Professional Documents
Culture Documents
Class Ex Ideal Gas Law
Class Ex Ideal Gas Law
Uploaded by
Mustapha BouregaaCopyright:
Available Formats
You might also like
- Music License AgreementDocument2 pagesMusic License AgreementAdrian Moscalu100% (1)
- Problem 2Document19 pagesProblem 2Aaliyahkaye JulaoNo ratings yet
- Formulas in Cooling TowerDocument6 pagesFormulas in Cooling TowerGringoNo ratings yet
- CH 01Document9 pagesCH 01Mary Lorelyn EscoteNo ratings yet
- Manual de Usuario Htw-Yan1f1-EngDocument10 pagesManual de Usuario Htw-Yan1f1-EngJonathan Hernán Valderrama RamírezNo ratings yet
- Topic 16:: Factor AnalysisDocument33 pagesTopic 16:: Factor AnalysisKelly WijayaNo ratings yet
- States of Matter - ThermodynamicsDocument146 pagesStates of Matter - ThermodynamicsbavisyaaaaNo ratings yet
- (Solved Problems) : Thermodynamics 01Document6 pages(Solved Problems) : Thermodynamics 01Ben0% (1)
- ClassWork CPD 2022Document20 pagesClassWork CPD 2022crazzyboy292No ratings yet
- Topic: Gaseous State: Chemistry Lecture NotesDocument32 pagesTopic: Gaseous State: Chemistry Lecture NotesDibya Ranjan BissoyiNo ratings yet
- Problemas Gases Ideales y Reales, Van Der WaalDocument9 pagesProblemas Gases Ideales y Reales, Van Der WaalmegustabajarinfoNo ratings yet
- Lecture3 PDFDocument8 pagesLecture3 PDFvignesh9489No ratings yet
- Solutions Manual For Fluid Mechanics 5th PDFDocument20 pagesSolutions Manual For Fluid Mechanics 5th PDFchala nigussieNo ratings yet
- Solutions Manual For Fluid Mechanics 5thDocument20 pagesSolutions Manual For Fluid Mechanics 5thhongjieNo ratings yet
- 06 - Pressure OLDocument4 pages06 - Pressure OLKNEWTON EducationNo ratings yet
- 06 PressureDocument9 pages06 PressureTahmedul Hasan TanvirNo ratings yet
- New-Problems-Chapter-21 Convective Mass TransferDocument2 pagesNew-Problems-Chapter-21 Convective Mass TransferKhanh NhiNo ratings yet
- Salinity ChartDocument17 pagesSalinity ChartWilmer CuicasNo ratings yet
- CY11001 ChemistryDocument2 pagesCY11001 ChemistryLakshay SinghalNo ratings yet
- A Study On Numerical Simulations and Experiments For Mass Transfer in Bubble Mode Absorber of Ammonia and WaterDocument8 pagesA Study On Numerical Simulations and Experiments For Mass Transfer in Bubble Mode Absorber of Ammonia and WaterusamaNo ratings yet
- Lecture 2Document12 pagesLecture 2Samaseen PrabhatNo ratings yet
- ADSORPSI II. 31 MAr 21Document15 pagesADSORPSI II. 31 MAr 21YumaNurAlfathNo ratings yet
- Coolship Calc - Cylindrical and RectangularDocument5 pagesCoolship Calc - Cylindrical and RectangularNicolas MossoNo ratings yet
- CH18ThermodynamicsP1-The First Law of ThermodynamicsDocument4 pagesCH18ThermodynamicsP1-The First Law of ThermodynamicsINDOMITABLENo ratings yet
- Dec 1984Document9 pagesDec 1984krishnaNo ratings yet
- Mass Transfer During Forest Biomass Particles Drying in A Fluidised BedDocument9 pagesMass Transfer During Forest Biomass Particles Drying in A Fluidised BedLAURA ISABEL ARIZA ARIZANo ratings yet
- Final ExamDocument5 pagesFinal ExamWaqasNo ratings yet
- Ch3 First TermDocument72 pagesCh3 First TermFBI AgentNo ratings yet
- C8 Cussler PDFDocument33 pagesC8 Cussler PDFRaisa LopezNo ratings yet
- Gaseous State PDFDocument16 pagesGaseous State PDFUditaNo ratings yet
- M17 Wolf57139 03 Se C17Document24 pagesM17 Wolf57139 03 Se C17c.s.kalkmanNo ratings yet
- Tuttherm2 PDFDocument6 pagesTuttherm2 PDFPrabir BanerjeeNo ratings yet
- Gaseous State (Package)Document55 pagesGaseous State (Package)Draw with Hassan JamalNo ratings yet
