Professional Documents
Culture Documents
Organic Chemistry I: CH H C CH D
Organic Chemistry I: CH H C CH D
Uploaded by
Sankar AdhikariCopyright:
Available Formats
You might also like
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- Reaction Guide by James Ashenhurst. 1-James AshenhurstDocument76 pagesReaction Guide by James Ashenhurst. 1-James AshenhurstSankar AdhikariNo ratings yet
- Organic Chemistry 1 Summary Sheets - James Ashenhurst - SS (2015)Document20 pagesOrganic Chemistry 1 Summary Sheets - James Ashenhurst - SS (2015)Sankar Adhikari100% (1)
- 3 Naming Alkynes Ws KeyDocument2 pages3 Naming Alkynes Ws KeyJaya Chitra Degala RamaluNo ratings yet
- 4 Roadtec 600 Cummins QSXDocument24 pages4 Roadtec 600 Cummins QSXdavidNo ratings yet
- Pyrolytic Elimination - Syn EliminationsDocument3 pagesPyrolytic Elimination - Syn EliminationssaheedvkNo ratings yet
- Section 3 - StereochemistryDocument21 pagesSection 3 - Stereochemistrysf9 fanfareNo ratings yet
- Trabajo Quimica Superior NAVIDADDocument13 pagesTrabajo Quimica Superior NAVIDADSebastian GuerraNo ratings yet
- Chemistry PracticeDocument13 pagesChemistry PracticeSiddharth KrishnamurthyNo ratings yet
- Ejercicios de Alcoholes y EteresDocument1 pageEjercicios de Alcoholes y EteresMaria Jose Duque AngelNo ratings yet
- A) OH B) OH C) OH D) OH E) OH F) OHDocument4 pagesA) OH B) OH C) OH D) OH E) OH F) OHRodrigo RVNo ratings yet
- CM1121 Tutorial 2 2006-7Document2 pagesCM1121 Tutorial 2 2006-7Tan Jun RongNo ratings yet
- Narayana Educational Soceity Organic ChemistryDocument25 pagesNarayana Educational Soceity Organic ChemistryNekhill KumarNo ratings yet
- Haloalcanos 3Document1 pageHaloalcanos 3MIRIAM SAN FRUTOS GARCÍANo ratings yet
- FitoquimiaDocument2 pagesFitoquimialuisgerardo000000No ratings yet
- JoDocument1 pageJoJoana Marie BeltranNo ratings yet
- Materi Reaksi KimiaDocument11 pagesMateri Reaksi KimiaRenn AgenaNo ratings yet
- Alcohol, Ether & PhenolDocument8 pagesAlcohol, Ether & Phenolshashwat.gupta.707No ratings yet
- CPP - HydrocarbonDocument13 pagesCPP - HydrocarbondivyanshjoshidpsjkpNo ratings yet
- Denumiți AlcaniiDocument2 pagesDenumiți AlcaniiCristianNo ratings yet
- DPP Optical+Isomer 4Document2 pagesDPP Optical+Isomer 4Shivam RoyNo ratings yet
- MechanismsDocument5 pagesMechanismsnajifaahmed223No ratings yet
- HC y AlcoholesDocument2 pagesHC y AlcoholesGonzalo HernandezNo ratings yet
- MULTIPLE CHOICE QUESTIONS Part 5: Stereochemistry: Topic: Identifications and ComparisonsDocument14 pagesMULTIPLE CHOICE QUESTIONS Part 5: Stereochemistry: Topic: Identifications and ComparisonsMoùümîtà KhäñráNo ratings yet
- 12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetDocument4 pages12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetZia Rathore100% (1)
- R Vs S AnsDocument2 pagesR Vs S AnsAtul SinghNo ratings yet
- Draw The Following Alkanes.: Hydrocarbon Nomenclature WorksheetDocument3 pagesDraw The Following Alkanes.: Hydrocarbon Nomenclature WorksheetCaseelyn Joy NantizaNo ratings yet
- PDFDocument30 pagesPDFAlok RanjanNo ratings yet
- Exercise StereochemistryDocument4 pagesExercise StereochemistryPuvaneswary LoganathanNo ratings yet
- Aqa Mechanisms A Level SummaryDocument5 pagesAqa Mechanisms A Level SummaryRS JNo ratings yet
- Answers To AssignmentDocument1 pageAnswers To AssignmentIgbereyivwe TejiriNo ratings yet
- Revision Notes Organic ChemistryDocument30 pagesRevision Notes Organic ChemistryAtharav Porwal100% (1)
- Isomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomersDocument11 pagesIsomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomerskamalNo ratings yet
- Organic Practice Set 9 - Chapter 7Document4 pagesOrganic Practice Set 9 - Chapter 7Aurora FerroNo ratings yet
- How To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inDocument2 pagesHow To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inmyiitchemistry81% (16)
