Professional Documents
Culture Documents
1455877785CHE P12 M35 Etext
1455877785CHE P12 M35 Etext
Uploaded by
Saurav PaulOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
1455877785CHE P12 M35 Etext
1455877785CHE P12 M35 Etext
Uploaded by
Saurav PaulCopyright:
Available Formats
Subject Chemistry
Paper No and Title 12: Organic Spectroscopy
Module No and 35: Combined problem on UV, IR, 1H NMR, 13C
Title NMR and Mass – Part VII
Module Tag CHE_P12_M35
CHEMISTRY PAPER No. 12: Organic Spectroscopy
MODULE No. 35: Combined problem on UV, IR, 1H NMR, 13C NMR
and Mass- Part VII
TABLE OF CONTENTS
1. Learning Outcomes
2. Introduction
3. Problems and their solutions
CHEMISTRY PAPER No. 12: Organic Spectroscopy
MODULE No. 35: Combined problem on UV, IR, 1H NMR, 13C NMR
and Mass- Part VII
1. Learning Outcomes
After studying this module, you shall be able to
Solve problem related with electronic transitions
Learn how to differentiate molecule on the basis of IR spectroscopy
Correlate spectra with structure of compound
Interpret the spectroscopic data
2. Introduction
The knowledge and concepts of UV-visible, IR, 1H NMR, 13
C NMR and Mass help us in
solving problems based on the experimental data. It will help us in analysing the
experimental data to elucidate the structure of any organic compound.
While analysing the data the following point must be kept in mind:
In UV-visible spectroscopy; the types of bonds and electrons plays important role in
understanding the electronic transitions.
UV-visible spectroscopy gives information regarding the presence of conjugation,
carbonyl group etc.
The IR values gives information regarding the functional group present in the
molecule
The 1H NMR tells us the number and environment of neighbouring hydrogens
present.
The 13
C NMR helps in getting the information about the type of carbon atom(s)
present in the molecule.
Mass spectral data gives information about the total mass and fragmentation pattern of
the molecule.
CHEMISTRY PAPER No. 12: Organic Spectroscopy
MODULE No. 35: Combined problem on UV, IR, 1H NMR, 13C NMR
and Mass- Part VII
By combining all the information one can find the structure of the molecule.
3. Problems and their solutions
Q. 1. Which of the following has the highest carbonyl stretching frequency.
A. 1. Except acetamide, the inductive effect is dominant and the highest carbonyl
stretching frequency will be shown by (b) because it involves the largest net inductive
withdrawal from the carbonyl group.
Q. 2. The proton-decoupled spectrum of a tribromobenzene (C6H3Br3) consists of two signals
only. What tribromobenzenes is it?
A. 2. There are three possible tribromobenzenes:
CHEMISTRY PAPER No. 12: Organic Spectroscopy
MODULE No. 35: Combined problem on UV, IR, 1H NMR, 13C NMR
and Mass- Part VII
The presence of two signals indicates that only two different types of carbon atoms are
present in the compound. Only 1,3,5-tribromobenzene has a degree of symmetry such that it
would give only two signals and, therefore, it is the correct structure.
Q. 3. Predict the structure of the compound whose peaks in the mass spectrum have m/e
values 57 (100% abundance), 41, 29 and 27.
A. 3. The predicted structure of the compound is:
Q. 4. A compound with molecular weight 116 gave the following general information:
(i) UV: 283 mµ Ɛmax 22.
(ii) IR: 3000-2500 (b) 1715 (s), 1342 cm-1 (w)
(iii) NMR: 7.88 τ singlet (3H), 7.40 τ triplet (2H), 7.75 τ triplet (2H) and -1.1 τ singlet (1H).
Find the structural formula of the compound. (Note: δ=10-τ)
A. 4. In the UV spectrum, the absorption at 283 mµ of low intensity indicates the
presence of carbonyl group.
The presence of carbonyl group is further confirmed by a strong band at 1715 cm-1. A very
broad band at 3000-2500 cm-1 is most characteristic of acids (O-H stretching and it appears as
a result of strong hydrogen bonding.