- L15 CRE II Heterogeneous Catalysis: Prof. K.K.Pant Department of Chemical Engineering IIT DelhiDocument15 pagesL15 CRE II Heterogeneous Catalysis: Prof. K.K.Pant Department of Chemical Engineering IIT DelhiMehul VarshneyNo ratings yet
- thay đổi PH đến Quang xúc tácDocument3 pagesthay đổi PH đến Quang xúc táckẻ khờ khạoNo ratings yet
- Oxygen Cylinder Duration CalculationDocument1 pageOxygen Cylinder Duration CalculationGetThisShitNo ratings yet
- The Journal of Supercritical Fluids: SciencedirectDocument11 pagesThe Journal of Supercritical Fluids: SciencedirectsutarsiNo ratings yet
- CBE 417 FlashDrumSizing Wankat PDFDocument7 pagesCBE 417 FlashDrumSizing Wankat PDFAbdul RehmanNo ratings yet
- CSEC Physics - SECTION B - THERMAL PHYSICS AND KINETIC THEORYDocument40 pagesCSEC Physics - SECTION B - THERMAL PHYSICS AND KINETIC THEORYBab50% (2)
- 11.1 - KTG & RadiationDocument11 pages11.1 - KTG & Radiationdevrarimanish72No ratings yet
- Chapter-3 PPTDocument109 pagesChapter-3 PPTnunuNo ratings yet
- DocxDocument11 pagesDocxNabila PutriNo ratings yet
- Technol03 PDFDocument9 pagesTechnol03 PDFHyunJoon, KimNo ratings yet
- Hno3 Baoh2 Neutralisation QDocument4 pagesHno3 Baoh2 Neutralisation QloxadegoNo ratings yet
- Chapter 1 (Part 2) : Basic Concepts 1.4 Process Variables: Marmy Roshaidah Mohd SallehDocument47 pagesChapter 1 (Part 2) : Basic Concepts 1.4 Process Variables: Marmy Roshaidah Mohd SallehAhmad Safwan HakimNo ratings yet
- Applications of First Order Differential Equations Discussion Part I PDFDocument22 pagesApplications of First Order Differential Equations Discussion Part I PDFErika Dawn Luciano AmbrayNo ratings yet
- P K Nag Exercise Problems Solved ThermodDocument265 pagesP K Nag Exercise Problems Solved ThermodSiddhant DeyNo ratings yet
- Chapter 9 The Gaseous State Edupdf 1Document64 pagesChapter 9 The Gaseous State Edupdf 1api-386303659No ratings yet
- 01 1stlaw ExessolsDocument29 pages01 1stlaw Exessolsblanca.pegueraNo ratings yet
- IES Conventional Mechanical Engineering 2007Document10 pagesIES Conventional Mechanical Engineering 2007eklavya koshtaNo ratings yet
- CPP Assignment 1Document2 pagesCPP Assignment 1AmandaEdwinNo ratings yet
- TD 1 Properties EnonceDocument2 pagesTD 1 Properties EnonceLuc AusterNo ratings yet
- Thiobacillus Ferrooxidans: Particle Size Effects in The Microbiological Eaching Sulfide Concentrates byDocument5 pagesThiobacillus Ferrooxidans: Particle Size Effects in The Microbiological Eaching Sulfide Concentrates byW ZuoNo ratings yet
- Unit 1 ProblemsDocument3 pagesUnit 1 Problemsgrace_juganNo ratings yet
- Single Phase SystemDocument61 pagesSingle Phase SystemNoorhalieza AliNo ratings yet
- Gaseous State PDFDocument32 pagesGaseous State PDFrockingrazzNo ratings yet
- Chapter 4 States of Matter 2021Document24 pagesChapter 4 States of Matter 2021suh mey chongNo ratings yet
- Problem Set 1Document4 pagesProblem Set 1ash jay100% (1)
- Ideal Gas Law 5684f195af3e6Document38 pagesIdeal Gas Law 5684f195af3e6Jose GulitiwNo ratings yet
- States of Matter by Rakshita SinghDocument14 pagesStates of Matter by Rakshita SinghFarzana ShaikNo ratings yet
- 11 GasesDocument17 pages11 Gasespuja ritongaNo ratings yet
- Carbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from -20° to 250°C and Pressures up to 1000 BarFrom EverandCarbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from -20° to 250°C and Pressures up to 1000 BarNo ratings yet
- the_regime_of_tectonic_stresses_and_fault_type_based_on_petrophysicalDocument13 pagesthe_regime_of_tectonic_stresses_and_fault_type_based_on_petrophysicalMustapha BouregaaNo ratings yet
- Petrographic_charcaterization_of_micropoDocument3 pagesPetrographic_charcaterization_of_micropoMustapha BouregaaNo ratings yet