- Estructura de Compuestos Biologicos-Parte 2: F.-EsteroidesDocument7 pagesEstructura de Compuestos Biologicos-Parte 2: F.-EsteroidesffhkeNo ratings yet
- PTM-1 ChemsitryDocument6 pagesPTM-1 ChemsitrymohakjainNo ratings yet
- Alkenes and Alkynes Worksheet PDFDocument3 pagesAlkenes and Alkynes Worksheet PDFDoha Hosam Shalaby HamamNo ratings yet
- Alcohol Nomenclature - Summative AssessmentDocument2 pagesAlcohol Nomenclature - Summative AssessmentDiana Carolina DuarteNo ratings yet
- Alcohol Nomenclature - Summative AssessmentDocument2 pagesAlcohol Nomenclature - Summative AssessmentDiana Carolina DuarteNo ratings yet
- Alcohol, Ether and PhenolDocument4 pagesAlcohol, Ether and PhenolKushagra SrivastavaNo ratings yet
- H NMR Problems: - How Many Unique Proton Environments Are There inDocument26 pagesH NMR Problems: - How Many Unique Proton Environments Are There inFatima AhmedNo ratings yet
- TPDocument39 pagesTPARAMAYO JuanNo ratings yet
- Basics of Photochemistry and Norrish Type I Reaction: Presented By: Dr. Nidhi VashisthaDocument12 pagesBasics of Photochemistry and Norrish Type I Reaction: Presented By: Dr. Nidhi Vashisthanidhi vashisthaNo ratings yet
- Ejercicios de Nomenclatura de Alcoholes.Document3 pagesEjercicios de Nomenclatura de Alcoholes.Diego Fernando Ardila ArizaNo ratings yet
- Naming AlkanesDocument3 pagesNaming Alkanesmercedes.caamalNo ratings yet
- Contoh: + Butan-2-On Metanol H SODocument2 pagesContoh: + Butan-2-On Metanol H SOElis TianiNo ratings yet
- Contoh: + Butan-2-On Metanol H SODocument2 pagesContoh: + Butan-2-On Metanol H SOElis TianiNo ratings yet
- OH A, A Is: : CHN 1 EqvDocument4 pagesOH A, A Is: : CHN 1 EqvAtharva GanjuNo ratings yet
- AlcoholsDocument7 pagesAlcoholsSuhaan GargNo ratings yet
- Organic Reactions Summary Alkenes, Alkynes and Variations For Use As A Study Guide BeauchampDocument45 pagesOrganic Reactions Summary Alkenes, Alkynes and Variations For Use As A Study Guide BeauchampShaker MahmoodNo ratings yet
- Org 1 PDFDocument4 pagesOrg 1 PDFTanmay KumarNo ratings yet
- Chapter 5 ProblemsDocument7 pagesChapter 5 Problemsdicoz diphaNo ratings yet
- Hidrocarburos MIX-01Document1 pageHidrocarburos MIX-01Abraham CastañedaNo ratings yet
- OrganicChemistryChapter7 PDFDocument30 pagesOrganicChemistryChapter7 PDFSeanne CruzNo ratings yet
- 314 Stereochem ProbsDocument14 pages314 Stereochem ProbsAtul SinghNo ratings yet
- Stereochemistry of OrganicDocument35 pagesStereochemistry of OrganicRams ChanderNo ratings yet
- S22 - URAIAN Dan KONTROL PROSES PEMBUATANDocument7 pagesS22 - URAIAN Dan KONTROL PROSES PEMBUATANTrisnawati AmaliaNo ratings yet
- Enabling AssessmentDocument3 pagesEnabling Assessmentlluvemnx takahashiNo ratings yet
- Summary of Alkyne Reactions: H H C H H C BR HDocument1 pageSummary of Alkyne Reactions: H H C H H C BR HKamelNo ratings yet
- Hidrocarbonetos 02Document2 pagesHidrocarbonetos 02Romário MelloNo ratings yet
- Mechanism Summary For A-Level AQA Chemistry: BR BRDocument5 pagesMechanism Summary For A-Level AQA Chemistry: BR BRamrhkmhNo ratings yet
- CO2 - 1S2 - 2011 - LSLL - CopieDocument5 pagesCO2 - 1S2 - 2011 - LSLL - CopiePFENo ratings yet
- Clock TricksDocument7 pagesClock TricksSankar AdhikariNo ratings yet
- MCQ On Problems On DiceDocument14 pagesMCQ On Problems On DiceSankar AdhikariNo ratings yet
- Fehling's Solution Is A Chemical Reagent Used To Differentiate BetweenDocument2 pagesFehling's Solution Is A Chemical Reagent Used To Differentiate BetweenSankar AdhikariNo ratings yet
- CSIR NET June 2021 InorganicDocument37 pagesCSIR NET June 2021 InorganicSankar AdhikariNo ratings yet
- Formulas For Clocks Questions - PrepInstaDocument3 pagesFormulas For Clocks Questions - PrepInstaSankar AdhikariNo ratings yet
- How To Solve Clocks Questions Easily - PrepInstaDocument4 pagesHow To Solve Clocks Questions Easily - PrepInstaSankar AdhikariNo ratings yet
