Professional Documents
Culture Documents
Determination of Ferrous Ion in Acidic Solutions With Permanganate Titrant
Determination of Ferrous Ion in Acidic Solutions With Permanganate Titrant
Uploaded by
Henrique PiaggioCopyright:
Available Formats
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5825)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- BKM Z60 80 B III Service Manual PDFDocument54 pagesBKM Z60 80 B III Service Manual PDFKelly VillamilNo ratings yet
- Brio UK Lit Dec 2007Document8 pagesBrio UK Lit Dec 2007Neil HoggNo ratings yet
- General: Method: M1 - Calibrating The PH Glass ElectrodeDocument6 pagesGeneral: Method: M1 - Calibrating The PH Glass ElectrodeHenrique PiaggioNo ratings yet
- General: Method: M3 - Titer Determination of Alkaline TitrantsDocument3 pagesGeneral: Method: M3 - Titer Determination of Alkaline TitrantsHenrique PiaggioNo ratings yet
- De Ac So Eterm Cidic Olutio Mina Solu On Ation Ution Noff Ns Wi Ferro TH Ce Us Io Eric On in NDocument3 pagesDe Ac So Eterm Cidic Olutio Mina Solu On Ation Ution Noff Ns Wi Ferro TH Ce Us Io Eric On in NHenrique PiaggioNo ratings yet
- Metrohm - Corrosion StudiesDocument15 pagesMetrohm - Corrosion StudiesHenrique PiaggioNo ratings yet
- T-64 Ti Application Note No.: Title: Titanium and Iron in MixturesDocument1 pageT-64 Ti Application Note No.: Title: Titanium and Iron in MixturesHenrique PiaggioNo ratings yet
- H-012 Thermo. Titr. Application Note No.: Title: Determination of Ferrous Ion Content of Heat Exchanger Wash SolutionsDocument3 pagesH-012 Thermo. Titr. Application Note No.: Title: Determination of Ferrous Ion Content of Heat Exchanger Wash SolutionsHenrique PiaggioNo ratings yet
- Electrochemical Properties of Graphene/epoxy CoatingDocument1 pageElectrochemical Properties of Graphene/epoxy CoatingHenrique PiaggioNo ratings yet
- Autolab - EC02 - Reference Electrodes PDFDocument3 pagesAutolab - EC02 - Reference Electrodes PDFHenrique PiaggioNo ratings yet
- Health Lesson PlanDocument26 pagesHealth Lesson Planapi-224842598No ratings yet
- All at Sea Caribbean 0917Document112 pagesAll at Sea Caribbean 0917Scott NeumanNo ratings yet
- Sound Proof Canopy Model:: Industrial RangeDocument1 pageSound Proof Canopy Model:: Industrial Rangesales3 iaescoNo ratings yet
- CH 11 Lab Human InheritanceDocument13 pagesCH 11 Lab Human InheritanceBao HoangNo ratings yet
- English To Marathi Animals NameDocument3 pagesEnglish To Marathi Animals Namesathishr_vtvplNo ratings yet
- HomoeopathyDocument354 pagesHomoeopathyEla Drzewiecka100% (4)
- 10-Formulation and Evaluation of Effervescent Tablets of ParacetamolDocument30 pages10-Formulation and Evaluation of Effervescent Tablets of ParacetamolRodrigo InostrozaNo ratings yet
- TariffDocument254 pagesTariffrehmannazirNo ratings yet
- Down SyndromeDocument3 pagesDown SyndromeMargie Ballesteros Manzano100% (1)
- Contraception Your Questions AnsweredDocument144 pagesContraception Your Questions AnsweredMohamed SaeedNo ratings yet
- Aptitude TestDocument8 pagesAptitude TestKhalidNo ratings yet
- Articulo 1Document8 pagesArticulo 1JESSICA ANDREA GUILOMBO GONZALEZNo ratings yet
- 2018 CONV Chassis 1 - DT - ABSDocument435 pages2018 CONV Chassis 1 - DT - ABSThanh HoangNo ratings yet
- Spark Plug Pictures For Lean To Rich Running EnginesDocument2 pagesSpark Plug Pictures For Lean To Rich Running EnginesSajjad Rasheed QureshiNo ratings yet
- Rili DLP MutationDocument16 pagesRili DLP MutationJohn Bernard RiliNo ratings yet
- Y2 Series: Three Phase Cast Iron MotorDocument4 pagesY2 Series: Three Phase Cast Iron MotorMark Christian TorresNo ratings yet
- Guidelines For Hard MaterialDocument30 pagesGuidelines For Hard MaterialMuhamad MuzakkirNo ratings yet