The presence of an acid group (-COOH) is also shown by NMR which gives a signal (singlet)
at the negative tau value. Thus, the compound under investigation contains:
(i) –CO- group and
(ii) –COOH group
Further, two triplets result at 7.40 τ and 7.75 τ having the same integral area. It must be due to
–CH2-CH2-, clearly, two methylene groups must be under the different environments and
thus, couple to give rise to two triplets. The appearance of a three proton singlet at 7.88 τ
CHEMISTRY PAPER No. 12: Organic Spectroscopy
MODULE No. 35: Combined problem on UV, IR, 1H NMR, 13C NMR
and Mass- Part VII
must be a methyl group attached with the carbonyl group. Hence, the compound under
investigation is
Q. 5. An organic compound forms a molecular ion peak in its mass spectrum at m/e 114. The
other prominent peaks appear at m/e 85, 72 (M. R. ion), 57, 41, 29. Name the compound.
A. 5. The possible structure of the compound would be:
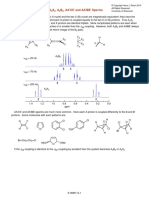
Q. 6. The compound has the molecular formula C5H7NO2. Following are the infrared, 1H
NMR and 13C NMR spectra.
CHEMISTRY PAPER No. 12: Organic Spectroscopy
MODULE No. 35: Combined problem on UV, IR, 1H NMR, 13C NMR
and Mass- Part VII
A. 6. We calculate the index of hydrogen deficiency of three. A quick glance at the
infrared spectrum reveals the source of unsaturation implied by an index of three: a
nitrile group at 2260 cm-1 (unsaturation index = two) and a carbonyl group at 1747
cm-1 (unsaturation index = one). The frequency of the carbonyl absorption indicates an
unconjugated ester. The appearance of several strong C-O bands near 1200 cm-1
confirms the presence of an ester functional group. We can rule out bond
because they usually absorb at a value of 2150 cm-1 and have a weaker intensity than
compounds that contain bond.
13
The C NMR spectrum shows five peaks and thus is consistent with the molecular
formula which contains five carbon atoms. Notice that the carbon atom in the
group has a characteristic value at 113 ppm. In addition, the carbon atom in the ester C=O
appears at 163 ppm. One of the remaining carbon atoms (63 ppm) probably lies next to an
CHEMISTRY PAPER No. 12: Organic Spectroscopy
MODULE No. 35: Combined problem on UV, IR, 1H NMR, 13C NMR
and Mass- Part VII
electronegative oxygen atom. The remaining two carbon atoms, which absorb at 25 and
14 ppm, are attributed to the remaining methylene and methyl carbons. The structure is:
The 1H NMR spectrum shows a classic ethyl pattern: a quartet (2H) at 4.3 ppm and a
triplet (3H) at 1.3 ppm. The quartet is strongly influenced by the electronegative oxygen
atom, which shifts it downfield. There is a two proton singlet at 3.5 ppm.
Q. 7. Calculate the chemical shift in parts per million (δ) for a proton that has a resonance
of 128 Hz downfield from TMS on a spectrometer that operates at 60 MHz.
A. 7. This can be calculated from the following formula:
ppm = Value of resonance frequency in Hz
Value of frequency that instrument operates on
Thus substituting the values in the above equation we get the value of ppm as 2.1 ppm.