- Math FundasDocument60 pagesMath FundasSankar AdhikariNo ratings yet
- DICE - Verbal Reasoning QuestionsDocument13 pagesDICE - Verbal Reasoning QuestionsSankar AdhikariNo ratings yet
- 100 Shortcuts To Quantitative Aptitude Speed MattersDocument102 pages100 Shortcuts To Quantitative Aptitude Speed Mattersbest commentator barack100% (3)
- Quenching PyrophoricsDocument3 pagesQuenching PyrophoricsSankar AdhikariNo ratings yet
- Dice, Cube ProblemsDocument13 pagesDice, Cube ProblemsSankar AdhikariNo ratings yet
- Tips & Tricks For Quantitative AptitudeDocument24 pagesTips & Tricks For Quantitative AptitudeSankar Adhikari0% (1)
- CSIR NET June 2021 Organic ChemistryDocument99 pagesCSIR NET June 2021 Organic ChemistrySankar AdhikariNo ratings yet
- Dr. Laurie S. Starkey, Cal Poly Pomona - NMR Spectroscopy: Spin-Spin CouplingDocument1 pageDr. Laurie S. Starkey, Cal Poly Pomona - NMR Spectroscopy: Spin-Spin CouplingSankar AdhikariNo ratings yet
- sp21 234 r10 Extra Problems Organometallics KeyDocument8 pagessp21 234 r10 Extra Problems Organometallics KeySankar AdhikariNo ratings yet
- Organometallics CH 8 NotesDocument11 pagesOrganometallics CH 8 NotesSankar AdhikariNo ratings yet
- 30 of The Most Common Grammatical Errors We All Need To Stop MakingDocument19 pages30 of The Most Common Grammatical Errors We All Need To Stop MakingSankar AdhikariNo ratings yet
- 22-Sharpless Asymmetric Epoxidation ReactionDocument5 pages22-Sharpless Asymmetric Epoxidation ReactionSankar AdhikariNo ratings yet
- CSIR Chemical Science ORGANICDocument492 pagesCSIR Chemical Science ORGANICSankar AdhikariNo ratings yet
- Introduction To Nuclear Magnetic Resonance (NMR) Spectroscopy Dr. Laurie S. Starkey, Cal Poly PomonaDocument1 pageIntroduction To Nuclear Magnetic Resonance (NMR) Spectroscopy Dr. Laurie S. Starkey, Cal Poly PomonaSankar AdhikariNo ratings yet
- Epoxidation ReviewDocument36 pagesEpoxidation ReviewSankar AdhikariNo ratings yet
- Oxidation of Primary Alcohols To Carboxylic Acids - A Guide To Current Common Practice-Gabriel Tojo - Marcos Fernández - Springer (2007)Document123 pagesOxidation of Primary Alcohols To Carboxylic Acids - A Guide To Current Common Practice-Gabriel Tojo - Marcos Fernández - Springer (2007)Sankar AdhikariNo ratings yet
- Layout of NykaaDocument12 pagesLayout of Nykaayogesh DivechaNo ratings yet
- Pizza Maker & Kitche N Crew Evaluations: Type of EvaluationDocument3 pagesPizza Maker & Kitche N Crew Evaluations: Type of Evaluationhari juharaNo ratings yet
- OR Review 3 Ppt.Document36 pagesOR Review 3 Ppt.jay kusainNo ratings yet
- Chapter Five Tractors and Related EquipmentDocument21 pagesChapter Five Tractors and Related EquipmentMicky AlemuNo ratings yet
- Director List of NishatDocument4 pagesDirector List of Nishatishipu14No ratings yet
- Sample Paper III (Science) - Question PaperDocument21 pagesSample Paper III (Science) - Question Paperfathima MiranNo ratings yet
- Ebook PDF Statistics Principles and Methods 8th Edition PDFDocument41 pagesEbook PDF Statistics Principles and Methods 8th Edition PDFjoseph.corey248100% (37)
- Oct-Chapter 1&2 - Practical ResearchDocument16 pagesOct-Chapter 1&2 - Practical ResearchJaimee Mojica TaligonNo ratings yet
- Ignou Regional and Study CentersDocument5 pagesIgnou Regional and Study Centersbtech_dksNo ratings yet
- Jeetendra Sharma ResumeDocument6 pagesJeetendra Sharma Resumer_awadhiyaNo ratings yet
- Quant Number Properties Part 1Document7 pagesQuant Number Properties Part 1Khurram AhmedNo ratings yet
- Week1 - FEE GIKIDocument38 pagesWeek1 - FEE GIKIHadeedAhmedSherNo ratings yet
- DzireDocument324 pagesDzireDaniel Lopez VenancioNo ratings yet
- Management Science: Definition, Characteristics and ToolsDocument6 pagesManagement Science: Definition, Characteristics and ToolsAmzelle Diego LaspiñasNo ratings yet
- SPM Unit 4 Notes-1Document27 pagesSPM Unit 4 Notes-1Ibrahim GadliNo ratings yet