- JPG-SDDocument15 pagesJPG-SDMustapha BouregaaNo ratings yet
- LotterDocument28 pagesLotterMustapha BouregaaNo ratings yet
- 48 - Geophysics, Geochemistry E-Books ListDocument31 pages48 - Geophysics, Geochemistry E-Books ListMustapha BouregaaNo ratings yet
- GeophysicsDocument22 pagesGeophysicsMustapha BouregaaNo ratings yet
- Intermedia - Exchange 2010 Pricing 12-15-09Document4 pagesIntermedia - Exchange 2010 Pricing 12-15-09Mustapha BouregaaNo ratings yet
- Introduction To Borehole GeophysicsDocument3 pagesIntroduction To Borehole GeophysicsMustapha BouregaaNo ratings yet
- DDR NZ25 - N045 - 26032011Document2 pagesDDR NZ25 - N045 - 26032011Mustapha BouregaaNo ratings yet
- 05 - Model Building Using Conditional Simulation Algorithms Such As SGS or SISDocument84 pages05 - Model Building Using Conditional Simulation Algorithms Such As SGS or SISMustapha BouregaaNo ratings yet
- 06 - Multiple-Poin Simulation (MPS)Document22 pages06 - Multiple-Poin Simulation (MPS)Mustapha BouregaaNo ratings yet
- Formation Evaluation Measurements: ENM237 Reservoir Geology and PetrophysicsDocument43 pagesFormation Evaluation Measurements: ENM237 Reservoir Geology and PetrophysicsMustapha BouregaaNo ratings yet
- Geostatistics and Reservoir Modeling Module: Review of Basic StatisticsDocument52 pagesGeostatistics and Reservoir Modeling Module: Review of Basic StatisticsMustapha BouregaaNo ratings yet
- Spatial StatisticsDocument96 pagesSpatial StatisticsMustapha BouregaaNo ratings yet
- ES476/576 Hydrology Review Problems: Physical Properties of WaterDocument2 pagesES476/576 Hydrology Review Problems: Physical Properties of WaterMustapha BouregaaNo ratings yet
- Formation Evaluation Measurements: ENM237 Reservoir Geology and PetrophysicsDocument67 pagesFormation Evaluation Measurements: ENM237 Reservoir Geology and PetrophysicsMustapha BouregaaNo ratings yet
- Journal of Natural Gas Science and Engineering: Shahab Dean MohagheghDocument9 pagesJournal of Natural Gas Science and Engineering: Shahab Dean MohagheghMustapha BouregaaNo ratings yet
- Formation Evaluation Measurements: ENM237 Reservoir Geology and PetrophysicsDocument57 pagesFormation Evaluation Measurements: ENM237 Reservoir Geology and PetrophysicsMustapha BouregaaNo ratings yet
- Geosciences 09 00078Document15 pagesGeosciences 09 00078Mustapha BouregaaNo ratings yet
- Journal of African Earth Sciences: Mohamed I. Abdel-Fattah, Farouk I. Metwalli, El Sayed I. MesilhiDocument13 pagesJournal of African Earth Sciences: Mohamed I. Abdel-Fattah, Farouk I. Metwalli, El Sayed I. MesilhiMustapha BouregaaNo ratings yet
- ES486 Key Word Search / Search Answer Exercise Hydrocarbon Production and Recovery TechniquesDocument1 pageES486 Key Word Search / Search Answer Exercise Hydrocarbon Production and Recovery TechniquesMustapha BouregaaNo ratings yet
- Exiway Easyled: Slim LED Emergency Lighting OfferDocument16 pagesExiway Easyled: Slim LED Emergency Lighting OfferDan PopescuNo ratings yet
- Digiflex KMTDocument58 pagesDigiflex KMTsarafciger12350% (2)
- CoreTec 4 - Transformer Monitoring PlatformDocument2 pagesCoreTec 4 - Transformer Monitoring PlatformJaime Vicuña CubillosNo ratings yet
- SITXWHS003 - Meeting Room Hazard Inspection Checklist.v1.0Document5 pagesSITXWHS003 - Meeting Room Hazard Inspection Checklist.v1.0মাহমুদুল হাসানNo ratings yet
- JYM-FN-6.65Operation and Maintenance ManualDocument32 pagesJYM-FN-6.65Operation and Maintenance ManualKunpeng ZhouNo ratings yet
- MX RM CameraSoftwareManual en 200131Document557 pagesMX RM CameraSoftwareManual en 200131atalincNo ratings yet