CHEMISTRY PAPER No. 12: Organic Spectroscopy
MODULE No. 35: Combined problem on UV, IR, 1H NMR, 13C NMR
and Mass- Part VII
You might also like
- (Organo) Part BDocument9 pages(Organo) Part BAmirul Amin Bin ShukriNo ratings yet
- Plugin EX 1-10-11 Practice AnswerDocument12 pagesPlugin EX 1-10-11 Practice Answervsuresh123456789No ratings yet
- SDBS Compounds and Spectral SearchDocument25 pagesSDBS Compounds and Spectral SearchKumara PrasannaNo ratings yet
- Paper 12: Organic Spectroscopy: Subject ChemistryDocument12 pagesPaper 12: Organic Spectroscopy: Subject ChemistryFaiza AnsariNo ratings yet
- 1455787242CHE P12 M30 EtextDocument8 pages1455787242CHE P12 M30 EtextSaurav PaulNo ratings yet
- NMR Spectroscopy - Short NoteDocument6 pagesNMR Spectroscopy - Short Notecoolhemakumar100% (4)
- 1455787138CHE P12 M29 EtextDocument7 pages1455787138CHE P12 M29 EtextSaurav PaulNo ratings yet
- Chemistry 318: Ir, MS, Uv, NMR SpectrosDocument17 pagesChemistry 318: Ir, MS, Uv, NMR Spectrosaamer_shahbaaz0% (4)
- Structure Determination of Organic Compounds - ChemvoiceDocument37 pagesStructure Determination of Organic Compounds - ChemvoiceJubin Kumar0% (1)
- TB Chapter12Document9 pagesTB Chapter12Luke SkywalkerNo ratings yet
- 1455787432CHE P12 M33 E-TextDocument12 pages1455787432CHE P12 M33 E-TextSaurav PaulNo ratings yet
- Chapter 15 - NMR SpectrosDocument13 pagesChapter 15 - NMR SpectrosHepi NuriyawanNo ratings yet
- 2d NMRDocument32 pages2d NMRDelicz TanNo ratings yet
- 6spectroscopy and ChromatographyDocument15 pages6spectroscopy and ChromatographyThinaya JayarathneNo ratings yet
- Informe 2 AnalisisDocument17 pagesInforme 2 AnalisisCarla ParraNo ratings yet
- Test Bank For Organic Chemistry 8th Edition McmurryDocument18 pagesTest Bank For Organic Chemistry 8th Edition McmurryAnthonyRogersydtfp100% (72)
- C NMR Lab ReportDocument14 pagesC NMR Lab ReportDominic BoyerNo ratings yet
- NMR Spectroscopy LabDocument7 pagesNMR Spectroscopy LabBriana Halbert100% (2)
- B.SC Organic Chemistry (Paper-5) - Q and ADocument59 pagesB.SC Organic Chemistry (Paper-5) - Q and ASyed furkhanNo ratings yet
- Probing Radical Chemistry in Salmonella Typhimurium Cells Under Oxidative Stress Using Spin Traps and Nitroxyl RadicalsDocument6 pagesProbing Radical Chemistry in Salmonella Typhimurium Cells Under Oxidative Stress Using Spin Traps and Nitroxyl RadicalsjwdaliNo ratings yet
- Mcmurry 12Document62 pagesMcmurry 12Ngurah MahasviraNo ratings yet
- Clayden 2e - End of Chapter Questions - Ch3Document4 pagesClayden 2e - End of Chapter Questions - Ch3Nikola NinkovNo ratings yet
- Lecture 1Document28 pagesLecture 1Sebastián Castro FragueiroNo ratings yet
- Reductions in Organic ChemistryDocument13 pagesReductions in Organic ChemistryNikola PudjaNo ratings yet
- Lab Report 6 Organic Chemistry 1Document4 pagesLab Report 6 Organic Chemistry 1Toria YoungNo ratings yet
- NMR Caracterization - 2022-2023Document21 pagesNMR Caracterization - 2022-2023Paula ArmendárizNo ratings yet
- InfraredDocument50 pagesInfraredChandra Sekhar ReddyNo ratings yet
- Organic Chemistry - Structural AnalysisDocument27 pagesOrganic Chemistry - Structural AnalysisRAIEL ALVARONo ratings yet
- IRtheory For StudentsDocument10 pagesIRtheory For StudentsNicolae PopaNo ratings yet
- Hafiz M. Asad NAwaz's AssignmentDocument8 pagesHafiz M. Asad NAwaz's AssignmentArslan ElahiNo ratings yet
- 3.09 InterpretationDocument10 pages3.09 Interpretationfouad elferdiNo ratings yet
- Assignment@SEM I@NMRDocument3 pagesAssignment@SEM I@NMRSoumyadeep BarmanNo ratings yet
- c13ppt 150515121301 Lva1 App6892Document16 pagesc13ppt 150515121301 Lva1 App6892kiran yaseenNo ratings yet