- Bhaktisiddhanta Appearance DayDocument5 pagesBhaktisiddhanta Appearance DaySanjeev NambalateNo ratings yet
- (20-58) Charging Case Firmware Update Guide For R180 - Rev1.1Document6 pages(20-58) Charging Case Firmware Update Guide For R180 - Rev1.1Brandon CifuentesNo ratings yet
- WWW Theprestigecity Gen inDocument81 pagesWWW Theprestigecity Gen inprestige cityNo ratings yet
- 99tata Motors Ltd. Letter of Offer 18.09.08Document403 pages99tata Motors Ltd. Letter of Offer 18.09.08Sharmilanoor Nurul BasharNo ratings yet
- First Semester Summary ReportDocument21 pagesFirst Semester Summary ReportAkhila JoseNo ratings yet
- C & C++ Interview Questions You'll Most Likely Be AskedDocument24 pagesC & C++ Interview Questions You'll Most Likely Be AskedVibrant PublishersNo ratings yet
- Pic E10224Document1 pagePic E10224santosh KumarNo ratings yet
- Fire Safety - Wikipedia PDFDocument29 pagesFire Safety - Wikipedia PDFAld rich NuñezNo ratings yet
- Sa Pula, Sa PutiDocument84 pagesSa Pula, Sa PutiLuvina Amor Belarma0% (2)
- Tracebility MatrixDocument4 pagesTracebility Matrixmkm969No ratings yet
- Cara Membaca Analisis Gas Darah Arteri (AGDA)Document27 pagesCara Membaca Analisis Gas Darah Arteri (AGDA)Martin Susanto, MD67% (3)
- Machine Learning in GeoscienceDocument22 pagesMachine Learning in GeoscienceAde PrayudaNo ratings yet
- India 2020 - A Vision For The New MilleniumDocument13 pagesIndia 2020 - A Vision For The New MilleniumsanjeevNo ratings yet
- ISOELECTRIC Insulators CatalogueDocument62 pagesISOELECTRIC Insulators CatalogueJoel Palomares100% (1)
Organic Chemistry I: CH H C CH D
Organic Chemistry I: CH H C CH D
Uploaded by
Sankar AdhikariOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Organic Chemistry I: CH H C CH D
Organic Chemistry I: CH H C CH D
Uploaded by
Sankar AdhikariCopyright:
Available Formats
lOMoARcPSD|5381057
ORGANIC CHEMISTRY I
STEREOCHEMISTRY EXERCISES – SET 2
PART A
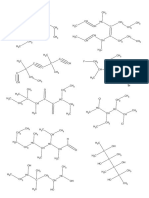
Consider the following molecules and answer the questions.
a) dichloromethane h) trans-1-bromo-2-chlorocyclobutane
b) 1-bromo-1-chloroethane i) cis-1-bromo-2-chloroethene
c) 2-bromopropane j) trans-1-bromo-2-chloroethene
d) 2-chlorobutane k) (2S, 3R)-2,3-dibromobutane
e) cis-1,2-dichlorocyclopropane l) (2R, 3R)-2,3-dibromobutane
f) trans-1,2-dichlorocyclopropane m) meso-1,3-dimethylcyclohexane
g) trans-1-bromo-3-chlorocyclobutane
1. Which of these molecules are chiral (i.e. asymmetric)?
2. Which of these molecules contain chiral carbons? In your drawings label them with an asterisk.
3. Which of these molecules can exist as enantiomeric pairs?
4. Which of these molecules represent meso compounds?
PART B
Indicate whether the following pairs of compounds represent the same molecule, pairs of enantiomers,
diastereomers, meso compounds, or stereochemically unrelated molecules.
CH3 CH3 CH3 HO Br
H H H C
C C C H
Br Br HO Br CH3
a) HO OH b)
CH3 H CH3 OH
H H3C H C Br
C C C
Br Br HO Br H3C H
c) HO HO d)
CH3 CH3 H H
H3C CH3
H OH HO
C C D Cl C C C C Cl
HO Br Br CH3 H3C
e) OH f) H H
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
lOMoARcPSD|5381057
H H
H OH H H
H3C H
OH H
Cl C C Cl
C C CH3 CH3
H CH3 H3C CH3
g) h) Br Br
H H OH OH
H Br H H
CH3 CH3 H H
i) Br H j) OH OH
OH H H OH OH H H
Br
Br H HO
H OH H H OH
k) l)
CH2CH 3 CH2CH 3
H OH HO H
CH2 CH2 NH2 CH3
H OH HO H H COOH HOOC H
m)
CH2CH 3 CH2CH 3 n)
CH3 NH2
CH3 Br
Br H Br H
NH2 COOH
H COOH H3C H H Br H CH3
CH3 NH2 CH3 CH3
o) p)
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
lOMoARcPSD|5381057
ANSWERS TO STEREOCHEMISTRY EXERCISE
PART A - Molecules (a) through (m) below have been drawn in a way that makes their symmetry apparent if
they are in fact symmetric. All the molecules labeled chiral can exist as enantiomeric pairs. All molecules with
two chiral carbons and a plane of symmetry represent meso compounds, namely (e), (k), and (m). Chiral
carbons have been marked with an asterisk.