- After Each Question Listed.: FFP 1612-Fire Behavior and Combustion Assignment #7-Modules 10 and 11Document5 pagesAfter Each Question Listed.: FFP 1612-Fire Behavior and Combustion Assignment #7-Modules 10 and 11Travis DavidNo ratings yet
- XtremIO Architecture and AllocationDocument27 pagesXtremIO Architecture and AllocationParthiNo ratings yet
- How To Return Multiple Values From A VBA Function (Part 1)Document4 pagesHow To Return Multiple Values From A VBA Function (Part 1)Ankit KumarNo ratings yet
- Analysis and Design of RC Tall Building Subjected To Wind and EarthquakeDocument10 pagesAnalysis and Design of RC Tall Building Subjected To Wind and EarthquakeAHSAN HABIB100% (1)
- 800C GSM Module 3.3 - 5V - Compatible - Micro RoboticsDocument5 pages800C GSM Module 3.3 - 5V - Compatible - Micro RoboticsJorge AdrianNo ratings yet
- Risk Management Plan - Company ABC Widget Production & Sales Project ManagerDocument20 pagesRisk Management Plan - Company ABC Widget Production & Sales Project ManageralkalkiaNo ratings yet
- Lecturer (BS-18/ Regular) : Government College Women University FaisalabadDocument2 pagesLecturer (BS-18/ Regular) : Government College Women University Faisalabadmuhammad shoaib khalidNo ratings yet
- Masterflow Heat Blower: Instruction Manual Mode D'emploi Manual de InstruccionesDocument28 pagesMasterflow Heat Blower: Instruction Manual Mode D'emploi Manual de InstruccionesRobNo ratings yet
- Powerroc T35 E: Surface Drill Rig For Construction, Road Construction and Urban DevelopmentDocument5 pagesPowerroc T35 E: Surface Drill Rig For Construction, Road Construction and Urban DevelopmentElgi Alam PangestuNo ratings yet
- Samsung F Series Complete-EcatalogueDocument28 pagesSamsung F Series Complete-EcatalogueA common people Residence of JadavpurNo ratings yet
- Manual Slim 5000 App La CD 53225 CurvasDocument8 pagesManual Slim 5000 App La CD 53225 CurvasJose ElenilsonNo ratings yet
- Cprog 2 - ComputerProgramming2 02 1920Document6 pagesCprog 2 - ComputerProgramming2 02 1920Glenn Josue FrencilloNo ratings yet
- ICTNWK604 - Class Activity 8Document9 pagesICTNWK604 - Class Activity 8MitchNo ratings yet
- AUH01-RED-D1-XX-RT-X-06000 - MEP Stage 4 Design Proposal - P7Document43 pagesAUH01-RED-D1-XX-RT-X-06000 - MEP Stage 4 Design Proposal - P7Abhilash100% (1)
- Topic 7. BIORISK MANAGEMENTDocument3 pagesTopic 7. BIORISK MANAGEMENTMark Theodore L. Gregas100% (1)
- GEO343 Final 3Document5 pagesGEO343 Final 3jameswell maestradoNo ratings yet
- Credit CardDocument15 pagesCredit CardAnant JainNo ratings yet
- Tom Wheatley Swing Check Valves (New)Document24 pagesTom Wheatley Swing Check Valves (New)Carlos Cardenas SochaNo ratings yet
- FPE Module 5Document35 pagesFPE Module 5RickNo ratings yet
- Sankhamul Squatter SettlementDocument41 pagesSankhamul Squatter SettlementAshru SigdelNo ratings yet
Class Ex Ideal Gas Law
Class Ex Ideal Gas Law
Uploaded by
Mustapha BouregaaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Class Ex Ideal Gas Law
Class Ex Ideal Gas Law
Uploaded by
Mustapha BouregaaCopyright:
Available Formats
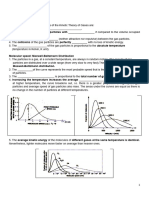
In-Class Exercise: Applica
ation of the Ideal Gas Law
L to Natu
ural Gas Exttraction
Volu
umes of natuural gas in fo
ormation are measured in cubic feet or millions of
o cubic feet (Mcf). The volume of
gas extracted fro endent upon temperature
om the reserrvoir is depe e and pressu ure at depth, according to
t the Idea
Gas Law:
wherre P is the pressure
p of th
he gas, V is the volume of the gas, n is the amo
ount of substtance of gass (measured
in moles), R is th
he ideal, or universal,
u ga
as constant, and T is the
e temperaturre of the gass.
