Professional Documents
Culture Documents
Reduction of Vanillin To Vanillyl Alcohol.: Background
Reduction of Vanillin To Vanillyl Alcohol.: Background
Uploaded by
Hawra JawadCopyright:
Available Formats
You might also like
- EnzymeDocument14 pagesEnzymeAthena Wars100% (1)
- Nitrogen Determination by Kjeldahl MethodDocument7 pagesNitrogen Determination by Kjeldahl MethodLinh VũNo ratings yet
- Williamson Ether Synthesis of An Expectorant - Guaifenesin - and Its Isolation From Guai-Aid Cough TabletsDocument8 pagesWilliamson Ether Synthesis of An Expectorant - Guaifenesin - and Its Isolation From Guai-Aid Cough TabletsAnna LuthfiahNo ratings yet
- Examiner Tips For O Level Chemistry 5070 FINALDocument10 pagesExaminer Tips For O Level Chemistry 5070 FINALMuhammad Bin Anis60% (5)
- Solutions To Crystallization ProblemsDocument5 pagesSolutions To Crystallization ProblemsmadhavanssnNo ratings yet
- Síntesis de Alcohol VainillílicoDocument6 pagesSíntesis de Alcohol VainillílicoYago L100% (1)
- Taller 2 FcoQca II 2022Document11 pagesTaller 2 FcoQca II 2022Natalia Petro RodríguezNo ratings yet
- Octyl AcetateDocument6 pagesOctyl AcetateKristine BautistaNo ratings yet
- Determination of The Differential Heat of SolutionDocument3 pagesDetermination of The Differential Heat of SolutionLoveFreequencyNo ratings yet
- Macrogol Cetostearyl EtherDocument1 pageMacrogol Cetostearyl EtherTeodor BoianovNo ratings yet
- Difusividad de Liquidos - PerryDocument3 pagesDifusividad de Liquidos - PerryGladys Ventura FloreNo ratings yet
- Tablas Alcoholimetricas BDocument3 pagesTablas Alcoholimetricas Bfgranobles100% (1)
- DMT Fumarate To FreebaseDocument3 pagesDMT Fumarate To Freebaseedmundo riveroNo ratings yet
- Acetanilida MSDSDocument5 pagesAcetanilida MSDStylerNo ratings yet
- Borohydride Reduction of Vanillin To Vanillyl AlcoholDocument8 pagesBorohydride Reduction of Vanillin To Vanillyl Alcoholandantino076657No ratings yet
- Extraction Organic Lab 3Document8 pagesExtraction Organic Lab 3jarissa bannerNo ratings yet
- Quantitative Reactions and Titrations ExperimentDocument5 pagesQuantitative Reactions and Titrations ExperimentJeremy BarrettNo ratings yet
- Experiment IV 143BDocument10 pagesExperiment IV 143BtrickkkpatNo ratings yet
- Preparation of B-NaphtholDocument4 pagesPreparation of B-NaphtholnithansaNo ratings yet
- Exp10 - Analysis of AspirinDocument7 pagesExp10 - Analysis of AspirinjemgeneNo ratings yet
- Experiment 4Document6 pagesExperiment 4Tèddy ÑawåNo ratings yet
- Common Ion EffectDocument2 pagesCommon Ion EffectRonnie AlfecheNo ratings yet
- Titration LabDocument2 pagesTitration LabAliayah RoweNo ratings yet
- Titration Lab 3Document2 pagesTitration Lab 3Aliayah RoweNo ratings yet
- KHP LabDocument5 pagesKHP LabSantino MusaNo ratings yet
- 25 Titration of VinegarDocument3 pages25 Titration of VinegarJuventie PrimastutiNo ratings yet
- Lab Report 3 Chm557Document9 pagesLab Report 3 Chm557Nur AqilahNo ratings yet
- Lab Report TitrationDocument7 pagesLab Report TitrationIanaNo ratings yet
- Experiment 30 - Volumetric Analysis: OBJECTIVE: To Become More Familiar With Volumetric Analysis. MaterialsDocument3 pagesExperiment 30 - Volumetric Analysis: OBJECTIVE: To Become More Familiar With Volumetric Analysis. MaterialsZari Sofia LevisteNo ratings yet
- Pre Lab: Example of Laboratory Write-Up - CHM 2201/2202Document5 pagesPre Lab: Example of Laboratory Write-Up - CHM 2201/2202Hawra JawadNo ratings yet
- Borohydride Reduction of Vanillin To Vanillyl AlcoholDocument6 pagesBorohydride Reduction of Vanillin To Vanillyl AlcoholHawra JawadNo ratings yet
