings yet
- Stereochemistry New L1-L3Document107 pagesStereochemistry New L1-L3Pareen5100% (2)
- Experiments For LBYCHEADocument26 pagesExperiments For LBYCHEABrille OzeniaNo ratings yet
- Chapter 16 OH LaNunDocument7 pagesChapter 16 OH LaNunshehdilanun100% (1)
- Alkyl Halide CompletedDocument26 pagesAlkyl Halide CompletedronNo ratings yet
- Hapter Stereochemistry: Lesson 1Document15 pagesHapter Stereochemistry: Lesson 1Ej FerrerNo ratings yet
- Chapter 2-StereochemistryDocument26 pagesChapter 2-Stereochemistrychalagirma95No ratings yet
- (Lecture 1) Molecular StructureDocument32 pages(Lecture 1) Molecular StructureKasraSrNo ratings yet
- Symmetry ElementsDocument27 pagesSymmetry Elementssekar20120No ratings yet
- Evaluation of Crude Drugs ..Document10 pagesEvaluation of Crude Drugs ..AMINA FAREEZANo ratings yet
- 2423 e 3Document21 pages2423 e 3Abdel Rahman MohamedNo ratings yet
- L5 Isomerism 3Document16 pagesL5 Isomerism 3Cheng FuNo ratings yet
- ExptIS PDFDocument6 pagesExptIS PDFMARIA EUGENIA ZOMETA CHOTONo ratings yet
- Carbohydrates: Organic Vs Inorganic CompoundsDocument16 pagesCarbohydrates: Organic Vs Inorganic CompoundsTsu Wei Chua100% (5)
- Wave Optics Theory MMDocument37 pagesWave Optics Theory MMphultushibls67% (3)
- PolarimetryDocument18 pagesPolarimetryLogavathanaa SamonNo ratings yet
- CLS Aipmt 19 20 XII Che Study Package 4 Level 1 Chapter 10Document36 pagesCLS Aipmt 19 20 XII Che Study Package 4 Level 1 Chapter 10Utkarsh KumarNo ratings yet
- D02IB011EN-E MCP100 150 ManualDocument88 pagesD02IB011EN-E MCP100 150 Manualzitouniianis2No ratings yet
- E4 StereoisomersDocument6 pagesE4 StereoisomersShaun Martel BantuganNo ratings yet
- AssignmentDocument18 pagesAssignmentசிவகுமரன் கார்த்திகாNo ratings yet
- The Volatile OilsDocument14 pagesThe Volatile OilsChatYaka AlvarezNo ratings yet
- P1-Hidrolisis de CarbohidratosDocument7 pagesP1-Hidrolisis de CarbohidratosgOnZo_86100% (1)
- StereoisomerismDocument32 pagesStereoisomerismbruno de jesus fontesNo ratings yet
- Physical Properties of Drug MoleculeDocument57 pagesPhysical Properties of Drug MoleculeNoorul AlamNo ratings yet
- 7 Revesion PDFDocument80 pages7 Revesion PDFMd Sakil AminNo ratings yet