- $4 Intro To Analytical TechniquesDocument15 pages$4 Intro To Analytical TechniquesAnonymous j6gqgCvNo ratings yet
- ORG-CHEM-LEC-CIP Rev1Document5 pagesORG-CHEM-LEC-CIP Rev1Arthur KirklandNo ratings yet
- 22 and Applications of C NMR: Subject ChemistryDocument13 pages22 and Applications of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- 11 (3) Spectroscopic ID SL HLDocument12 pages11 (3) Spectroscopic ID SL HLmickey mouseNo ratings yet
- Guía de Estudio EspectrosDocument5 pagesGuía de Estudio EspectrosCésar CidNo ratings yet
- Spectroscopy and Chromatography 1 Unit 4 New SpecificationsDocument11 pagesSpectroscopy and Chromatography 1 Unit 4 New SpecificationsLoh Jun XianNo ratings yet
- Dominikus - Jurnal Reaksi Perisiklik 2Document3 pagesDominikus - Jurnal Reaksi Perisiklik 2Ekin Dwi ArifNo ratings yet
- Ch-05-Spectroscopy of Organic CompoundsDocument10 pagesCh-05-Spectroscopy of Organic CompoundsRuxhiNo ratings yet
- CHEM220 SI Mini Revision TestDocument4 pagesCHEM220 SI Mini Revision TestSthokomele ZuluNo ratings yet
- CHM 3402 Experiment 8Document11 pagesCHM 3402 Experiment 8Uma Villashini GunasekaranNo ratings yet
- Experiment 8Document8 pagesExperiment 8NathanianNo ratings yet
- Basic Concept of C NMR: Subject ChemistryDocument15 pagesBasic Concept of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- Revised Preliminary Introduction of SpectrosDocument29 pagesRevised Preliminary Introduction of SpectrosRevathiNo ratings yet
- Lecture 5 Molecular SpectrosDocument97 pagesLecture 5 Molecular Spectroshoboslayer97No ratings yet
- IR and NMR SpectrosDocument13 pagesIR and NMR SpectrosAnand BarapatreNo ratings yet
- NMR Multiple Choice Questions PDFDocument71 pagesNMR Multiple Choice Questions PDFDeepak SinghNo ratings yet
- Stucture DeterminationDocument7 pagesStucture DeterminationFiaz medicoNo ratings yet
- Lai 1992Document7 pagesLai 1992Saurav PaulNo ratings yet
- IR SpectrosDocument27 pagesIR SpectrosAlyssa NesianandaNo ratings yet
- Interpreting and Predicting Proton NMR Spectra - View As Single PageDocument64 pagesInterpreting and Predicting Proton NMR Spectra - View As Single PageMaxi MaNo ratings yet
- Structural and Spectroscopic Investigations of Redox Active Seven Coordinate Luminescent Lanthanide ComplexesDocument9 pagesStructural and Spectroscopic Investigations of Redox Active Seven Coordinate Luminescent Lanthanide ComplexesHoracio Piña SpeziaNo ratings yet
- IR SpectrometryDocument46 pagesIR Spectrometryortizan8No ratings yet
- Lab Manual Metal Acetylacetonate Complexes Web PDFDocument22 pagesLab Manual Metal Acetylacetonate Complexes Web PDFYan Jie ChongNo ratings yet
- UV Spectroscopy QuestionsDocument3 pagesUV Spectroscopy QuestionsRashmiNo ratings yet
- 9701 - Nos - As - 3 Applications of Analytial ChemistryDocument53 pages9701 - Nos - As - 3 Applications of Analytial ChemistryWilliam SkyNo ratings yet
- Modern Vibrational Spectroscopy and Micro-Spectroscopy: Theory, Instrumentation and Biomedical ApplicationsFrom EverandModern Vibrational Spectroscopy and Micro-Spectroscopy: Theory, Instrumentation and Biomedical ApplicationsNo ratings yet
- Experimental and Theoretical Approaches to Actinide ChemistryFrom EverandExperimental and Theoretical Approaches to Actinide ChemistryJohn K. GibsonNo ratings yet
- Reactive Oxygen Species: Signaling Between Hierarchical Levels in PlantsFrom EverandReactive Oxygen Species: Signaling Between Hierarchical Levels in PlantsFranz-Josef SchmittNo ratings yet
