Professional Documents
Culture Documents
Bocii 3 Assignment PDF
Bocii 3 Assignment PDF
Uploaded by
lakhuindiaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Bocii 3 Assignment PDF
Bocii 3 Assignment PDF
Uploaded by
lakhuindiaCopyright:
Available Formats
ASSIGNMENT - 3 (BOCII) XI
Q.1. What is the mass percentage of carbon in one mol- Q.7. The formula of ferric sulphate is
ecule of calcium carbonate
(a) Fe(SO)4 (b) Fe2(SO4)3
(a) 26% (b)12% (c) 48% (c) 24%
(c) Fe3(SO4)2 (d) Fe3SO4
Q.2. What is the formula of sodium phosphate
Q.8. What is the molecular mass of sulphuric acid
(a) Na3PO4 (b) Na2(PO4)2
(a) 98 (b) 49 (c) 80 (d) 120
(c) Na3(PO4)2 (d) Na2(PO4)3
Q.9. The Empirical formula of a compound of molecular
Q.3. What is the molecular mass of glucose?
formula C6 H12 O6 is
(a)190 (b)180 (c)260 (d)60
(a) C6 H12 O6 (b) C3 H6 O3
Q.4. What is the atomicity of sulphur?
(c) CH 2 O (d) C12 H 24 O12
(a) 6 (b) 4 (c) 8 (d) 7
Q.5. The number of electrons in Na is x. The charge on Q.10. The formula of mercuric chloride is
sodium ion is y. Then x + y is
(a) HgCl2 (b) Hg 2Cl2 (c) HgClO3 (d) AgCl
(a) 11 (b) 10 (c) 12 (d) 13
Q.6. The formula of magnesium perchlorate is
(a) Mg(ClO4)3 (b) MgClO4
(c) Mg2ClO4 (d) Mg(ClO4)2
ASSIGNMENT - 3 (BOCII) XI - ANSWER KEY
Q.1. (b) Q.2. (a) Q.3. (b) Q.4. (c) Q.5. (c) Q.6. (d) Q.7. (b)
Q.8. (a) Q.9. (c) Q.10. (a)
MITESH RATHI CLASSES R-5, S.B.I, COLONY OPP. RLY TRACK, ZONE-II M.P. NAGAR, BHOPAL PHONE-4269261, 2550238 1
You might also like
- Foundations of College Chemistry 14th Edition Hein Solutions Manual DownloadDocument9 pagesFoundations of College Chemistry 14th Edition Hein Solutions Manual DownloadJohn Gaudreau100% (25)
- Bocii 3 Assignment PDFDocument1 pageBocii 3 Assignment PDFlakhuindiaNo ratings yet
- Bocii 3 Assignment PDFDocument1 pageBocii 3 Assignment PDFlakhuindiaNo ratings yet
- KJB Answersheet Test Objective Coordination CompoundsDocument2 pagesKJB Answersheet Test Objective Coordination CompoundsLALITA KUMARINo ratings yet
- Dokumen - Tips Chapter 3 Compounds and Molecules What Is The Correct 1020 Gmol ChapterDocument10 pagesDokumen - Tips Chapter 3 Compounds and Molecules What Is The Correct 1020 Gmol ChapterInfernape IncineroarNo ratings yet
- Mole Concept @kvpy - AspirantsDocument7 pagesMole Concept @kvpy - AspirantssagarNo ratings yet
- Coordination Compound (Xii 2020-22) (Ans) 19 08 21Document2 pagesCoordination Compound (Xii 2020-22) (Ans) 19 08 21ombendarkarNo ratings yet
- MCQ Coordination CompoundDocument3 pagesMCQ Coordination Compoundharshdadhich2006No ratings yet
- Du M.SC - Entrance Chemistry 2015Document8 pagesDu M.SC - Entrance Chemistry 2015Priyabrata debnathNo ratings yet
- 9th+class Symbols+and+formulae Chemistry Quiz+ (CMD)Document2 pages9th+class Symbols+and+formulae Chemistry Quiz+ (CMD)aveerareddy9No ratings yet
- Dated: 02-01-2019 TIME: 3 Hours M.M.: 360 (Full Test - 8) (Jee-Main)Document10 pagesDated: 02-01-2019 TIME: 3 Hours M.M.: 360 (Full Test - 8) (Jee-Main)Dikshit AroraNo ratings yet
- Single Answer Type Questions: (+4, - 1)Document5 pagesSingle Answer Type Questions: (+4, - 1)Aadish JainNo ratings yet
- By Pri Nce Sir: ChemistryDocument3 pagesBy Pri Nce Sir: ChemistryThapliyal PrakashNo ratings yet
- Test 36 - Coordination Compounds - Middle of PyramidDocument5 pagesTest 36 - Coordination Compounds - Middle of Pyramiditsrudra39No ratings yet
- Which of The Following Has Square Planar StructureDocument8 pagesWhich of The Following Has Square Planar StructureUmesh ShivappaNo ratings yet
- 레이먼드 창의 대학기초화학 7판 선택된 문제의 정답Document4 pages레이먼드 창의 대학기초화학 7판 선택된 문제의 정답hjw5835209No ratings yet