H Cl H H
* *
Cl C Cl H CH3 H3C CH3 H3C CH2 CH3
H Br Br Cl
dichloromethane 1-bromo-1-chloroethane 2-bromopropane 2-chlorobutane
achiral one chiral carbon achiral one chiral carbon
chiral chiral
Br Cl
Cl Cl Cl Cl * *
* * * *
Br Cl
cis-1,2-dichloro- trans-1,2-dichloro- trans-1-bromo-3-chloro- trans-1-bromo-2-chloro-
cyclopropane cyclopropane cyclobutane cyclobutane
2 chiral carbons 2 chiral carbons achiral 2 chiral carbons
achiral chiral chiral
CH3 CH3
2 2
H Br Br H
Br Cl Br H
3 3
H Br H Br
H H H Cl CH3 CH3
cis-1-bromo-2-chloro- trans-1-bromo-2-chloro- (2S,3R)-2,3-dibromo- (2R,3R)-2,3-dibromo-
ethene ethene butane butane
achiral (planar) achiral (planar) 2 chiral carbons 2 chiral carbons
achiral chiral
H3C CH3
* *
meso-1,3-dimethyl-
cyclohexane
2 chiral carbons
achiral
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
lOMoARcPSD|5381057
PART B - Before assigning configuration to carbons, make sure they are chiral!
a) Chiral molecule and its mirror image - enantiomers.
b) R-isomer on the left, S-isomer on the right - enantiomers.
c) -OH and -Br are in the same positions, but -H and -CH3 have been exchanged - enantiomers.
d) R-isomer on the left, R-isomer on the right - same molecule.
e) Both molecules are chiral, but they do not have the same groups attached to the chiral carbon - unrelated.
f) Each molecule is chiral (no plane of symmetry) and they are mirror images - enantiomers.
g) The easiest way to approach this one is to assign configurations to the chiral carbons. The molecules have
the same molecular formula and the same connectivities, but their 3D arrangement is different. They are
stereoisomers. The configurations of their chiral centers mirror each other, which makes them enantiomers.
1
H H OH
H3C S S R
R OH C H
Cl C C Cl C 2 3
2 3 CH3
H CH3 H3C
4 1 4
(2R, 3S) (2S, 3R)
h) The molecule is not chiral (easiest to see in top view) and they’re both cis-isomers - same molecule.
Br CH3 cis-1-bromo-3-methylcyclobutane
i) A pair of cis/trans isomers - diastereomers.
j) Two trans-isomers (chiral) and mirror images - enantiomers.
k) Rotating the molecule on the left as shown leads to the molecule on the right - same molecule.
OH H
H Br
Br
H
OH H
l) Cis-isomers, same substituents on the same carbons (1 and 3), but different conformations - same molecule.
m) Each molecule has two chiral carbons and a plane of symmetry. Although they mirror each other, the are the
same molecule (a meso compound).
n) Both structures represent the S-isomer - same molecule.
o) S-isomer on the left, R-isomer on the right - enantiomers.
p) Both molecules represent 2,3-dibromobutane, but the molecule on the left has all the groups (-H, -Br, -CH3)
anti to each other. One can rotate the front carbon until all the groups eclipse and match each other.
That is not the case with the molecule on the right. Both molecules have two chiral carbons. The one on the left
has a plane of symmetry, which makes it a meso compound. The molecule on the right has no symmetry and
therefore it is chiral. They are diastereomers. For added clarity turn the molecules around to view them from
the side.
Downloaded by Sankar Adhikari (66adhikarisankar@gmail.com)
You might also like
- Solution Manual for The Elements of Polymer Science and EngineeringFrom EverandSolution Manual for The Elements of Polymer Science and EngineeringRating: 4 out of 5 stars4/5 (3)
- Reaction Guide by James Ashenhurst. 1-James AshenhurstDocument76 pagesReaction Guide by James Ashenhurst. 1-James AshenhurstSankar AdhikariNo ratings yet
- Organic Chemistry 1 Summary Sheets - James Ashenhurst - SS (2015)Document20 pagesOrganic Chemistry 1 Summary Sheets - James Ashenhurst - SS (2015)Sankar Adhikari100% (1)
- 3 Naming Alkynes Ws KeyDocument2 pages3 Naming Alkynes Ws KeyJaya Chitra Degala RamaluNo ratings yet
- 4 Roadtec 600 Cummins QSXDocument24 pages4 Roadtec 600 Cummins QSXdavidNo ratings yet
- Pyrolytic Elimination - Syn EliminationsDocument3 pagesPyrolytic Elimination - Syn EliminationssaheedvkNo ratings yet
- Section 3 - StereochemistryDocument21 pagesSection 3 - Stereochemistrysf9 fanfareNo ratings yet