- Lab 8 - Solutions and Titration 2023-1Document8 pagesLab 8 - Solutions and Titration 2023-12021155224No ratings yet
- Ach Lab ReportDocument6 pagesAch Lab ReportTiofelus H. HamutenyaNo ratings yet
- Acid-Base TitrationDocument7 pagesAcid-Base TitrationPok Wan SoonNo ratings yet
- Experiment # 01: Calculations Related To Organic ChemistryDocument12 pagesExperiment # 01: Calculations Related To Organic ChemistryAbdullah JavedNo ratings yet
- Lab Manual CMT450Document32 pagesLab Manual CMT450Mohamad Hanafi Bin AbdullahNo ratings yet
- ProcedureDocument3 pagesProcedureMadhav VenugopalanNo ratings yet
- Lab Manual FGS0074Document8 pagesLab Manual FGS0074hash117No ratings yet
- Standardization of NaohDocument3 pagesStandardization of NaohsadyaNo ratings yet
- Exp-1 Dye Synthesis Phenyl Azo B-Napthol DyeDocument6 pagesExp-1 Dye Synthesis Phenyl Azo B-Napthol DyeKhadija ShirgarNo ratings yet
- POC-II Tutorial MMP PDFDocument29 pagesPOC-II Tutorial MMP PDFmehuNo ratings yet
- Standardization of Acid and Base Solutions PDFDocument3 pagesStandardization of Acid and Base Solutions PDFKassim100% (1)
- GEMs ID90Document8 pagesGEMs ID90Andre PNo ratings yet
- Experiment Number 5Document16 pagesExperiment Number 5najjar119No ratings yet
- Titration Group 1Document4 pagesTitration Group 1brydonsantosNo ratings yet
- Synthesis of Benzilic Acid: Experiment 17Document9 pagesSynthesis of Benzilic Acid: Experiment 17Ayattolla KachingweNo ratings yet
- Acetophenone Reduction by Sodium BorohydrideDocument2 pagesAcetophenone Reduction by Sodium BorohydrideAravind Rao Karanam67% (3)
- Experiment: Title: ObjectiveDocument23 pagesExperiment: Title: Objectiveapi-3734333No ratings yet
- Standardization of A Strong Base NaOH WiDocument4 pagesStandardization of A Strong Base NaOH Wi232449045No ratings yet
- StandardizationDocument18 pagesStandardizationCHEA MICH L. ABELLANONo ratings yet
- Experiment 4 Preparation of Standardized SolutionsDocument10 pagesExperiment 4 Preparation of Standardized SolutionsJohn Dy100% (1)
- 07 Experiment 07 Salicylic Acid - Molecular Weight DeterminationDocument5 pages07 Experiment 07 Salicylic Acid - Molecular Weight DeterminationTaffazzul ShaukatNo ratings yet
- Chemistry Project Dheekshanya KumariDocument21 pagesChemistry Project Dheekshanya KumariDheekshith KumarNo ratings yet
- 2004 Chem 2OB3 Lab Manual - Experiment 4. Reactions of Carboxylic AcidsDocument4 pages2004 Chem 2OB3 Lab Manual - Experiment 4. Reactions of Carboxylic AcidsNstm3No ratings yet
- Project of Chemistry 11thDocument9 pagesProject of Chemistry 11thNitin ChandwaniNo ratings yet
- Ms Ijbpas 2021 Dec SPCL 2005Document5 pagesMs Ijbpas 2021 Dec SPCL 2005kannnan768402No ratings yet
- Experiment No. 3 Volumetric TransferDocument14 pagesExperiment No. 3 Volumetric TransferJoemar SubongNo ratings yet
- BP406P Medicinal Chemistry1Document30 pagesBP406P Medicinal Chemistry1Anit DubeyNo ratings yet
- LidocaineDocument6 pagesLidocaineg20kpNo ratings yet
- Titration - Dry Lab - 2020Document6 pagesTitration - Dry Lab - 2020MariaPaulaGonzalezRojasNo ratings yet
- Titration - Dry Lab - 2020Document6 pagesTitration - Dry Lab - 2020MariaPaulaGonzalezRojasNo ratings yet
- Cracking Chemistry - Titration 2 - NaOH Against Ethanoic AcidDocument3 pagesCracking Chemistry - Titration 2 - NaOH Against Ethanoic Acidmarco.chiappero.ieNo ratings yet
- Experiment 1: Preparation of 2-Iodobenzoic Acid From Anthranilic Acid (2-Amino Benzoic Acid)Document11 pagesExperiment 1: Preparation of 2-Iodobenzoic Acid From Anthranilic Acid (2-Amino Benzoic Acid)Sanjida Khandoker 1911009049No ratings yet
- Assim Exp 1Document87 pagesAssim Exp 1Hawra JawadNo ratings yet