P = pressure
p in atm
a V = Volume in literss n = mass in moles
m
T = temperature
t in degree Kelvin
K R = 0.0 m/oK-mole
0821 liter-atm
Wheen natural gaas is extracteed from the reservoir
r at depths of thousands of feet, and deelivered to th
he surface off
the Earth,
E the temperature and
a pressure e changes will
w result in volume
v channge during thhe process. Volume-
tempperature-preessure are immportant as they
t will dicttate how much the naturral gas sells per cubic fo
oot on the
markket. Let’s exxplore the efffects of thesse relationshhips in a hyp
pothetical sce
enario descrribed below:
Our goal is to exxtract 10 moles of metha
ane (CH4) fro
om a gas we ell located in a reservoir at a depth of
o 7000 ft
below
w the surfacce. The follo
owing inform
mation is available related
d to the cond ditions:
Drill Hole Depth = 7000 ft e Temperatu
Surface ure = 25 oC
Masss = 10 moless of methane Bottom
m Hole Tempperature = 1225 oC
Surfaace Pressurre = 1 bar m Hole Presssure = 2700 psi
Bottom
____ _____________________ __________
__________
___________ __________ __________
__________
Taskk 1. Using th
he periodic chart,
c determ
mine the masss of 10 mole
es of CH4 in grams.
Taskk 2. Calculatte the volume of methan
ne occupied by 10 moless at the bottoom of the ho
ole, vs. the vo
olume of
meth
hane occupie ed by the sa
ame 10 mole
es at the top of the hole (surface).
Taskk 3. Determine the perce
ent change in volume fro
om subsurface to surface. SHOW ALL
A OF YOU
UR MATH
WOR RK AND UN NIT ALGEBR RA.
____
__________
___________
__________
__________
___________
__________
__________
__________
Convversion facto
ors and cons
stants that may
m be helpfful:
R = 0.0821
0 liter-atm/oK-mole
e o
K = 273 + oC 1 atm = 1013 mb 1 bar = 1000 mb
1 bar = 100,000 Pa = 14.5 5 lb/in2
You might also like
- Music License AgreementDocument2 pagesMusic License AgreementAdrian Moscalu100% (1)
- Problem 2Document19 pagesProblem 2Aaliyahkaye JulaoNo ratings yet
- Formulas in Cooling TowerDocument6 pagesFormulas in Cooling TowerGringoNo ratings yet
- CH 01Document9 pagesCH 01Mary Lorelyn EscoteNo ratings yet
- Manual de Usuario Htw-Yan1f1-EngDocument10 pagesManual de Usuario Htw-Yan1f1-EngJonathan Hernán Valderrama RamírezNo ratings yet
- Topic 16:: Factor AnalysisDocument33 pagesTopic 16:: Factor AnalysisKelly WijayaNo ratings yet
- States of Matter - ThermodynamicsDocument146 pagesStates of Matter - ThermodynamicsbavisyaaaaNo ratings yet
- (Solved Problems) : Thermodynamics 01Document6 pages(Solved Problems) : Thermodynamics 01Ben0% (1)
- ClassWork CPD 2022Document20 pagesClassWork CPD 2022crazzyboy292No ratings yet
- Topic: Gaseous State: Chemistry Lecture NotesDocument32 pagesTopic: Gaseous State: Chemistry Lecture NotesDibya Ranjan BissoyiNo ratings yet
- Problemas Gases Ideales y Reales, Van Der WaalDocument9 pagesProblemas Gases Ideales y Reales, Van Der WaalmegustabajarinfoNo ratings yet
- Lecture3 PDFDocument8 pagesLecture3 PDFvignesh9489No ratings yet
- Solutions Manual For Fluid Mechanics 5th PDFDocument20 pagesSolutions Manual For Fluid Mechanics 5th PDFchala nigussieNo ratings yet
- Solutions Manual For Fluid Mechanics 5thDocument20 pagesSolutions Manual For Fluid Mechanics 5thhongjieNo ratings yet
- 06 - Pressure OLDocument4 pages06 - Pressure OLKNEWTON EducationNo ratings yet
- 06 PressureDocument9 pages06 PressureTahmedul Hasan TanvirNo ratings yet
- New-Problems-Chapter-21 Convective Mass TransferDocument2 pagesNew-Problems-Chapter-21 Convective Mass TransferKhanh NhiNo ratings yet
- Salinity ChartDocument17 pagesSalinity ChartWilmer CuicasNo ratings yet
- CY11001 ChemistryDocument2 pagesCY11001 ChemistryLakshay SinghalNo ratings yet
- A Study On Numerical Simulations and Experiments For Mass Transfer in Bubble Mode Absorber of Ammonia and WaterDocument8 pagesA Study On Numerical Simulations and Experiments For Mass Transfer in Bubble Mode Absorber of Ammonia and WaterusamaNo ratings yet