- Trabajo Quimica Superior NAVIDADDocument13 pagesTrabajo Quimica Superior NAVIDADSebastian GuerraNo ratings yet
- Chemistry PracticeDocument13 pagesChemistry PracticeSiddharth KrishnamurthyNo ratings yet
- Ejercicios de Alcoholes y EteresDocument1 pageEjercicios de Alcoholes y EteresMaria Jose Duque AngelNo ratings yet
- A) OH B) OH C) OH D) OH E) OH F) OHDocument4 pagesA) OH B) OH C) OH D) OH E) OH F) OHRodrigo RVNo ratings yet
- CM1121 Tutorial 2 2006-7Document2 pagesCM1121 Tutorial 2 2006-7Tan Jun RongNo ratings yet
- Narayana Educational Soceity Organic ChemistryDocument25 pagesNarayana Educational Soceity Organic ChemistryNekhill KumarNo ratings yet
- Haloalcanos 3Document1 pageHaloalcanos 3MIRIAM SAN FRUTOS GARCÍANo ratings yet
- FitoquimiaDocument2 pagesFitoquimialuisgerardo000000No ratings yet
- JoDocument1 pageJoJoana Marie BeltranNo ratings yet
- Materi Reaksi KimiaDocument11 pagesMateri Reaksi KimiaRenn AgenaNo ratings yet
- Alcohol, Ether & PhenolDocument8 pagesAlcohol, Ether & Phenolshashwat.gupta.707No ratings yet
- CPP - HydrocarbonDocument13 pagesCPP - HydrocarbondivyanshjoshidpsjkpNo ratings yet
- Denumiți AlcaniiDocument2 pagesDenumiți AlcaniiCristianNo ratings yet
- DPP Optical+Isomer 4Document2 pagesDPP Optical+Isomer 4Shivam RoyNo ratings yet
- MechanismsDocument5 pagesMechanismsnajifaahmed223No ratings yet
- HC y AlcoholesDocument2 pagesHC y AlcoholesGonzalo HernandezNo ratings yet
- MULTIPLE CHOICE QUESTIONS Part 5: Stereochemistry: Topic: Identifications and ComparisonsDocument14 pagesMULTIPLE CHOICE QUESTIONS Part 5: Stereochemistry: Topic: Identifications and ComparisonsMoùümîtà KhäñráNo ratings yet
- 12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetDocument4 pages12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetZia Rathore100% (1)
- R Vs S AnsDocument2 pagesR Vs S AnsAtul SinghNo ratings yet
- Draw The Following Alkanes.: Hydrocarbon Nomenclature WorksheetDocument3 pagesDraw The Following Alkanes.: Hydrocarbon Nomenclature WorksheetCaseelyn Joy NantizaNo ratings yet
- PDFDocument30 pagesPDFAlok RanjanNo ratings yet
- Exercise StereochemistryDocument4 pagesExercise StereochemistryPuvaneswary LoganathanNo ratings yet
- Aqa Mechanisms A Level SummaryDocument5 pagesAqa Mechanisms A Level SummaryRS JNo ratings yet
- Answers To AssignmentDocument1 pageAnswers To AssignmentIgbereyivwe TejiriNo ratings yet
- Revision Notes Organic ChemistryDocument30 pagesRevision Notes Organic ChemistryAtharav Porwal100% (1)
- Isomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomersDocument11 pagesIsomerism: One or More Than One Answer Type Questions: 1. Which One of The Following Pairs of Isomers Are EnentiomerskamalNo ratings yet
- Organic Practice Set 9 - Chapter 7Document4 pagesOrganic Practice Set 9 - Chapter 7Aurora FerroNo ratings yet
- How To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inDocument2 pagesHow To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inmyiitchemistry81% (16)
- Estructura de Compuestos Biologicos-Parte 2: F.-EsteroidesDocument7 pagesEstructura de Compuestos Biologicos-Parte 2: F.-EsteroidesffhkeNo ratings yet
- PTM-1 ChemsitryDocument6 pagesPTM-1 ChemsitrymohakjainNo ratings yet
- Alkenes and Alkynes Worksheet PDFDocument3 pagesAlkenes and Alkynes Worksheet PDFDoha Hosam Shalaby HamamNo ratings yet
- Alcohol Nomenclature - Summative AssessmentDocument2 pagesAlcohol Nomenclature - Summative AssessmentDiana Carolina DuarteNo ratings yet
- Alcohol Nomenclature - Summative AssessmentDocument2 pagesAlcohol Nomenclature - Summative AssessmentDiana Carolina DuarteNo ratings yet
- Alcohol, Ether and PhenolDocument4 pagesAlcohol, Ether and PhenolKushagra SrivastavaNo ratings yet
- H NMR Problems: - How Many Unique Proton Environments Are There inDocument26 pagesH NMR Problems: - How Many Unique Proton Environments Are There inFatima AhmedNo ratings yet
- TPDocument39 pagesTPARAMAYO JuanNo ratings yet
- Basics of Photochemistry and Norrish Type I Reaction: Presented By: Dr. Nidhi VashisthaDocument12 pagesBasics of Photochemistry and Norrish Type I Reaction: Presented By: Dr. Nidhi Vashisthanidhi vashisthaNo ratings yet