- Experiment 1. Ketone Reduction by Sodium Borohydride: Butyrophenone and AcetophenoneDocument3 pagesExperiment 1. Ketone Reduction by Sodium Borohydride: Butyrophenone and AcetophenoneHawra JawadNo ratings yet
- Borohydride Reduction of Vanillin To Vanillyl AlcoholDocument6 pagesBorohydride Reduction of Vanillin To Vanillyl AlcoholHawra JawadNo ratings yet
- EXPERIMENT 7: Reduction of Carbonyl Compounds - Achiral and Chiral ReductionDocument13 pagesEXPERIMENT 7: Reduction of Carbonyl Compounds - Achiral and Chiral ReductionHawra JawadNo ratings yet
- Soduim Borohydride Reduction of CyclohexanoneDocument10 pagesSoduim Borohydride Reduction of CyclohexanoneHawra JawadNo ratings yet
- Assim ExpDocument10 pagesAssim ExpHawra JawadNo ratings yet
- Convenient Reduction of Carbonyl Compounds To Their Corresponding Alcohols With Nabh / (NH) C O SystemDocument7 pagesConvenient Reduction of Carbonyl Compounds To Their Corresponding Alcohols With Nabh / (NH) C O SystemHawra JawadNo ratings yet
- Pre Lab: Example of Laboratory Write-Up - CHM 2201/2202Document5 pagesPre Lab: Example of Laboratory Write-Up - CHM 2201/2202Hawra JawadNo ratings yet
- Stereochemistry of The Sodium Borohydride Reduction of BenzoinDocument8 pagesStereochemistry of The Sodium Borohydride Reduction of BenzoinHawra JawadNo ratings yet
- Reduction of Aldehydes Using Sodium Borohydride Under Ultrasonic IrradiationDocument4 pagesReduction of Aldehydes Using Sodium Borohydride Under Ultrasonic IrradiationHawra JawadNo ratings yet
- NorSaliyana 21 5 22Document7 pagesNorSaliyana 21 5 22Hawra JawadNo ratings yet
- Reduction of Aldehydes, Ketones, To: (1) Clemmsens MethodDocument9 pagesReduction of Aldehydes, Ketones, To: (1) Clemmsens MethodHawra JawadNo ratings yet
- ZN (BH) /2nacl: A Novel Reducing System For Efficient Reduction of Organic Carbonyl Compounds To Their Corresponding AlcoholsDocument8 pagesZN (BH) /2nacl: A Novel Reducing System For Efficient Reduction of Organic Carbonyl Compounds To Their Corresponding AlcoholsHawra JawadNo ratings yet
- SRI CO2 ManualDocument28 pagesSRI CO2 ManualChhomNo ratings yet
- Earth Science 4Document3 pagesEarth Science 4Sean CaloyloyNo ratings yet
- THERMIT-Presentasi Lengkap Thermit-Oxy LanceDocument14 pagesTHERMIT-Presentasi Lengkap Thermit-Oxy LanceFirdaus AdenNo ratings yet
- AIGA 090 - 14 - Reference Guide For Requalification of Gas CylindersDocument13 pagesAIGA 090 - 14 - Reference Guide For Requalification of Gas CylindersEmran UmerNo ratings yet
- Science 7 - Quarter 1 - Lesson 3 - Concentrations of SolutionsDocument72 pagesScience 7 - Quarter 1 - Lesson 3 - Concentrations of SolutionsTrisphere Media TechnologiesNo ratings yet
- Combustion of Gaseous and Liquid FuelsDocument2 pagesCombustion of Gaseous and Liquid FuelsLouie G Navalta0% (1)
- API 570 Point Recall 4Document3 pagesAPI 570 Point Recall 4Ravindra S. Jivani100% (2)
- 1.2 Assessed HomeworkDocument8 pages1.2 Assessed HomeworkNavine NavNo ratings yet
- TS STAB 34 Crude Oil Demulsification PDFDocument2 pagesTS STAB 34 Crude Oil Demulsification PDFMo OsNo ratings yet
- Alcohols, Phenols and Ethers (CBSE)Document25 pagesAlcohols, Phenols and Ethers (CBSE)Vishu Bansal100% (1)
- Studies The Mechanism of Action Of: 6-MercaptopurineDocument5 pagesStudies The Mechanism of Action Of: 6-MercaptopurineBelladonna Perdana PutraNo ratings yet
- Self-Healing CrackingDocument13 pagesSelf-Healing CrackingSujan NeupaneNo ratings yet
- ValenceDocument218 pagesValenceSubhabrata MabhaiNo ratings yet
- Alloy Design For Additive Manufacturing: Eric. A. JägleDocument43 pagesAlloy Design For Additive Manufacturing: Eric. A. JäglePopa Nicolea MirelaNo ratings yet
