Professional Documents
Culture Documents
Zeeman Effect Index Atomic Structure Concepts: Herzberg
Zeeman Effect Index Atomic Structure Concepts: Herzberg
Uploaded by
Sreedevi Krishnakumar0 ratings0% found this document useful (0 votes)
27 views1 pageThe Stark effect describes the splitting of atomic spectral lines when an externally applied electric field is present. The splitting is caused by the electric field first polarizing the atom, then interacting with the induced electric dipole moment. This interaction splits the energy levels in a way that depends on the magnitude of the quantum number J, but not its sign, resulting in splittings proportional to J+1 or J+1/2. While the Stark effect has been of limited use for atomic spectra, it has been widely used to study molecular rotational spectra.
Original Description:
Original Title
Stark Effect
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentThe Stark effect describes the splitting of atomic spectral lines when an externally applied electric field is present. The splitting is caused by the electric field first polarizing the atom, then interacting with the induced electric dipole moment. This interaction splits the energy levels in a way that depends on the magnitude of the quantum number J, but not its sign, resulting in splittings proportional to J+1 or J+1/2. While the Stark effect has been of limited use for atomic spectra, it has been widely used to study molecular rotational spectra.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
27 views1 pageZeeman Effect Index Atomic Structure Concepts: Herzberg
Zeeman Effect Index Atomic Structure Concepts: Herzberg
Uploaded by
Sreedevi KrishnakumarThe Stark effect describes the splitting of atomic spectral lines when an externally applied electric field is present. The splitting is caused by the electric field first polarizing the atom, then interacting with the induced electric dipole moment. This interaction splits the energy levels in a way that depends on the magnitude of the quantum number J, but not its sign, resulting in splittings proportional to J+1 or J+1/2. While the Stark effect has been of limited use for atomic spectra, it has been widely used to study molecular rotational spectra.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 1
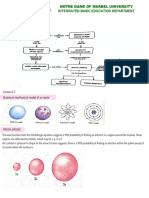
Stark Effect in Atomic Spectra
The splitting of atomic spectral lines as a
result of an externally applied electric field
was discovered by Stark, and is called the
Stark effect. As the splitting of a line of the Index
helium spectrum shows, the splitting is not
symmetric like that of the Zeeman effect. Atomic
Structure
The splitting of the energy levels by an
Concepts
electric field first requires that the field
polarizes the atom and then interacts with
Reference
the resulting electric dipole moment. That
Herzberg
dipole moment depends upon the
Atomic
magnitude of Mj, but not its sign, so that
Spectra
the energy levels show splitting Ch 2, p
proportional to quantum numbers J+1 or 114.
J+1/2, for integer and half-integer spins
respectively.
The Stark effect has been of marginal
benefit in the analysis of atomic spectra, but
has been a major tool for molecular
rotational spectra.
Go Back
HyperPhysics***** Quantum Physics R Nave
You might also like
- Exploring Gamma RayDocument9 pagesExploring Gamma RayChloe SoleNo ratings yet
- Magnetic Moment Magnetic Field Torque Vector Product Magnetic Force Applications Magnetic Field ConceptsDocument2 pagesMagnetic Moment Magnetic Field Torque Vector Product Magnetic Force Applications Magnetic Field ConceptsSreedevi Krishnakumar100% (1)
- Reviewer in ChemistryDocument6 pagesReviewer in ChemistryStella SalvadorNo ratings yet
- Chapter 2 Structure of AtomDocument2 pagesChapter 2 Structure of Atomgibinshaji2No ratings yet
- Nuclear Magnetic Resonance (NMR) SpectrosDocument52 pagesNuclear Magnetic Resonance (NMR) Spectrossharifah sakinah syed soffianNo ratings yet
- Spectroscopy 01 Introduction 23 24Document88 pagesSpectroscopy 01 Introduction 23 24ramonrubio2004No ratings yet
- Unit Code: APH306 Unit Title: Atomic PhysicsDocument20 pagesUnit Code: APH306 Unit Title: Atomic PhysicsAbel peakNo ratings yet