- Ejercicios de Nomenclatura de Alcoholes.Document3 pagesEjercicios de Nomenclatura de Alcoholes.Diego Fernando Ardila ArizaNo ratings yet
- Naming AlkanesDocument3 pagesNaming Alkanesmercedes.caamalNo ratings yet
- Contoh: + Butan-2-On Metanol H SODocument2 pagesContoh: + Butan-2-On Metanol H SOElis TianiNo ratings yet
- Contoh: + Butan-2-On Metanol H SODocument2 pagesContoh: + Butan-2-On Metanol H SOElis TianiNo ratings yet
- OH A, A Is: : CHN 1 EqvDocument4 pagesOH A, A Is: : CHN 1 EqvAtharva GanjuNo ratings yet
- AlcoholsDocument7 pagesAlcoholsSuhaan GargNo ratings yet
- Organic Reactions Summary Alkenes, Alkynes and Variations For Use As A Study Guide BeauchampDocument45 pagesOrganic Reactions Summary Alkenes, Alkynes and Variations For Use As A Study Guide BeauchampShaker MahmoodNo ratings yet
- Org 1 PDFDocument4 pagesOrg 1 PDFTanmay KumarNo ratings yet
- Chapter 5 ProblemsDocument7 pagesChapter 5 Problemsdicoz diphaNo ratings yet
- Hidrocarburos MIX-01Document1 pageHidrocarburos MIX-01Abraham CastañedaNo ratings yet
- OrganicChemistryChapter7 PDFDocument30 pagesOrganicChemistryChapter7 PDFSeanne CruzNo ratings yet
- 314 Stereochem ProbsDocument14 pages314 Stereochem ProbsAtul SinghNo ratings yet
- Stereochemistry of OrganicDocument35 pagesStereochemistry of OrganicRams ChanderNo ratings yet
- S22 - URAIAN Dan KONTROL PROSES PEMBUATANDocument7 pagesS22 - URAIAN Dan KONTROL PROSES PEMBUATANTrisnawati AmaliaNo ratings yet
- Enabling AssessmentDocument3 pagesEnabling Assessmentlluvemnx takahashiNo ratings yet
- Summary of Alkyne Reactions: H H C H H C BR HDocument1 pageSummary of Alkyne Reactions: H H C H H C BR HKamelNo ratings yet
- Hidrocarbonetos 02Document2 pagesHidrocarbonetos 02Romário MelloNo ratings yet
- Mechanism Summary For A-Level AQA Chemistry: BR BRDocument5 pagesMechanism Summary For A-Level AQA Chemistry: BR BRamrhkmhNo ratings yet
- CO2 - 1S2 - 2011 - LSLL - CopieDocument5 pagesCO2 - 1S2 - 2011 - LSLL - CopiePFENo ratings yet
- Clock TricksDocument7 pagesClock TricksSankar AdhikariNo ratings yet
- MCQ On Problems On DiceDocument14 pagesMCQ On Problems On DiceSankar AdhikariNo ratings yet
- Fehling's Solution Is A Chemical Reagent Used To Differentiate BetweenDocument2 pagesFehling's Solution Is A Chemical Reagent Used To Differentiate BetweenSankar AdhikariNo ratings yet
- CSIR NET June 2021 InorganicDocument37 pagesCSIR NET June 2021 InorganicSankar AdhikariNo ratings yet
- Formulas For Clocks Questions - PrepInstaDocument3 pagesFormulas For Clocks Questions - PrepInstaSankar AdhikariNo ratings yet
- How To Solve Clocks Questions Easily - PrepInstaDocument4 pagesHow To Solve Clocks Questions Easily - PrepInstaSankar AdhikariNo ratings yet
- Math FundasDocument60 pagesMath FundasSankar AdhikariNo ratings yet
- DICE - Verbal Reasoning QuestionsDocument13 pagesDICE - Verbal Reasoning QuestionsSankar AdhikariNo ratings yet
- 100 Shortcuts To Quantitative Aptitude Speed MattersDocument102 pages100 Shortcuts To Quantitative Aptitude Speed Mattersbest commentator barack100% (3)
- Quenching PyrophoricsDocument3 pagesQuenching PyrophoricsSankar AdhikariNo ratings yet
- Dice, Cube ProblemsDocument13 pagesDice, Cube ProblemsSankar AdhikariNo ratings yet
- Tips & Tricks For Quantitative AptitudeDocument24 pagesTips & Tricks For Quantitative AptitudeSankar Adhikari0% (1)
- CSIR NET June 2021 Organic ChemistryDocument99 pagesCSIR NET June 2021 Organic ChemistrySankar AdhikariNo ratings yet
- Dr. Laurie S. Starkey, Cal Poly Pomona - NMR Spectroscopy: Spin-Spin CouplingDocument1 pageDr. Laurie S. Starkey, Cal Poly Pomona - NMR Spectroscopy: Spin-Spin CouplingSankar AdhikariNo ratings yet
- sp21 234 r10 Extra Problems Organometallics KeyDocument8 pagessp21 234 r10 Extra Problems Organometallics KeySankar AdhikariNo ratings yet
- Organometallics CH 8 NotesDocument11 pagesOrganometallics CH 8 NotesSankar AdhikariNo ratings yet
- 30 of The Most Common Grammatical Errors We All Need To Stop MakingDocument19 pages30 of The Most Common Grammatical Errors We All Need To Stop MakingSankar AdhikariNo ratings yet