- Unit 7E Acids and Alkalis: Name: .Document21 pagesUnit 7E Acids and Alkalis: Name: .irene9tan9ailianNo ratings yet
- Advances in Replacements For Cadmium Plating in Aerospace ApplicationsDocument9 pagesAdvances in Replacements For Cadmium Plating in Aerospace ApplicationsNguyễn Ngọc SơnNo ratings yet
- The Extractive Metallurgy of South Africa's Platinum Ores: L.A. CramerDocument5 pagesThe Extractive Metallurgy of South Africa's Platinum Ores: L.A. CramerTeererai KaguraNo ratings yet
- Nano Emulsions Formation by Low Energy Methods PDFDocument9 pagesNano Emulsions Formation by Low Energy Methods PDFCarmen RaveNo ratings yet
- Thermal Analysis-Frying PanDocument12 pagesThermal Analysis-Frying PanAkshay BorateNo ratings yet
- Applied Thermal Engineering: S. Srinivasa Murthy, E. Anil KumarDocument14 pagesApplied Thermal Engineering: S. Srinivasa Murthy, E. Anil KumarGopakumarKuttappanNo ratings yet
- Mixtures Notes PDFDocument5 pagesMixtures Notes PDFKhaled ElsayedNo ratings yet
- LEAD ACETATE (Basic) ARDocument1 pageLEAD ACETATE (Basic) ARMimma afrinNo ratings yet
- CH 19 Chemical Handling and StorageDocument9 pagesCH 19 Chemical Handling and StoragedesosanNo ratings yet
- Final Appendix K Ageing TGX Sep 08Document30 pagesFinal Appendix K Ageing TGX Sep 08Perkresht PawarNo ratings yet
- 3 NomenclatureDocument45 pages3 Nomenclaturerusnah chungNo ratings yet
- Cryogenic Heat TreatmentDocument3 pagesCryogenic Heat Treatmentsri78770% (1)
- Catalysts v12 I12 - 20230622Document1 pageCatalysts v12 I12 - 20230622Editor IJDMNo ratings yet
Reduction of Vanillin To Vanillyl Alcohol.: Background
Reduction of Vanillin To Vanillyl Alcohol.: Background
Uploaded by
Hawra JawadOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Reduction of Vanillin To Vanillyl Alcohol.: Background
Reduction of Vanillin To Vanillyl Alcohol.: Background
Uploaded by
Hawra JawadCopyright:
Available Formats
Reduction of Vanillin to Vanillyl Alcohol.
Background
In this lab you will reduce vanillin (4-hydroxy-3-methoxybenzaldehyde) with sodium
borohydride (NaBH4) to produce vanillyl alcohol (4-hydroxy-3-methoxybenzyl alcohol),
scheme 1. After isolating the product, you will determine its melting point, determine the
isolated yield of the reaction and discuss how NMR may be used to verify the reaction.
O H OH
NaBH4
NaOH H3BO4
H2O
OCH3 OCH3
OH OH
Vanillin Vanillyl alcohol
M.W. = 152.14 g/mole M.W. = 154.16 g/mole
m.p. = 82 oC m.p. = 115 oC
Scheme 1.
Vanillin is an aromatic compound that can be isolated from the cured fruit of Vanilla
planifolia. It is mostly used in foods (killer French toast) and perfumes (Ode de Keibler).
However, most commercial vanilla flavoring is derived from the lignin of wood pulp,
yummm... We will be using vanillin as a starting material for the production of vanillyl
alcohol, a compound that can serve as a valuable intermediate in the production of novel
flavorings and perfumes. An excess of sodium borohydride, a convenient and mild
reducing agent, will be used as the reductant in this reaction. The experimental apparatus
and a list of reagents are provided in figure 1, below.
Procedure
1. Place 2g (13.2 mmol) of vanillin in a 25 mL round bottom flask followed by 4 mL
ethanol. Add a stir bar, clamp the flask above a stir plate and commence stirring at room
temperature to solublize the vanillin.
2. After the vanillin goes into solution, add an
REAGENTS ice bath under your flask to cool the solution.
25 mL round
bottom flask 1) Vanillin
2) Ethanol 3. In a separate reaction vial, dissolve 0.5 g
Ice bath
3) NaBH4 of NaBH4 in 3.8 mL of 1M NaOH.