- HANSE 341 EnglDocument2 pagesHANSE 341 EnglMiguel ToqueNo ratings yet
- Dental ProblemsDocument18 pagesDental ProblemsRenellie Quiñonez TrimidalNo ratings yet
- Vaillant Commercial Range BrochureDocument45 pagesVaillant Commercial Range Brochureandreiasbd100% (1)
- 411UDAC Battery Calculation: Secondary Power Source RequirementsDocument1 page411UDAC Battery Calculation: Secondary Power Source RequirementsFire ChileNo ratings yet
- Concrete Structures For Retaining Aqueous Liquids - Code of PracticeDocument28 pagesConcrete Structures For Retaining Aqueous Liquids - Code of PracticeProject ManagerStructuresNo ratings yet
- First Step: Prepare All The Ingredients andDocument4 pagesFirst Step: Prepare All The Ingredients andMerriel Joy GarinoNo ratings yet
- Acute Lymphoblastic Leukemia RadiopediaDocument2 pagesAcute Lymphoblastic Leukemia RadiopediaRani Henty NovitaNo ratings yet
- Post Incident Damage Assessment ChecklistDocument9 pagesPost Incident Damage Assessment ChecklistFerdinand M. TurbanosNo ratings yet
- TR MassageTherapy NCIIDocument91 pagesTR MassageTherapy NCIIRebu Leno Rnlptlmtcssi100% (2)
- Victor Leborgne (Nickname "Tan") : The Wild ChildDocument2 pagesVictor Leborgne (Nickname "Tan") : The Wild ChildWajeeha RizwanNo ratings yet
- Tourniquet Conversion Drew JSOM Fall 2015 Edition-2Document5 pagesTourniquet Conversion Drew JSOM Fall 2015 Edition-2Oleg ShubinNo ratings yet
Determination of Ferrous Ion in Acidic Solutions With Permanganate Titrant
Determination of Ferrous Ion in Acidic Solutions With Permanganate Titrant
Uploaded by
Henrique PiaggioOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Determination of Ferrous Ion in Acidic Solutions With Permanganate Titrant
Determination of Ferrous Ion in Acidic Solutions With Permanganate Titrant
Uploaded by
Henrique PiaggioCopyright:
Available Formats
Titration Application Note H–128
Determination of ferrous ion in
acidic solutions with
permanganate titrant
This Application Note looks at the determination of ferrous ion in acidic
solutions by redox titration with a permanganate titrant using
thermometric titration.
Method description
Principle freshly for each batch of
KMnO4 solution to be
Permanganate ion in strongly acidic solution is a suitable
standardized. Calculate
titrant for the direct determination of ferrous ion.
amount of FAS to be
weighed, taking into
MnO4– + 8 H+ + 5 e– ↔ Mn2+ + 4 H2O account the stated purity of
[Fe 2+ ↔ Fe 3+ + e–] × 5 the reagent. Note: FAS is
not a primary standard, but
------------------------------
is suitable for
5 Fe2+ + MnO4– + 8 H+ ↔ 5 Fe3+ + Mn2+ + 4 H2O standardizing KMnO4
solutions used for routine
Thus, 1 mol MnO4– ≡ 5 mol Fe2+. The reaction is analysis.
strongly exothermic. A disadvantage of permanganate is “Test solution” A test solution of 1 g/L
that solutions have no long-term stability and should be Fe2+ was prepared from
standardized for good accuracy. Solutions should be FAS to simulate a customer
prepared with freshly produced DI water and stored in solution containing a low
brown glass bottles with minimum headspace. level of Fe2+. This was
prepared by weighing
Samples approximately 1.77 g FAS
“Test solution” into a 250 mL volumetric
flask containing 10 mL 10%
Sample preparation (w/v) H2SO4 and making to
No sample preparation required volume with DI water.
Configuration
Analysis
Basic equipment list for automated titration
Basic method (for 1 g/L Fe2+ solution):
814 USB Sample Processor 2.814.0030 Pipette a 20 mL aliquot of test solution into a titration
859 Titrotherm 2.859.0010 vessel, add 10 mL 10% (w/v) H2SO4, and titrate with
c(KMnO4) = 0.25 mol/L solution to an exothermic
Sample rack 24 × 75 mL 6.2041.340 endpoint.