- 22-Sharpless Asymmetric Epoxidation ReactionDocument5 pages22-Sharpless Asymmetric Epoxidation ReactionSankar AdhikariNo ratings yet
- CSIR Chemical Science ORGANICDocument492 pagesCSIR Chemical Science ORGANICSankar AdhikariNo ratings yet
- Introduction To Nuclear Magnetic Resonance (NMR) Spectroscopy Dr. Laurie S. Starkey, Cal Poly PomonaDocument1 pageIntroduction To Nuclear Magnetic Resonance (NMR) Spectroscopy Dr. Laurie S. Starkey, Cal Poly PomonaSankar AdhikariNo ratings yet
- Epoxidation ReviewDocument36 pagesEpoxidation ReviewSankar AdhikariNo ratings yet
- Oxidation of Primary Alcohols To Carboxylic Acids - A Guide To Current Common Practice-Gabriel Tojo - Marcos Fernández - Springer (2007)Document123 pagesOxidation of Primary Alcohols To Carboxylic Acids - A Guide To Current Common Practice-Gabriel Tojo - Marcos Fernández - Springer (2007)Sankar AdhikariNo ratings yet
- Layout of NykaaDocument12 pagesLayout of Nykaayogesh DivechaNo ratings yet
- Pizza Maker & Kitche N Crew Evaluations: Type of EvaluationDocument3 pagesPizza Maker & Kitche N Crew Evaluations: Type of Evaluationhari juharaNo ratings yet
- OR Review 3 Ppt.Document36 pagesOR Review 3 Ppt.jay kusainNo ratings yet
- Chapter Five Tractors and Related EquipmentDocument21 pagesChapter Five Tractors and Related EquipmentMicky AlemuNo ratings yet
- Director List of NishatDocument4 pagesDirector List of Nishatishipu14No ratings yet
- Sample Paper III (Science) - Question PaperDocument21 pagesSample Paper III (Science) - Question Paperfathima MiranNo ratings yet
- Ebook PDF Statistics Principles and Methods 8th Edition PDFDocument41 pagesEbook PDF Statistics Principles and Methods 8th Edition PDFjoseph.corey248100% (37)
- Oct-Chapter 1&2 - Practical ResearchDocument16 pagesOct-Chapter 1&2 - Practical ResearchJaimee Mojica TaligonNo ratings yet
- Ignou Regional and Study CentersDocument5 pagesIgnou Regional and Study Centersbtech_dksNo ratings yet
- Jeetendra Sharma ResumeDocument6 pagesJeetendra Sharma Resumer_awadhiyaNo ratings yet
- Quant Number Properties Part 1Document7 pagesQuant Number Properties Part 1Khurram AhmedNo ratings yet
- Week1 - FEE GIKIDocument38 pagesWeek1 - FEE GIKIHadeedAhmedSherNo ratings yet
- DzireDocument324 pagesDzireDaniel Lopez VenancioNo ratings yet
- Management Science: Definition, Characteristics and ToolsDocument6 pagesManagement Science: Definition, Characteristics and ToolsAmzelle Diego LaspiñasNo ratings yet
- SPM Unit 4 Notes-1Document27 pagesSPM Unit 4 Notes-1Ibrahim GadliNo ratings yet
- Bhaktisiddhanta Appearance DayDocument5 pagesBhaktisiddhanta Appearance DaySanjeev NambalateNo ratings yet
- (20-58) Charging Case Firmware Update Guide For R180 - Rev1.1Document6 pages(20-58) Charging Case Firmware Update Guide For R180 - Rev1.1Brandon CifuentesNo ratings yet
- WWW Theprestigecity Gen inDocument81 pagesWWW Theprestigecity Gen inprestige cityNo ratings yet
- 99tata Motors Ltd. Letter of Offer 18.09.08Document403 pages99tata Motors Ltd. Letter of Offer 18.09.08Sharmilanoor Nurul BasharNo ratings yet
- First Semester Summary ReportDocument21 pagesFirst Semester Summary ReportAkhila JoseNo ratings yet
- C & C++ Interview Questions You'll Most Likely Be AskedDocument24 pagesC & C++ Interview Questions You'll Most Likely Be AskedVibrant PublishersNo ratings yet
- Pic E10224Document1 pagePic E10224santosh KumarNo ratings yet
- Fire Safety - Wikipedia PDFDocument29 pagesFire Safety - Wikipedia PDFAld rich NuñezNo ratings yet
- Sa Pula, Sa PutiDocument84 pagesSa Pula, Sa PutiLuvina Amor Belarma0% (2)
- Tracebility MatrixDocument4 pagesTracebility Matrixmkm969No ratings yet
- Cara Membaca Analisis Gas Darah Arteri (AGDA)Document27 pagesCara Membaca Analisis Gas Darah Arteri (AGDA)Martin Susanto, MD67% (3)
- Machine Learning in GeoscienceDocument22 pagesMachine Learning in GeoscienceAde PrayudaNo ratings yet
- India 2020 - A Vision For The New MilleniumDocument13 pagesIndia 2020 - A Vision For The New MilleniumsanjeevNo ratings yet
- ISOELECTRIC Insulators CatalogueDocument62 pagesISOELECTRIC Insulators CatalogueJoel Palomares100% (1)