4) 1M NaOH
Stir Plate 5) 6M HCl
4. Using a glass pipet, SLOWLY add the
6) pH paper
7) Ice cold water NaBH4 solution DROPWISE to the vanillin
Experimental solution over a period of 10 minutes. This is
Apparatus
an exothermic reaction, so be careful to add
Figure 1.
the NaBH4 solution slowly.
5. After the addition is complete, remove the ice bath and stir the resulting mixture for 10
minutes at room temperature.
6. Replace the ice bath to again cool the mixture. While stirring with the stir bar, add 6M
HCl DROPWISE until the evolution of H2 gas stops (you are decomposing the excess
NaBH4). Check the pH by removing a drop of the solution and adding it to a strip of pH
paper to make sure the reaction is acidic (do not dip the pH paper into the reaction).
7. Stir for an addition 10 minutes while cooling to allow the product to precipitate from
solution.
8. Collect the product by filtration using ice-cold water to aid the transfer of the material
to the filter funnel. Wash the product twice with ice-cold water and then transfer the
product to a dry piece of filter paper to air dry.
9. After your product has dried, weigh it to calculate the isolated yield and collect a
melting point.
10. Give your product to the TA for collection.
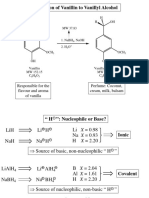
Pre-lab questions
1. Calculate the molarity of the NaBH4 solution that you prepared in step 3 above.
2. How many equivalents of hydride (H-) are you adding to vanillin in this reaction?
3. Please draw a reasonable mechanism for this reaction.
4. What is the pKa of the phenolic functional group of vanillin. Predict the protonation
state (e.g. is it protonated or deprotonated) of this functional group during the course of
this experiment e.g. when 1. vanillin is first dissolved in ethanol. 2. After the addition of
the NaBH4 solution. 3. During the decomposition of excess NaBH4. 4. After isolation by
filtration.
Post lab questions
1. How could you modify your procedure to increase your isolated yield.
2. How did your melting point compare to the reported value? If lower, what can you do
to increase the m.p.
O
3. Draw the NMR chemical shift splitting pattern (tree diagram) for
H Ha, Hb and Hc, labeling the appropriate coupling constants. Assume
Ha Hc Jbc = 1 Hz
Hb OCH3 4. If you had to use 1HNMR to verify that you actually synthesized
OH vanillyl alcohol from vanillin, describe at least two key differences in
the 1HNMR spectra of these two compounds that would support your
Vanillin
claim.
You might also like
- EnzymeDocument14 pagesEnzymeAthena Wars100% (1)
- Nitrogen Determination by Kjeldahl MethodDocument7 pagesNitrogen Determination by Kjeldahl MethodLinh VũNo ratings yet
- Williamson Ether Synthesis of An Expectorant - Guaifenesin - and Its Isolation From Guai-Aid Cough TabletsDocument8 pagesWilliamson Ether Synthesis of An Expectorant - Guaifenesin - and Its Isolation From Guai-Aid Cough TabletsAnna LuthfiahNo ratings yet
- Examiner Tips For O Level Chemistry 5070 FINALDocument10 pagesExaminer Tips For O Level Chemistry 5070 FINALMuhammad Bin Anis60% (5)
- Solutions To Crystallization ProblemsDocument5 pagesSolutions To Crystallization ProblemsmadhavanssnNo ratings yet
- Síntesis de Alcohol VainillílicoDocument6 pagesSíntesis de Alcohol VainillílicoYago L100% (1)
- Taller 2 FcoQca II 2022Document11 pagesTaller 2 FcoQca II 2022Natalia Petro RodríguezNo ratings yet
- Octyl AcetateDocument6 pagesOctyl AcetateKristine BautistaNo ratings yet
- Determination of The Differential Heat of SolutionDocument3 pagesDetermination of The Differential Heat of SolutionLoveFreequencyNo ratings yet
- Macrogol Cetostearyl EtherDocument1 pageMacrogol Cetostearyl EtherTeodor BoianovNo ratings yet