- Lecture 2Document12 pagesLecture 2Samaseen PrabhatNo ratings yet
- ADSORPSI II. 31 MAr 21Document15 pagesADSORPSI II. 31 MAr 21YumaNurAlfathNo ratings yet
- Coolship Calc - Cylindrical and RectangularDocument5 pagesCoolship Calc - Cylindrical and RectangularNicolas MossoNo ratings yet
- CH18ThermodynamicsP1-The First Law of ThermodynamicsDocument4 pagesCH18ThermodynamicsP1-The First Law of ThermodynamicsINDOMITABLENo ratings yet
- Dec 1984Document9 pagesDec 1984krishnaNo ratings yet
- Mass Transfer During Forest Biomass Particles Drying in A Fluidised BedDocument9 pagesMass Transfer During Forest Biomass Particles Drying in A Fluidised BedLAURA ISABEL ARIZA ARIZANo ratings yet
- Final ExamDocument5 pagesFinal ExamWaqasNo ratings yet
- Ch3 First TermDocument72 pagesCh3 First TermFBI AgentNo ratings yet
- C8 Cussler PDFDocument33 pagesC8 Cussler PDFRaisa LopezNo ratings yet
- Gaseous State PDFDocument16 pagesGaseous State PDFUditaNo ratings yet
- M17 Wolf57139 03 Se C17Document24 pagesM17 Wolf57139 03 Se C17c.s.kalkmanNo ratings yet
- Tuttherm2 PDFDocument6 pagesTuttherm2 PDFPrabir BanerjeeNo ratings yet
- Gaseous State (Package)Document55 pagesGaseous State (Package)Draw with Hassan JamalNo ratings yet
- L15 CRE II Heterogeneous Catalysis: Prof. K.K.Pant Department of Chemical Engineering IIT DelhiDocument15 pagesL15 CRE II Heterogeneous Catalysis: Prof. K.K.Pant Department of Chemical Engineering IIT DelhiMehul VarshneyNo ratings yet
- thay đổi PH đến Quang xúc tácDocument3 pagesthay đổi PH đến Quang xúc táckẻ khờ khạoNo ratings yet
- Oxygen Cylinder Duration CalculationDocument1 pageOxygen Cylinder Duration CalculationGetThisShitNo ratings yet
- The Journal of Supercritical Fluids: SciencedirectDocument11 pagesThe Journal of Supercritical Fluids: SciencedirectsutarsiNo ratings yet
- CBE 417 FlashDrumSizing Wankat PDFDocument7 pagesCBE 417 FlashDrumSizing Wankat PDFAbdul RehmanNo ratings yet
- CSEC Physics - SECTION B - THERMAL PHYSICS AND KINETIC THEORYDocument40 pagesCSEC Physics - SECTION B - THERMAL PHYSICS AND KINETIC THEORYBab50% (2)
- 11.1 - KTG & RadiationDocument11 pages11.1 - KTG & Radiationdevrarimanish72No ratings yet
- Chapter-3 PPTDocument109 pagesChapter-3 PPTnunuNo ratings yet
- DocxDocument11 pagesDocxNabila PutriNo ratings yet
- Technol03 PDFDocument9 pagesTechnol03 PDFHyunJoon, KimNo ratings yet
- Hno3 Baoh2 Neutralisation QDocument4 pagesHno3 Baoh2 Neutralisation QloxadegoNo ratings yet
- Chapter 1 (Part 2) : Basic Concepts 1.4 Process Variables: Marmy Roshaidah Mohd SallehDocument47 pagesChapter 1 (Part 2) : Basic Concepts 1.4 Process Variables: Marmy Roshaidah Mohd SallehAhmad Safwan HakimNo ratings yet
- Applications of First Order Differential Equations Discussion Part I PDFDocument22 pagesApplications of First Order Differential Equations Discussion Part I PDFErika Dawn Luciano AmbrayNo ratings yet
- P K Nag Exercise Problems Solved ThermodDocument265 pagesP K Nag Exercise Problems Solved ThermodSiddhant DeyNo ratings yet
- Chapter 9 The Gaseous State Edupdf 1Document64 pagesChapter 9 The Gaseous State Edupdf 1api-386303659No ratings yet
- 01 1stlaw ExessolsDocument29 pages01 1stlaw Exessolsblanca.pegueraNo ratings yet
- IES Conventional Mechanical Engineering 2007Document10 pagesIES Conventional Mechanical Engineering 2007eklavya koshtaNo ratings yet
- CPP Assignment 1Document2 pagesCPP Assignment 1AmandaEdwinNo ratings yet
- TD 1 Properties EnonceDocument2 pagesTD 1 Properties EnonceLuc AusterNo ratings yet
- Thiobacillus Ferrooxidans: Particle Size Effects in The Microbiological Eaching Sulfide Concentrates byDocument5 pagesThiobacillus Ferrooxidans: Particle Size Effects in The Microbiological Eaching Sulfide Concentrates byW ZuoNo ratings yet