- Voltagedependent Optical Activity of A Twisted Nematic Liquid CrystalDocument3 pagesVoltagedependent Optical Activity of A Twisted Nematic Liquid CrystalSaurav PaulNo ratings yet
- 1455787242CHE P12 M30 EtextDocument8 pages1455787242CHE P12 M30 EtextSaurav PaulNo ratings yet
- Funahashi 1997Document7 pagesFunahashi 1997Saurav PaulNo ratings yet
- Dielectric Materials: Dielectric Materials Are and Used Principally in andDocument7 pagesDielectric Materials: Dielectric Materials Are and Used Principally in andSaurav PaulNo ratings yet
- Coates 1973Document2 pagesCoates 1973Saurav PaulNo ratings yet
- Structure Problem Solving Using H and C NMR Spectral Data Tutorial SessionDocument12 pagesStructure Problem Solving Using H and C NMR Spectral Data Tutorial SessionSaurav PaulNo ratings yet
- Nakanishi 2004Document9 pagesNakanishi 2004Saurav PaulNo ratings yet
- A Highly Selective and Sensitive Fluorescent Chemosensor For Fe in Physiological Aqueous SolutionDocument2 pagesA Highly Selective and Sensitive Fluorescent Chemosensor For Fe in Physiological Aqueous SolutionSaurav PaulNo ratings yet
- 1455787138CHE P12 M29 EtextDocument7 pages1455787138CHE P12 M29 EtextSaurav PaulNo ratings yet
- 22 and Applications of C NMR: Subject ChemistryDocument13 pages22 and Applications of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- 1455786764CHE P12 M24 E-TextDocument10 pages1455786764CHE P12 M24 E-TextSaurav PaulNo ratings yet
- Account Statement From 1 Sep 2019 To 11 Aug 2020: TXN Date Value Date Description Ref No./Cheque No. Debit Credit BalanceDocument2 pagesAccount Statement From 1 Sep 2019 To 11 Aug 2020: TXN Date Value Date Description Ref No./Cheque No. Debit Credit BalanceSaurav PaulNo ratings yet
- Basic Concept of C NMR: Subject ChemistryDocument15 pagesBasic Concept of C NMR: Subject ChemistrySaurav PaulNo ratings yet
- 1455787432CHE P12 M33 E-TextDocument12 pages1455787432CHE P12 M33 E-TextSaurav PaulNo ratings yet
- Account Statement From 1 Oct 2020 To 6 Oct 2020Document1 pageAccount Statement From 1 Oct 2020 To 6 Oct 2020Saurav PaulNo ratings yet
- AA'BB' SpectraDocument11 pagesAA'BB' SpectraSaurav PaulNo ratings yet
- Towards Complex Matter: Supramolecular Chemistry and Self-OrganizationDocument19 pagesTowards Complex Matter: Supramolecular Chemistry and Self-OrganizationSaurav PaulNo ratings yet
- The Relative NMR Sensitivity of Nucleus at Constant Magnetic FieldDocument4 pagesThe Relative NMR Sensitivity of Nucleus at Constant Magnetic FieldSaurav PaulNo ratings yet
- Chapter 6 Introduction To Thermodynamics PDFDocument17 pagesChapter 6 Introduction To Thermodynamics PDFSaurav PaulNo ratings yet
- The Structure of The Atom: Randima Piyumalie GalhenageDocument5 pagesThe Structure of The Atom: Randima Piyumalie GalhenageSaurav PaulNo ratings yet
- Novel Bunyavirus in Domestic and Captive Farmed Animals, Minnesota, USADocument4 pagesNovel Bunyavirus in Domestic and Captive Farmed Animals, Minnesota, USASaurav PaulNo ratings yet
- 41 Topics in Organometallic ChemistryDocument11 pages41 Topics in Organometallic ChemistrySaurav PaulNo ratings yet
- Schroeder 2020Document11 pagesSchroeder 2020Quí HuỳnhNo ratings yet
- 10 1016@j Molliq 2019 111008Document8 pages10 1016@j Molliq 2019 111008muh. almusyafirNo ratings yet
- CBZ CaracteristicasDocument189 pagesCBZ CaracteristicasEduardo TarangoNo ratings yet
- Two-Step Protic Solvent-Catalyzed Reaction of Phenylethylamine With Methyl Acrylate (Organic Preparations and Procedures International, 2005, 37, 6, 579-584 10.1080@00304940509354990)Document7 pagesTwo-Step Protic Solvent-Catalyzed Reaction of Phenylethylamine With Methyl Acrylate (Organic Preparations and Procedures International, 2005, 37, 6, 579-584 10.1080@00304940509354990)DmitryNo ratings yet