- Difusividad de Liquidos - PerryDocument3 pagesDifusividad de Liquidos - PerryGladys Ventura FloreNo ratings yet
- Tablas Alcoholimetricas BDocument3 pagesTablas Alcoholimetricas Bfgranobles100% (1)
- DMT Fumarate To FreebaseDocument3 pagesDMT Fumarate To Freebaseedmundo riveroNo ratings yet
- Acetanilida MSDSDocument5 pagesAcetanilida MSDStylerNo ratings yet
- Borohydride Reduction of Vanillin To Vanillyl AlcoholDocument8 pagesBorohydride Reduction of Vanillin To Vanillyl Alcoholandantino076657No ratings yet
- Extraction Organic Lab 3Document8 pagesExtraction Organic Lab 3jarissa bannerNo ratings yet
- Quantitative Reactions and Titrations ExperimentDocument5 pagesQuantitative Reactions and Titrations ExperimentJeremy BarrettNo ratings yet
- Experiment IV 143BDocument10 pagesExperiment IV 143BtrickkkpatNo ratings yet
- Preparation of B-NaphtholDocument4 pagesPreparation of B-NaphtholnithansaNo ratings yet
- Exp10 - Analysis of AspirinDocument7 pagesExp10 - Analysis of AspirinjemgeneNo ratings yet
- Experiment 4Document6 pagesExperiment 4Tèddy ÑawåNo ratings yet
- Common Ion EffectDocument2 pagesCommon Ion EffectRonnie AlfecheNo ratings yet
- Titration LabDocument2 pagesTitration LabAliayah RoweNo ratings yet
- Titration Lab 3Document2 pagesTitration Lab 3Aliayah RoweNo ratings yet
- KHP LabDocument5 pagesKHP LabSantino MusaNo ratings yet
- 25 Titration of VinegarDocument3 pages25 Titration of VinegarJuventie PrimastutiNo ratings yet
- Lab Report 3 Chm557Document9 pagesLab Report 3 Chm557Nur AqilahNo ratings yet
- Lab Report TitrationDocument7 pagesLab Report TitrationIanaNo ratings yet
- Experiment 30 - Volumetric Analysis: OBJECTIVE: To Become More Familiar With Volumetric Analysis. MaterialsDocument3 pagesExperiment 30 - Volumetric Analysis: OBJECTIVE: To Become More Familiar With Volumetric Analysis. MaterialsZari Sofia LevisteNo ratings yet
- Pre Lab: Example of Laboratory Write-Up - CHM 2201/2202Document5 pagesPre Lab: Example of Laboratory Write-Up - CHM 2201/2202Hawra JawadNo ratings yet
- Borohydride Reduction of Vanillin To Vanillyl AlcoholDocument6 pagesBorohydride Reduction of Vanillin To Vanillyl AlcoholHawra JawadNo ratings yet
- Lab 8 - Solutions and Titration 2023-1Document8 pagesLab 8 - Solutions and Titration 2023-12021155224No ratings yet
- Ach Lab ReportDocument6 pagesAch Lab ReportTiofelus H. HamutenyaNo ratings yet
- Acid-Base TitrationDocument7 pagesAcid-Base TitrationPok Wan SoonNo ratings yet
- Experiment # 01: Calculations Related To Organic ChemistryDocument12 pagesExperiment # 01: Calculations Related To Organic ChemistryAbdullah JavedNo ratings yet
- Lab Manual CMT450Document32 pagesLab Manual CMT450Mohamad Hanafi Bin AbdullahNo ratings yet
- ProcedureDocument3 pagesProcedureMadhav VenugopalanNo ratings yet
- Lab Manual FGS0074Document8 pagesLab Manual FGS0074hash117No ratings yet
- Standardization of NaohDocument3 pagesStandardization of NaohsadyaNo ratings yet
- Exp-1 Dye Synthesis Phenyl Azo B-Napthol DyeDocument6 pagesExp-1 Dye Synthesis Phenyl Azo B-Napthol DyeKhadija ShirgarNo ratings yet
- POC-II Tutorial MMP PDFDocument29 pagesPOC-II Tutorial MMP PDFmehuNo ratings yet
- Standardization of Acid and Base Solutions PDFDocument3 pagesStandardization of Acid and Base Solutions PDFKassim100% (1)
- GEMs ID90Document8 pagesGEMs ID90Andre PNo ratings yet
- Experiment Number 5Document16 pagesExperiment Number 5najjar119No ratings yet
- Titration Group 1Document4 pagesTitration Group 1brydonsantosNo ratings yet
- Synthesis of Benzilic Acid: Experiment 17Document9 pagesSynthesis of Benzilic Acid: Experiment 17Ayattolla KachingweNo ratings yet