- Flurocense SpectrosDocument6 pagesFlurocense SpectrosRahul DebNo ratings yet
- Magnetism SC L1-3Document24 pagesMagnetism SC L1-3Abhishek tiwariNo ratings yet
- NMRDocument173 pagesNMRঋ ত্বিকNo ratings yet
- Spin PDFDocument7 pagesSpin PDFMaarioNo ratings yet
- Bab 6 Magnetic Fields in MatterDocument19 pagesBab 6 Magnetic Fields in MatterAfdal Wiranu PutraNo ratings yet
- Analisis SPM 2021Document2 pagesAnalisis SPM 2021Pavina baskyNo ratings yet
- Atoms in Complex Twisted Light: Journal of OpticsDocument68 pagesAtoms in Complex Twisted Light: Journal of Opticsfabian correaNo ratings yet
- Gamma Ray Spectroscopy Assignment PDFDocument13 pagesGamma Ray Spectroscopy Assignment PDFMADHAVA MOORTHINo ratings yet
- PhysRevA 14 2061Document3 pagesPhysRevA 14 2061Meidk LoreNo ratings yet
- Basic Concepts of Analytical Chemistry - (Chapter 28. Atomic Emission Spectroscopy)Document9 pagesBasic Concepts of Analytical Chemistry - (Chapter 28. Atomic Emission Spectroscopy)Sushma Somnath TiwariNo ratings yet
- Tunable Magnetic Relaxation in MagneticDocument4 pagesTunable Magnetic Relaxation in MagneticProfirNo ratings yet
- R N N Z: Rydberg Formula: Limitations of Bohr's ModelDocument2 pagesR N N Z: Rydberg Formula: Limitations of Bohr's ModelisaacNo ratings yet
- Particle Wave DualityDocument24 pagesParticle Wave DualityIsrael PopeNo ratings yet
- ESCA Electron Spectroscopy For Chemical Analysis XPS: X-Ray Photoelectron SpectrosDocument72 pagesESCA Electron Spectroscopy For Chemical Analysis XPS: X-Ray Photoelectron SpectrosAnurag TiwariNo ratings yet
- On The Role of Potentials in The Aharonov-Bohm Effect: PACS Numbers: 03.65.-w, 03.65.Vf, 03.65.ta, 03.65.udDocument4 pagesOn The Role of Potentials in The Aharonov-Bohm Effect: PACS Numbers: 03.65.-w, 03.65.Vf, 03.65.ta, 03.65.udnigel agrippaNo ratings yet
- NMR 3Document29 pagesNMR 3knkoradiyaNo ratings yet
- Electron Spin ResonanceDocument8 pagesElectron Spin ResonanceGaluh PrameswariNo ratings yet
- ELECTRICITYDocument17 pagesELECTRICITYTest Music CopyrightNo ratings yet
- 03 Chapter 2 New Methods of Modern Logging and Geological ApplicationDocument91 pages03 Chapter 2 New Methods of Modern Logging and Geological ApplicationalphaidedjibrillaNo ratings yet
- Physical Revie% December Atomic: FirstDocument3 pagesPhysical Revie% December Atomic: FirstMeidk LoreNo ratings yet
- ( (USEFULL) ) Raman Spectroscopy Umea5handoutsDocument6 pages( (USEFULL) ) Raman Spectroscopy Umea5handoutsshadyghanemNo ratings yet
- 546 5686798651313311232164897987lijdcoihsdiuhgfieygDocument24 pages546 5686798651313311232164897987lijdcoihsdiuhgfieygędën hãzárdNo ratings yet
- Structure Of Atom: Rutherford's Experiment on Scattering of α-Particles Nuclear Model of AtomDocument6 pagesStructure Of Atom: Rutherford's Experiment on Scattering of α-Particles Nuclear Model of Atomnandarak4No ratings yet
- Semiconductor Cheat Sheet Ver 1Document18 pagesSemiconductor Cheat Sheet Ver 1testerJesterNo ratings yet
- Lecture 2 (A)Document63 pagesLecture 2 (A)Yingqi SuNo ratings yet
- Charles Kittel Intro Solid State Physics PDFDocument27 pagesCharles Kittel Intro Solid State Physics PDFJahan ClaesNo ratings yet
- Background You Need To Know: 3.1 The AtomDocument24 pagesBackground You Need To Know: 3.1 The AtomDuong AnhNo ratings yet
- Zeeman Effect PDFDocument6 pagesZeeman Effect PDFQaisar ShahNo ratings yet
- MIT6 007S11 Lec39Document25 pagesMIT6 007S11 Lec39THARMARAJNo ratings yet
- Structure of The Atom: Sub-Atomic Particles Atomic Models Characteristics of AtomDocument1 pageStructure of The Atom: Sub-Atomic Particles Atomic Models Characteristics of AtomJitendra KumarNo ratings yet
- State and Explain Franck Condon Principle.: Discuss The Feature of Vibrational Raman SpectraDocument25 pagesState and Explain Franck Condon Principle.: Discuss The Feature of Vibrational Raman SpectraThakuri Praveen ChandNo ratings yet
- Stark EffectDocument16 pagesStark EffectBharath Manchikodi100% (1)
- Introduction To Computational Chemistry: For The BeginnerDocument16 pagesIntroduction To Computational Chemistry: For The BeginnerNaga ChaNo ratings yet
- Schrodinger Wave EquationDocument25 pagesSchrodinger Wave EquationGowri SankarNo ratings yet