- Unit 1 ProblemsDocument3 pagesUnit 1 Problemsgrace_juganNo ratings yet
- Single Phase SystemDocument61 pagesSingle Phase SystemNoorhalieza AliNo ratings yet
- Gaseous State PDFDocument32 pagesGaseous State PDFrockingrazzNo ratings yet
- Chapter 4 States of Matter 2021Document24 pagesChapter 4 States of Matter 2021suh mey chongNo ratings yet
- Problem Set 1Document4 pagesProblem Set 1ash jay100% (1)
- Ideal Gas Law 5684f195af3e6Document38 pagesIdeal Gas Law 5684f195af3e6Jose GulitiwNo ratings yet
- States of Matter by Rakshita SinghDocument14 pagesStates of Matter by Rakshita SinghFarzana ShaikNo ratings yet
- 11 GasesDocument17 pages11 Gasespuja ritongaNo ratings yet
- Carbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from -20° to 250°C and Pressures up to 1000 BarFrom EverandCarbon Dioxide Thermodynamic Properties Handbook: Covering Temperatures from -20° to 250°C and Pressures up to 1000 BarNo ratings yet
- the_regime_of_tectonic_stresses_and_fault_type_based_on_petrophysicalDocument13 pagesthe_regime_of_tectonic_stresses_and_fault_type_based_on_petrophysicalMustapha BouregaaNo ratings yet
- Petrographic_charcaterization_of_micropoDocument3 pagesPetrographic_charcaterization_of_micropoMustapha BouregaaNo ratings yet
- JPG-SDDocument15 pagesJPG-SDMustapha BouregaaNo ratings yet
- LotterDocument28 pagesLotterMustapha BouregaaNo ratings yet
- 48 - Geophysics, Geochemistry E-Books ListDocument31 pages48 - Geophysics, Geochemistry E-Books ListMustapha BouregaaNo ratings yet
- GeophysicsDocument22 pagesGeophysicsMustapha BouregaaNo ratings yet
- Intermedia - Exchange 2010 Pricing 12-15-09Document4 pagesIntermedia - Exchange 2010 Pricing 12-15-09Mustapha BouregaaNo ratings yet
- Introduction To Borehole GeophysicsDocument3 pagesIntroduction To Borehole GeophysicsMustapha BouregaaNo ratings yet
- DDR NZ25 - N045 - 26032011Document2 pagesDDR NZ25 - N045 - 26032011Mustapha BouregaaNo ratings yet
- 05 - Model Building Using Conditional Simulation Algorithms Such As SGS or SISDocument84 pages05 - Model Building Using Conditional Simulation Algorithms Such As SGS or SISMustapha BouregaaNo ratings yet
- 06 - Multiple-Poin Simulation (MPS)Document22 pages06 - Multiple-Poin Simulation (MPS)Mustapha BouregaaNo ratings yet
- Formation Evaluation Measurements: ENM237 Reservoir Geology and PetrophysicsDocument43 pagesFormation Evaluation Measurements: ENM237 Reservoir Geology and PetrophysicsMustapha BouregaaNo ratings yet
- Geostatistics and Reservoir Modeling Module: Review of Basic StatisticsDocument52 pagesGeostatistics and Reservoir Modeling Module: Review of Basic StatisticsMustapha BouregaaNo ratings yet
- Spatial StatisticsDocument96 pagesSpatial StatisticsMustapha BouregaaNo ratings yet
- ES476/576 Hydrology Review Problems: Physical Properties of WaterDocument2 pagesES476/576 Hydrology Review Problems: Physical Properties of WaterMustapha BouregaaNo ratings yet
- Formation Evaluation Measurements: ENM237 Reservoir Geology and PetrophysicsDocument67 pagesFormation Evaluation Measurements: ENM237 Reservoir Geology and PetrophysicsMustapha BouregaaNo ratings yet
- Journal of Natural Gas Science and Engineering: Shahab Dean MohagheghDocument9 pagesJournal of Natural Gas Science and Engineering: Shahab Dean MohagheghMustapha BouregaaNo ratings yet
- Formation Evaluation Measurements: ENM237 Reservoir Geology and PetrophysicsDocument57 pagesFormation Evaluation Measurements: ENM237 Reservoir Geology and PetrophysicsMustapha BouregaaNo ratings yet
- Geosciences 09 00078Document15 pagesGeosciences 09 00078Mustapha BouregaaNo ratings yet
- Journal of African Earth Sciences: Mohamed I. Abdel-Fattah, Farouk I. Metwalli, El Sayed I. MesilhiDocument13 pagesJournal of African Earth Sciences: Mohamed I. Abdel-Fattah, Farouk I. Metwalli, El Sayed I. MesilhiMustapha BouregaaNo ratings yet