- Acetophenone Reduction by Sodium BorohydrideDocument2 pagesAcetophenone Reduction by Sodium BorohydrideAravind Rao Karanam67% (3)
- Experiment: Title: ObjectiveDocument23 pagesExperiment: Title: Objectiveapi-3734333No ratings yet
- Standardization of A Strong Base NaOH WiDocument4 pagesStandardization of A Strong Base NaOH Wi232449045No ratings yet
- StandardizationDocument18 pagesStandardizationCHEA MICH L. ABELLANONo ratings yet
- Experiment 4 Preparation of Standardized SolutionsDocument10 pagesExperiment 4 Preparation of Standardized SolutionsJohn Dy100% (1)
- 07 Experiment 07 Salicylic Acid - Molecular Weight DeterminationDocument5 pages07 Experiment 07 Salicylic Acid - Molecular Weight DeterminationTaffazzul ShaukatNo ratings yet
- Chemistry Project Dheekshanya KumariDocument21 pagesChemistry Project Dheekshanya KumariDheekshith KumarNo ratings yet
- 2004 Chem 2OB3 Lab Manual - Experiment 4. Reactions of Carboxylic AcidsDocument4 pages2004 Chem 2OB3 Lab Manual - Experiment 4. Reactions of Carboxylic AcidsNstm3No ratings yet
- Project of Chemistry 11thDocument9 pagesProject of Chemistry 11thNitin ChandwaniNo ratings yet
- Ms Ijbpas 2021 Dec SPCL 2005Document5 pagesMs Ijbpas 2021 Dec SPCL 2005kannnan768402No ratings yet
- Experiment No. 3 Volumetric TransferDocument14 pagesExperiment No. 3 Volumetric TransferJoemar SubongNo ratings yet
- BP406P Medicinal Chemistry1Document30 pagesBP406P Medicinal Chemistry1Anit DubeyNo ratings yet
- LidocaineDocument6 pagesLidocaineg20kpNo ratings yet
- Titration - Dry Lab - 2020Document6 pagesTitration - Dry Lab - 2020MariaPaulaGonzalezRojasNo ratings yet
- Titration - Dry Lab - 2020Document6 pagesTitration - Dry Lab - 2020MariaPaulaGonzalezRojasNo ratings yet
- Cracking Chemistry - Titration 2 - NaOH Against Ethanoic AcidDocument3 pagesCracking Chemistry - Titration 2 - NaOH Against Ethanoic Acidmarco.chiappero.ieNo ratings yet
- Experiment 1: Preparation of 2-Iodobenzoic Acid From Anthranilic Acid (2-Amino Benzoic Acid)Document11 pagesExperiment 1: Preparation of 2-Iodobenzoic Acid From Anthranilic Acid (2-Amino Benzoic Acid)Sanjida Khandoker 1911009049No ratings yet
- Assim Exp 1Document87 pagesAssim Exp 1Hawra JawadNo ratings yet
- Experiment 1. Ketone Reduction by Sodium Borohydride: Butyrophenone and AcetophenoneDocument3 pagesExperiment 1. Ketone Reduction by Sodium Borohydride: Butyrophenone and AcetophenoneHawra JawadNo ratings yet
- Borohydride Reduction of Vanillin To Vanillyl AlcoholDocument6 pagesBorohydride Reduction of Vanillin To Vanillyl AlcoholHawra JawadNo ratings yet
- EXPERIMENT 7: Reduction of Carbonyl Compounds - Achiral and Chiral ReductionDocument13 pagesEXPERIMENT 7: Reduction of Carbonyl Compounds - Achiral and Chiral ReductionHawra JawadNo ratings yet
- Soduim Borohydride Reduction of CyclohexanoneDocument10 pagesSoduim Borohydride Reduction of CyclohexanoneHawra JawadNo ratings yet
- Assim ExpDocument10 pagesAssim ExpHawra JawadNo ratings yet
- Convenient Reduction of Carbonyl Compounds To Their Corresponding Alcohols With Nabh / (NH) C O SystemDocument7 pagesConvenient Reduction of Carbonyl Compounds To Their Corresponding Alcohols With Nabh / (NH) C O SystemHawra JawadNo ratings yet
- Pre Lab: Example of Laboratory Write-Up - CHM 2201/2202Document5 pagesPre Lab: Example of Laboratory Write-Up - CHM 2201/2202Hawra JawadNo ratings yet
- Stereochemistry of The Sodium Borohydride Reduction of BenzoinDocument8 pagesStereochemistry of The Sodium Borohydride Reduction of BenzoinHawra JawadNo ratings yet
- Reduction of Aldehydes Using Sodium Borohydride Under Ultrasonic IrradiationDocument4 pagesReduction of Aldehydes Using Sodium Borohydride Under Ultrasonic IrradiationHawra JawadNo ratings yet