Thermoprobe, 6.9011.040 Standardization:
fluoride resistant Using a specially constructed tiamo™ program, aliquots
ranging from 4 to 20 mL in 4 mL increments were
Titration Head for Titrotherm 6.9914.159
dispensed from a Dosino into titration vessels containing
Sample beaker/75 mL 6.1459.400 10 mL 10% (w/v) H2SO4 and volumes of DI water to
802 Stirrer 2.802.0010 give a total volume of approximately 30 mL at the start
of the titration. The tiamo program performs the
Stirring propeller (104 mm) 6.1909.020 regression analysis and calculates the molarity, titration
1 × 800 Dosino 2.800.0010 blank and correlation coefficient of the regression line
automatically.
1 × Dosing unit 10 mL 6.3032.210
tiamo™ 6.6056.222 Parameters
Solutions Titrant dose rate
4
(mL/min)
Titrant c(KMnO4) = 0.25 mol/L
potassium permanganate ERC EP1 (exothermic) -100
acid: 10% (w/v) H2SO4 Data smooting (“filter
solution 45
factor”)
Acid 10% (w/v) H2SO4 solution
Stirring speed
Standard solution c(Fe2+) = 0.3 mol/L ferrous 14
(802 Rod stirrer)
Titration Application Note H–128
ammonium sulfate (FAS),
Evaluation start (mL) 0.5
(NH4)2SO4FeSO46H2O,
prepared in 0.1 mol/L Damping until (mL) 0.5
H2SO4 solution. Prepare
Version 1
Method description
Calculations
g/L Fe2+ = ((EP1 - blank) × C01 × C02 × 5)/C00
EP1 = endpoint in mL
C00 = sample weight in mL
C01 = concentration of permanganate titrant in mol/L
C02 = molecular weight of Fe (55.845 g/mol)
Results
“Test solution” Fe2+ = 0.99 ± 0.00 g/L
(theoretical = 1.01 g/L)
Standardization:
c(KMnO4) = 0.2550 mol/L
blank = 0.043 mL
coefficient of determination, R2 = 1.0000
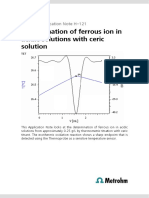
Titration plot
Fig. 1. Titration plot, Fe2+ with KMnO4
Titration Application Note H–128
Version 1
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5825)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (349)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (823)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (403)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- BKM Z60 80 B III Service Manual PDFDocument54 pagesBKM Z60 80 B III Service Manual PDFKelly VillamilNo ratings yet
- Brio UK Lit Dec 2007Document8 pagesBrio UK Lit Dec 2007Neil HoggNo ratings yet
- General: Method: M1 - Calibrating The PH Glass ElectrodeDocument6 pagesGeneral: Method: M1 - Calibrating The PH Glass ElectrodeHenrique PiaggioNo ratings yet
- General: Method: M3 - Titer Determination of Alkaline TitrantsDocument3 pagesGeneral: Method: M3 - Titer Determination of Alkaline TitrantsHenrique PiaggioNo ratings yet
- De Ac So Eterm Cidic Olutio Mina Solu On Ation Ution Noff Ns Wi Ferro TH Ce Us Io Eric On in NDocument3 pagesDe Ac So Eterm Cidic Olutio Mina Solu On Ation Ution Noff Ns Wi Ferro TH Ce Us Io Eric On in NHenrique PiaggioNo ratings yet
- Metrohm - Corrosion StudiesDocument15 pagesMetrohm - Corrosion StudiesHenrique PiaggioNo ratings yet
- T-64 Ti Application Note No.: Title: Titanium and Iron in MixturesDocument1 pageT-64 Ti Application Note No.: Title: Titanium and Iron in MixturesHenrique PiaggioNo ratings yet
- H-012 Thermo. Titr. Application Note No.: Title: Determination of Ferrous Ion Content of Heat Exchanger Wash SolutionsDocument3 pagesH-012 Thermo. Titr. Application Note No.: Title: Determination of Ferrous Ion Content of Heat Exchanger Wash SolutionsHenrique PiaggioNo ratings yet