- WWW Myengg Com JEE Main Chemistry Model Paper 6Document8 pagesWWW Myengg Com JEE Main Chemistry Model Paper 6Senthil PNo ratings yet
- NeetDocument3 pagesNeetd anjilappaNo ratings yet
- Co-Ordination Chemistry and Organometallics Assignment: Gravity ClassesDocument3 pagesCo-Ordination Chemistry and Organometallics Assignment: Gravity ClassesGopal PenjarlaNo ratings yet
- MCQ Chapter 8 Coordination CompoundDocument7 pagesMCQ Chapter 8 Coordination CompoundSavien Brandan100% (3)
- +2 Chemistry (One Mar) - Feb 2023 - EMDocument4 pages+2 Chemistry (One Mar) - Feb 2023 - EMAdlin PertishyaNo ratings yet
- Du Entrance Chemistry 2017Document15 pagesDu Entrance Chemistry 2017Arnav ChakrabortyNo ratings yet
- Stoichiometry RevisionDocument4 pagesStoichiometry RevisionAshwin Balaji0% (1)
- Coordination WSDocument3 pagesCoordination WSDeena chemistNo ratings yet
- Yti 45 Tu VD0 QKJ Beh Ekm LDocument46 pagesYti 45 Tu VD0 QKJ Beh Ekm LAradhana GuptaNo ratings yet
- Redox Reactions-T-5Document2 pagesRedox Reactions-T-5Soham SagaonkarNo ratings yet
- Instruction: Answer Number 1 and Any 2 Questions.: TheoryDocument2 pagesInstruction: Answer Number 1 and Any 2 Questions.: TheoryPrince CarrintonNo ratings yet
- Che Neet 3Document5 pagesChe Neet 3pinnaacleclasses salemNo ratings yet
- Inorganic Chemistry: SO H O S H SO H O S HDocument11 pagesInorganic Chemistry: SO H O S H SO H O S Hwickdexter86No ratings yet
- Sheet St-2: 1. S Fe (CN)Document2 pagesSheet St-2: 1. S Fe (CN)vivek mishraNo ratings yet
- Chemistry: Space For Rough WorkDocument3 pagesChemistry: Space For Rough WorkSachjith MNo ratings yet
- Revision Sheet (SRP-1) (With Ans.) (Solid State + Nitrogen Family) 20.12.22 Tiwari SirDocument3 pagesRevision Sheet (SRP-1) (With Ans.) (Solid State + Nitrogen Family) 20.12.22 Tiwari SirShashank YadavNo ratings yet
- Coordination Compounds-T-4Document3 pagesCoordination Compounds-T-4sharavaravindNo ratings yet
- Stoichiometry 1Document4 pagesStoichiometry 1hey mama don’t stress your mindNo ratings yet
- Practice Exam 2Document6 pagesPractice Exam 2Erik StyürNo ratings yet
- 9th+class Symbols+and+formulae Chemistry CPP+ (CMD)Document1 page9th+class Symbols+and+formulae Chemistry CPP+ (CMD)aveerareddy9No ratings yet
- Part - I: Only One Option Correct Type: Basic Inorganic NomenclatureDocument3 pagesPart - I: Only One Option Correct Type: Basic Inorganic NomenclaturewanderedNo ratings yet
- Senior - 2020 - Class - 12 - Chemistry - Objective Questions - Coordination CompoundsDocument4 pagesSenior - 2020 - Class - 12 - Chemistry - Objective Questions - Coordination CompoundsJijendarNo ratings yet
- Ox. No & StateDocument2 pagesOx. No & StateajaxNo ratings yet
- Practice Sheet - CHEMICAL BONDINGDocument2 pagesPractice Sheet - CHEMICAL BONDINGRachna JainNo ratings yet
- JEE Main Online Exam 2019: Questions & Solutions (Memory Based)Document5 pagesJEE Main Online Exam 2019: Questions & Solutions (Memory Based)Ihtisham Ul HaqNo ratings yet
- Cat 9Document3 pagesCat 9Ravi Kiran KoduriNo ratings yet
- P Block ElementsDocument10 pagesP Block ElementsEzhil MukilNo ratings yet
- Hcu Chemistry 2018 PDFDocument9 pagesHcu Chemistry 2018 PDFSatyajit biswasNo ratings yet
- Cord Compd, Alc, Ether, PhenolDocument20 pagesCord Compd, Alc, Ether, PhenolRajendra ChikkamathNo ratings yet
- Coordination Compounds Teaching PDFDocument6 pagesCoordination Compounds Teaching PDFNeel PatelNo ratings yet
- Chemistry - Mains1 (Entire 11th)Document9 pagesChemistry - Mains1 (Entire 11th)Ravi Kiran KoduriNo ratings yet
- Section-A Multiple Choice Questins (MCQ) Q.1-Q.10 Carry One Mark EachDocument6 pagesSection-A Multiple Choice Questins (MCQ) Q.1-Q.10 Carry One Mark EachParul kandolaNo ratings yet