- NorSaliyana 21 5 22Document7 pagesNorSaliyana 21 5 22Hawra JawadNo ratings yet
- Reduction of Aldehydes, Ketones, To: (1) Clemmsens MethodDocument9 pagesReduction of Aldehydes, Ketones, To: (1) Clemmsens MethodHawra JawadNo ratings yet
- ZN (BH) /2nacl: A Novel Reducing System For Efficient Reduction of Organic Carbonyl Compounds To Their Corresponding AlcoholsDocument8 pagesZN (BH) /2nacl: A Novel Reducing System For Efficient Reduction of Organic Carbonyl Compounds To Their Corresponding AlcoholsHawra JawadNo ratings yet
- SRI CO2 ManualDocument28 pagesSRI CO2 ManualChhomNo ratings yet
- Earth Science 4Document3 pagesEarth Science 4Sean CaloyloyNo ratings yet
- THERMIT-Presentasi Lengkap Thermit-Oxy LanceDocument14 pagesTHERMIT-Presentasi Lengkap Thermit-Oxy LanceFirdaus AdenNo ratings yet
- AIGA 090 - 14 - Reference Guide For Requalification of Gas CylindersDocument13 pagesAIGA 090 - 14 - Reference Guide For Requalification of Gas CylindersEmran UmerNo ratings yet
- Science 7 - Quarter 1 - Lesson 3 - Concentrations of SolutionsDocument72 pagesScience 7 - Quarter 1 - Lesson 3 - Concentrations of SolutionsTrisphere Media TechnologiesNo ratings yet
- Combustion of Gaseous and Liquid FuelsDocument2 pagesCombustion of Gaseous and Liquid FuelsLouie G Navalta0% (1)
- API 570 Point Recall 4Document3 pagesAPI 570 Point Recall 4Ravindra S. Jivani100% (2)
- 1.2 Assessed HomeworkDocument8 pages1.2 Assessed HomeworkNavine NavNo ratings yet
- TS STAB 34 Crude Oil Demulsification PDFDocument2 pagesTS STAB 34 Crude Oil Demulsification PDFMo OsNo ratings yet
- Alcohols, Phenols and Ethers (CBSE)Document25 pagesAlcohols, Phenols and Ethers (CBSE)Vishu Bansal100% (1)
- Studies The Mechanism of Action Of: 6-MercaptopurineDocument5 pagesStudies The Mechanism of Action Of: 6-MercaptopurineBelladonna Perdana PutraNo ratings yet
- Self-Healing CrackingDocument13 pagesSelf-Healing CrackingSujan NeupaneNo ratings yet
- ValenceDocument218 pagesValenceSubhabrata MabhaiNo ratings yet
- Alloy Design For Additive Manufacturing: Eric. A. JägleDocument43 pagesAlloy Design For Additive Manufacturing: Eric. A. JäglePopa Nicolea MirelaNo ratings yet
- Unit 7E Acids and Alkalis: Name: .Document21 pagesUnit 7E Acids and Alkalis: Name: .irene9tan9ailianNo ratings yet
- Advances in Replacements For Cadmium Plating in Aerospace ApplicationsDocument9 pagesAdvances in Replacements For Cadmium Plating in Aerospace ApplicationsNguyễn Ngọc SơnNo ratings yet
- The Extractive Metallurgy of South Africa's Platinum Ores: L.A. CramerDocument5 pagesThe Extractive Metallurgy of South Africa's Platinum Ores: L.A. CramerTeererai KaguraNo ratings yet
- Nano Emulsions Formation by Low Energy Methods PDFDocument9 pagesNano Emulsions Formation by Low Energy Methods PDFCarmen RaveNo ratings yet
- Thermal Analysis-Frying PanDocument12 pagesThermal Analysis-Frying PanAkshay BorateNo ratings yet
- Applied Thermal Engineering: S. Srinivasa Murthy, E. Anil KumarDocument14 pagesApplied Thermal Engineering: S. Srinivasa Murthy, E. Anil KumarGopakumarKuttappanNo ratings yet
- Mixtures Notes PDFDocument5 pagesMixtures Notes PDFKhaled ElsayedNo ratings yet
- LEAD ACETATE (Basic) ARDocument1 pageLEAD ACETATE (Basic) ARMimma afrinNo ratings yet
- CH 19 Chemical Handling and StorageDocument9 pagesCH 19 Chemical Handling and StoragedesosanNo ratings yet
- Final Appendix K Ageing TGX Sep 08Document30 pagesFinal Appendix K Ageing TGX Sep 08Perkresht PawarNo ratings yet
- 3 NomenclatureDocument45 pages3 Nomenclaturerusnah chungNo ratings yet
- Cryogenic Heat TreatmentDocument3 pagesCryogenic Heat Treatmentsri78770% (1)
- Catalysts v12 I12 - 20230622Document1 pageCatalysts v12 I12 - 20230622Editor IJDMNo ratings yet