- Theory of A Quantum Critical Phenomenon in A Topological Insulator: (3+1) Dimensional Quantum Electrodynamics in SolidsDocument6 pagesTheory of A Quantum Critical Phenomenon in A Topological Insulator: (3+1) Dimensional Quantum Electrodynamics in SolidsNetto HolandaNo ratings yet
- EprDocument8 pagesEprcyrimathewNo ratings yet
- Different Magnetic Interaction MechanismsDocument5 pagesDifferent Magnetic Interaction MechanismsJoshuaNo ratings yet
- 무기화학 1 ch2Document5 pages무기화학 1 ch2최마리아No ratings yet
- General Chemistry: More Than Two Electrons (With Opposite Spin) ."Document5 pagesGeneral Chemistry: More Than Two Electrons (With Opposite Spin) ."Marc Vincent CastilloNo ratings yet
- Quantum Physics: By: Louisa, Angelica, CalistaDocument17 pagesQuantum Physics: By: Louisa, Angelica, Calistaahmad fatoniNo ratings yet
- Lecture 6 Radiation-Matter Interactions - 2Document25 pagesLecture 6 Radiation-Matter Interactions - 2Yun WangNo ratings yet
- KAA 502 - Note 5 - Chapter 8 - Intro To Optical Spectrometric Methods 212Document5 pagesKAA 502 - Note 5 - Chapter 8 - Intro To Optical Spectrometric Methods 212Jun MingNo ratings yet
- Vector Model For Orbital Angular MomentumDocument6 pagesVector Model For Orbital Angular MomentumAgrupación Astronomica de Alicante100% (2)
- Adobe Scan 09 Feb 2024Document2 pagesAdobe Scan 09 Feb 2024ANIRUDDHA MONDALNo ratings yet
- JC Chapter 6 HandoutDocument6 pagesJC Chapter 6 HandoutAdi PrabowoNo ratings yet
- The Electron Spin Resonance (ESR) : Principle Theory and ApplicationsDocument11 pagesThe Electron Spin Resonance (ESR) : Principle Theory and ApplicationsGaluh PrameswariNo ratings yet
- The Lorentz Force and Superconductivity: PACS NumbersDocument4 pagesThe Lorentz Force and Superconductivity: PACS NumberspippoNo ratings yet
- Stark Effect in Atomic SpectraDocument6 pagesStark Effect in Atomic Spectraflamesup41No ratings yet
- (B) Orbital and Spin Magnetic MomentDocument3 pages(B) Orbital and Spin Magnetic MomentSURESH SURAGANINo ratings yet
- DetectorsDocument282 pagesDetectorsMoch. Syamsul AlamsyahNo ratings yet
- GenChem ReviewerDocument6 pagesGenChem ReviewersoleiliaaaaaNo ratings yet
- Molecules in Electromagnetic Fields: From Ultracold Physics to Controlled ChemistryFrom EverandMolecules in Electromagnetic Fields: From Ultracold Physics to Controlled ChemistryNo ratings yet
- Negative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 2: Gravitational and Inertial Control, #2From EverandNegative Mass and Negative Refractive Index in Atom Nuclei - Nuclear Wave Equation - Gravitational and Inertial Control: Part 2: Gravitational and Inertial Control, #2No ratings yet
- Exchange Reactions - Part 3Document6 pagesExchange Reactions - Part 3Sreedevi KrishnakumarNo ratings yet
- 388QRB2013Document133 pages388QRB2013Sreedevi KrishnakumarNo ratings yet
- Recimization ReactionsDocument4 pagesRecimization ReactionsSreedevi KrishnakumarNo ratings yet
- Reaction Mechanisms of Inorganic and Organometallic Systems by Robert B. JordanDocument532 pagesReaction Mechanisms of Inorganic and Organometallic Systems by Robert B. JordanSreedevi Krishnakumar100% (1)
- ColourDocument21 pagesColourSreedevi KrishnakumarNo ratings yet
- Paschen-Back EffectDocument3 pagesPaschen-Back EffectSreedevi KrishnakumarNo ratings yet
- Retrosynthetic Analysis: Synthesis BackwardDocument12 pagesRetrosynthetic Analysis: Synthesis BackwardSreedevi KrishnakumarNo ratings yet
- 28.3 - Organic Photochemistry - Chemistry LibreTexts PDFDocument8 pages28.3 - Organic Photochemistry - Chemistry LibreTexts PDFSreedevi KrishnakumarNo ratings yet
- Full Paper: Han Liu and Da-Ming DuDocument11 pagesFull Paper: Han Liu and Da-Ming DuSreedevi KrishnakumarNo ratings yet
- Comparison of Raman and IR SpectrosDocument2 pagesComparison of Raman and IR SpectrosSreedevi KrishnakumarNo ratings yet
- CHEM 430 NMR Spectroscopy Chapter 3Document97 pagesCHEM 430 NMR Spectroscopy Chapter 3Sreedevi KrishnakumarNo ratings yet
- Kumar 2012Document12 pagesKumar 2012Sreedevi KrishnakumarNo ratings yet
- Indian Baby Names Boys & Girls Beginning With A-Z WWW - BabynamesdirectDocument1 pageIndian Baby Names Boys & Girls Beginning With A-Z WWW - BabynamesdirectSreedevi KrishnakumarNo ratings yet