- Electrochemical Properties of Graphene/epoxy CoatingDocument1 pageElectrochemical Properties of Graphene/epoxy CoatingHenrique PiaggioNo ratings yet
- Autolab - EC02 - Reference Electrodes PDFDocument3 pagesAutolab - EC02 - Reference Electrodes PDFHenrique PiaggioNo ratings yet
- Health Lesson PlanDocument26 pagesHealth Lesson Planapi-224842598No ratings yet
- All at Sea Caribbean 0917Document112 pagesAll at Sea Caribbean 0917Scott NeumanNo ratings yet
- Sound Proof Canopy Model:: Industrial RangeDocument1 pageSound Proof Canopy Model:: Industrial Rangesales3 iaescoNo ratings yet
- CH 11 Lab Human InheritanceDocument13 pagesCH 11 Lab Human InheritanceBao HoangNo ratings yet
- English To Marathi Animals NameDocument3 pagesEnglish To Marathi Animals Namesathishr_vtvplNo ratings yet
- HomoeopathyDocument354 pagesHomoeopathyEla Drzewiecka100% (4)
- 10-Formulation and Evaluation of Effervescent Tablets of ParacetamolDocument30 pages10-Formulation and Evaluation of Effervescent Tablets of ParacetamolRodrigo InostrozaNo ratings yet
- TariffDocument254 pagesTariffrehmannazirNo ratings yet
- Down SyndromeDocument3 pagesDown SyndromeMargie Ballesteros Manzano100% (1)
- Contraception Your Questions AnsweredDocument144 pagesContraception Your Questions AnsweredMohamed SaeedNo ratings yet
- Aptitude TestDocument8 pagesAptitude TestKhalidNo ratings yet
- Articulo 1Document8 pagesArticulo 1JESSICA ANDREA GUILOMBO GONZALEZNo ratings yet
- 2018 CONV Chassis 1 - DT - ABSDocument435 pages2018 CONV Chassis 1 - DT - ABSThanh HoangNo ratings yet
- Spark Plug Pictures For Lean To Rich Running EnginesDocument2 pagesSpark Plug Pictures For Lean To Rich Running EnginesSajjad Rasheed QureshiNo ratings yet
- Rili DLP MutationDocument16 pagesRili DLP MutationJohn Bernard RiliNo ratings yet
- Y2 Series: Three Phase Cast Iron MotorDocument4 pagesY2 Series: Three Phase Cast Iron MotorMark Christian TorresNo ratings yet
- Guidelines For Hard MaterialDocument30 pagesGuidelines For Hard MaterialMuhamad MuzakkirNo ratings yet
- HANSE 341 EnglDocument2 pagesHANSE 341 EnglMiguel ToqueNo ratings yet
- Dental ProblemsDocument18 pagesDental ProblemsRenellie Quiñonez TrimidalNo ratings yet
- Vaillant Commercial Range BrochureDocument45 pagesVaillant Commercial Range Brochureandreiasbd100% (1)
- 411UDAC Battery Calculation: Secondary Power Source RequirementsDocument1 page411UDAC Battery Calculation: Secondary Power Source RequirementsFire ChileNo ratings yet
- Concrete Structures For Retaining Aqueous Liquids - Code of PracticeDocument28 pagesConcrete Structures For Retaining Aqueous Liquids - Code of PracticeProject ManagerStructuresNo ratings yet
- First Step: Prepare All The Ingredients andDocument4 pagesFirst Step: Prepare All The Ingredients andMerriel Joy GarinoNo ratings yet
- Acute Lymphoblastic Leukemia RadiopediaDocument2 pagesAcute Lymphoblastic Leukemia RadiopediaRani Henty NovitaNo ratings yet
- Post Incident Damage Assessment ChecklistDocument9 pagesPost Incident Damage Assessment ChecklistFerdinand M. TurbanosNo ratings yet
- TR MassageTherapy NCIIDocument91 pagesTR MassageTherapy NCIIRebu Leno Rnlptlmtcssi100% (2)
- Victor Leborgne (Nickname "Tan") : The Wild ChildDocument2 pagesVictor Leborgne (Nickname "Tan") : The Wild ChildWajeeha RizwanNo ratings yet
- Tourniquet Conversion Drew JSOM Fall 2015 Edition-2Document5 pagesTourniquet Conversion Drew JSOM Fall 2015 Edition-2Oleg ShubinNo ratings yet