- Aloe Vera QNMR Method - Poster PresentationDocument1 pageAloe Vera QNMR Method - Poster Presentationjcepna5397No ratings yet
- Problems Related To Spectroscopy (Solution)Document9 pagesProblems Related To Spectroscopy (Solution)Nana BadrNo ratings yet
- Anna Zurek Chem 231 Lab Report - Portfolio VersionDocument31 pagesAnna Zurek Chem 231 Lab Report - Portfolio VersionAnna ZurekNo ratings yet
- CHEM F111: General Chemistry: PilaniDocument25 pagesCHEM F111: General Chemistry: Pilanicukdbjsisns shsusbsbvzNo ratings yet
- Lab Report Tips!!Document12 pagesLab Report Tips!!Diana SekarNo ratings yet
- Predict 1H Proton NMR SpectraDocument1 pagePredict 1H Proton NMR SpectraSarathy Hari KumarNo ratings yet
- European Journal of Medicinal Chemistry: Yan Zhu, Nannan Sun, Mingcheng Yu, Huimin Guo, Qiong Xie, Yonghui WangDocument16 pagesEuropean Journal of Medicinal Chemistry: Yan Zhu, Nannan Sun, Mingcheng Yu, Huimin Guo, Qiong Xie, Yonghui WangWalid Ebid ElgammalNo ratings yet
- Phmz.61.7pheno Glycosides From Exostema Mexicanum LeavesDocument4 pagesPhmz.61.7pheno Glycosides From Exostema Mexicanum LeavesMarco AC HernándezNo ratings yet
- Problem 1 (Author Khvalyuk V.N.) : 54th International Mendeleev Olympiad, 2020 1 Theoretical Tour SolutionsDocument15 pagesProblem 1 (Author Khvalyuk V.N.) : 54th International Mendeleev Olympiad, 2020 1 Theoretical Tour SolutionsQuốc NguyễnNo ratings yet
- H/NMR MetodaDocument31 pagesH/NMR MetodaAlexandra MilenkovicNo ratings yet
- Influence of Poly Methacrylate Co Maleic Anhydride Pour Point Depre - 2018 - FDocument10 pagesInfluence of Poly Methacrylate Co Maleic Anhydride Pour Point Depre - 2018 - FKhairul Amirin100% (1)
- Organic Chemistry NotesDocument45 pagesOrganic Chemistry NotesJasmine Sloan100% (1)
- 19p NMR Part 7 2Document26 pages19p NMR Part 7 2Go RikanNo ratings yet
- Chemical and Biological Investigation of Erythrina Variegata (Fabaceae)Document63 pagesChemical and Biological Investigation of Erythrina Variegata (Fabaceae)zakx24x7bdNo ratings yet
- Applications of NMR Spectroscopy in Inorganic ChemistryDocument11 pagesApplications of NMR Spectroscopy in Inorganic ChemistryDhanaswamy Ilangeswaran92% (12)
- Lipase Inhibitors From Nigella Sativa and Punica Granatum As An Effective Approach Towards Controlling ObesityDocument19 pagesLipase Inhibitors From Nigella Sativa and Punica Granatum As An Effective Approach Towards Controlling ObesityGlobal Research and Development ServicesNo ratings yet
- A.K. Sider Et Al - Hexanitrohexaazaisowurtzitane or CL-20 in India: Synthesis and CharacterisationDocument11 pagesA.K. Sider Et Al - Hexanitrohexaazaisowurtzitane or CL-20 in India: Synthesis and CharacterisationKommissar1981No ratings yet
- NMR Chemical Shifts of Common Laboratory SolventsDocument4 pagesNMR Chemical Shifts of Common Laboratory Solventspharmacysmile8049No ratings yet
- Artikel ENNRJ SyarifahDocument16 pagesArtikel ENNRJ SyarifahKurratul 'AiniNo ratings yet
- Nanogels CurcuminDocument7 pagesNanogels CurcuminLê Huyền PhụngNo ratings yet
- 4.9 Spectroscopy and Chromatography-2Document45 pages4.9 Spectroscopy and Chromatography-2Michael Angelo FilomenoNo ratings yet
- Natural Product Research: Formerly Natural Product LettersDocument14 pagesNatural Product Research: Formerly Natural Product LettersRIVALDO MARSEL TNo ratings yet
- Fasanello MJ5.1-4 41 2007Document12 pagesFasanello MJ5.1-4 41 2007rubeushagrid526No ratings yet
- Questions For Assignment 1 CHM557Document8 pagesQuestions For Assignment 1 CHM557SITI NUR AFIQAH MAHAZANNo ratings yet