- ES486 Key Word Search / Search Answer Exercise Hydrocarbon Production and Recovery TechniquesDocument1 pageES486 Key Word Search / Search Answer Exercise Hydrocarbon Production and Recovery TechniquesMustapha BouregaaNo ratings yet
- Exiway Easyled: Slim LED Emergency Lighting OfferDocument16 pagesExiway Easyled: Slim LED Emergency Lighting OfferDan PopescuNo ratings yet
- Digiflex KMTDocument58 pagesDigiflex KMTsarafciger12350% (2)
- CoreTec 4 - Transformer Monitoring PlatformDocument2 pagesCoreTec 4 - Transformer Monitoring PlatformJaime Vicuña CubillosNo ratings yet
- SITXWHS003 - Meeting Room Hazard Inspection Checklist.v1.0Document5 pagesSITXWHS003 - Meeting Room Hazard Inspection Checklist.v1.0মাহমুদুল হাসানNo ratings yet
- JYM-FN-6.65Operation and Maintenance ManualDocument32 pagesJYM-FN-6.65Operation and Maintenance ManualKunpeng ZhouNo ratings yet
- MX RM CameraSoftwareManual en 200131Document557 pagesMX RM CameraSoftwareManual en 200131atalincNo ratings yet
- After Each Question Listed.: FFP 1612-Fire Behavior and Combustion Assignment #7-Modules 10 and 11Document5 pagesAfter Each Question Listed.: FFP 1612-Fire Behavior and Combustion Assignment #7-Modules 10 and 11Travis DavidNo ratings yet
- XtremIO Architecture and AllocationDocument27 pagesXtremIO Architecture and AllocationParthiNo ratings yet
- How To Return Multiple Values From A VBA Function (Part 1)Document4 pagesHow To Return Multiple Values From A VBA Function (Part 1)Ankit KumarNo ratings yet
- Analysis and Design of RC Tall Building Subjected To Wind and EarthquakeDocument10 pagesAnalysis and Design of RC Tall Building Subjected To Wind and EarthquakeAHSAN HABIB100% (1)
- 800C GSM Module 3.3 - 5V - Compatible - Micro RoboticsDocument5 pages800C GSM Module 3.3 - 5V - Compatible - Micro RoboticsJorge AdrianNo ratings yet
- Risk Management Plan - Company ABC Widget Production & Sales Project ManagerDocument20 pagesRisk Management Plan - Company ABC Widget Production & Sales Project ManageralkalkiaNo ratings yet
- Lecturer (BS-18/ Regular) : Government College Women University FaisalabadDocument2 pagesLecturer (BS-18/ Regular) : Government College Women University Faisalabadmuhammad shoaib khalidNo ratings yet
- Masterflow Heat Blower: Instruction Manual Mode D'emploi Manual de InstruccionesDocument28 pagesMasterflow Heat Blower: Instruction Manual Mode D'emploi Manual de InstruccionesRobNo ratings yet
- Powerroc T35 E: Surface Drill Rig For Construction, Road Construction and Urban DevelopmentDocument5 pagesPowerroc T35 E: Surface Drill Rig For Construction, Road Construction and Urban DevelopmentElgi Alam PangestuNo ratings yet
- Samsung F Series Complete-EcatalogueDocument28 pagesSamsung F Series Complete-EcatalogueA common people Residence of JadavpurNo ratings yet
- Manual Slim 5000 App La CD 53225 CurvasDocument8 pagesManual Slim 5000 App La CD 53225 CurvasJose ElenilsonNo ratings yet
- Cprog 2 - ComputerProgramming2 02 1920Document6 pagesCprog 2 - ComputerProgramming2 02 1920Glenn Josue FrencilloNo ratings yet
- ICTNWK604 - Class Activity 8Document9 pagesICTNWK604 - Class Activity 8MitchNo ratings yet
- AUH01-RED-D1-XX-RT-X-06000 - MEP Stage 4 Design Proposal - P7Document43 pagesAUH01-RED-D1-XX-RT-X-06000 - MEP Stage 4 Design Proposal - P7Abhilash100% (1)
- Topic 7. BIORISK MANAGEMENTDocument3 pagesTopic 7. BIORISK MANAGEMENTMark Theodore L. Gregas100% (1)
- GEO343 Final 3Document5 pagesGEO343 Final 3jameswell maestradoNo ratings yet
- Credit CardDocument15 pagesCredit CardAnant JainNo ratings yet
- Tom Wheatley Swing Check Valves (New)Document24 pagesTom Wheatley Swing Check Valves (New)Carlos Cardenas SochaNo ratings yet
- FPE Module 5Document35 pagesFPE Module 5RickNo ratings yet
- Sankhamul Squatter SettlementDocument41 pagesSankhamul Squatter SettlementAshru SigdelNo ratings yet