- Chemical Sciences Test Series II 24-11-2013Document10 pagesChemical Sciences Test Series II 24-11-2013ImranNo ratings yet
- Ics - 8 Test Paper: Semester - 3Document7 pagesIcs - 8 Test Paper: Semester - 3xanshahNo ratings yet
- Test Bansal Coordinationcompounds PDFDocument10 pagesTest Bansal Coordinationcompounds PDFAdityaNo ratings yet
- Geol P 20 Paper II CHEMISTRY PDFDocument32 pagesGeol P 20 Paper II CHEMISTRY PDFRambo FeverNo ratings yet
- UNIT - 10 Redox Reactions: Multiple Choice QuestionsDocument9 pagesUNIT - 10 Redox Reactions: Multiple Choice QuestionsYogy YNo ratings yet
- Chemistry (Question Paper)Document5 pagesChemistry (Question Paper)Jay Doshi ShashikantNo ratings yet
- 3 - Molecules and Compounds: Practice TestDocument2 pages3 - Molecules and Compounds: Practice Testfamily_jvcNo ratings yet
- Dec Chem 2015Document26 pagesDec Chem 2015maheshNo ratings yet
- Chemistry 100 Days Challenge CPPDocument141 pagesChemistry 100 Days Challenge CPPFardeen MalickNo ratings yet
- Graphene Oxide: Fundamentals and ApplicationsFrom EverandGraphene Oxide: Fundamentals and ApplicationsAyrat M. DimievNo ratings yet
- Main Group Metal Coordination Polymers: Structures and NanostructuresFrom EverandMain Group Metal Coordination Polymers: Structures and NanostructuresNo ratings yet
- Assignment - 2 (Calculus) Xi: y X y X y X y XDocument2 pagesAssignment - 2 (Calculus) Xi: y X y X y X y XlakhuindiaNo ratings yet
- Test Correction PDFDocument1 pageTest Correction PDFlakhuindiaNo ratings yet
- Calculus 1 AssignmentDocument3 pagesCalculus 1 AssignmentlakhuindiaNo ratings yet
- Jee Mains Level - QEEDocument5 pagesJee Mains Level - QEElakhuindiaNo ratings yet
- Vector Assignment 3 XiDocument1 pageVector Assignment 3 XilakhuindiaNo ratings yet
- C. R. Q.-Sequence & Series: F HG I KJDocument2 pagesC. R. Q.-Sequence & Series: F HG I KJlakhuindiaNo ratings yet
- Assignment - 1 (Boc-1) : Log Log LogDocument1 pageAssignment - 1 (Boc-1) : Log Log LoglakhuindiaNo ratings yet
- As 5 AssignmentDocument2 pagesAs 5 AssignmentlakhuindiaNo ratings yet
- Mitesh Rathi Classes Test Given Student List: S-2 ID Batch Name of Student Phy/ChemDocument2 pagesMitesh Rathi Classes Test Given Student List: S-2 ID Batch Name of Student Phy/ChemlakhuindiaNo ratings yet
- Daily Class Test - I - Logarithm (Class 11th) : Ac C Abc Ac C Abc Ac C Abc X yDocument1 pageDaily Class Test - I - Logarithm (Class 11th) : Ac C Abc Ac C Abc Ac C Abc X ylakhuindiaNo ratings yet
- Bocii 3 Assignment PDFDocument1 pageBocii 3 Assignment PDFlakhuindiaNo ratings yet
- Bocii 3 Assignment PDFDocument1 pageBocii 3 Assignment PDFlakhuindiaNo ratings yet
- Time Table XI 29-3 AUG 2020Document1 pageTime Table XI 29-3 AUG 2020lakhuindiaNo ratings yet
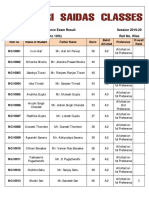
- 10th Final External Entrance Exam Result 2019-20 (Girls) PDFDocument3 pages10th Final External Entrance Exam Result 2019-20 (Girls) PDFlakhuindiaNo ratings yet
- Anant Utsav School Half Yearly Examination: Multiple Choice QuestionDocument3 pagesAnant Utsav School Half Yearly Examination: Multiple Choice QuestionlakhuindiaNo ratings yet
- Mitesh Rathi Classes: Test Given Students ListDocument2 pagesMitesh Rathi Classes: Test Given Students ListlakhuindiaNo ratings yet
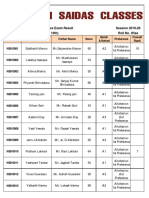
- 10th Final External Entrance Exam Result 2019-20 (Boys) PDFDocument4 pages10th Final External Entrance Exam Result 2019-20 (Boys) PDFlakhuindiaNo ratings yet
- C.R.Q. - Laws of Motion (Xi) : Board LevelDocument4 pagesC.R.Q. - Laws of Motion (Xi) : Board LevellakhuindiaNo ratings yet