Professional Documents
Culture Documents
The Anaerobic Oxidation of Hydrazine - A Novel Re
The Anaerobic Oxidation of Hydrazine - A Novel Re
Uploaded by
LuqmanOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
The Anaerobic Oxidation of Hydrazine - A Novel Re
The Anaerobic Oxidation of Hydrazine - A Novel Re
Uploaded by
LuqmanCopyright:
Available Formats
FEMS Microbiology Letters 158 (1998) 61^67
The anaerobic oxidation of hydrazine:
a novel reaction in microbial nitrogen metabolism
Jos Schalk a , Hege Oustad b , J. Gijs Kuenen a , Mike S.M. Jetten a; *
a
Kluyver Laboratory for Biotechnology, Delft University of Technology, Julianalaan 67, 2628 BC Delft, The Netherlands
b
Technical University of Trondheim, Department for Biotechnology, Trondheim, Norway
Received 14 October 1997; revised 28 October 1997; accepted 28 October 1997
Abstract
Hydrazine is rarely found as an intermediate in microbial nitrogen conversions. In this study the conversion of hydrazine by
the anaerobic ammonium oxidation (Anammox) culture, in which hydrazine has been proposed as an intermediate, was
investigated. This study demonstrated the biological nature of hydrazine conversion by the Anammox culture. In batch
cultures with hydrazine it was observed that 3 mol N2 H4 was disproportionated to 4 mol NH 4 and 1 mol N2 . Hydrazine with
nitrite as an electron acceptor showed a conversion of 3 mol N2 H4 and 4 mol NO3 2 to 5 mol N2 , with a specific activity of 5.5
nmol min31 (mg volatile suspended solids)31 . Addition of hydrazine to a biofilm reactor for 80 days showed that it was not
possible to grow Anammox with hydrazine.
ß 1998 Federation of European Microbiological Societies. Published by Elsevier Science B.V.
Keywords : Anaerobic ammonium oxidation; Hydrazine; Nitrogen removal ; Nitrite ; Bio¢lm reactor; Nitri¢cation
1. Introduction bic conditions, hydrazine can be converted to N2 via
a two-electron oxidation (N2 H4 CN2 H2 CN2 ) [1,3].
Hydrazine is a hazardous compound, which can In contrast to the numerous chemical reactions,
be readily oxidized. It is used as a rocket fuel, as hydrazine is rarely observed as an intermediate in
an antioxidant or as an intermediate for the produc- microbial nitrogen conversions. Hydrazine is pro-
tion of explosives and pesticides [1]. Hydrazine is a posed to be an enzyme-bound intermediate in the
strong reducing agent, which reacts easily with metal reduction of dinitrogen gas to ammonium by nitro-
ions [2]. In the presence of catalysts like Cu(II) and genase [4]. In Nitrosomonas europaea cells, hydrazine
phosphate ions hydrazine radicals are formed via can be used as an external energy source, e.g. for the
a one-electron oxidation, which ultimately are de novo synthesis of polypeptides [5]. Under anaero-
converted into ammonia and dinitrogen gas bic conditions hydrazine can be used as an electron
(2N2 H4 C2N2 H3 CN4 H6 CN2 +2NH3 . Under aero- donor for the reduction of nitrite to NO and N2 O
[6]. In addition, it was shown that the enzyme hy-
droxylamine oxidoreductase (HAO) from N. euro-
* Corresponding author. Tel.: +31 (15) 2781193; Fax: paea was capable of converting hydrazine to dinitro-
+31 (15) 2782355; E-mail: M.Jetten@STM.TUDelft.NL gen gas [7,8]. A di¡erent enzyme system, the R2
0378-1097 / 98 / $19.00 ß 1998 Federation of European Microbiological Societies. Published by Elsevier Science B.V.
PII S 0 3 7 8 - 1 0 9 7 ( 9 7 ) 0 0 5 0 1 - 6
FEMSLE 7929 5-1-98
62 J. Schalk et al. / FEMS Microbiology Letters 158 (1998) 61^67
2. Materials and methods
2.1. Origin of biomass and cultivation
An Anammox culture, grown in a £uidized bed
reactor on synthetic medium containing 45 mM am-
monium and nitrite, was used for batch culture and
continuous culture experiments. The £uidized bed
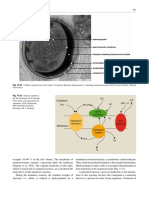
Fig. 1. Possible reaction mechanism for anaerobic ammonium
oxidation. Hydrazine (N2 H4 ) is formed by the oxidation of am-
reactor was fed with an anaerobic autotrophic syn-
monium with hydroxylamine. Hydrazine is then oxidized to dini- thetic medium [10]. Before use, the biomass was ho-
trogen generating four reduction equivalents, which can be used mogenized and washed with 20 mM anaerobic
for the reduction of nitrite to hydroxylamine [12]. KHCO3 bu¡er (pH 7.5).
Alcaligenes faecalis TUD (LMD 89.147), an or-
subunit of ribonucleotide reductase from Escherichia ganism capable of denitri¢cation and heterotrophic
coli, was shown to catalyze a disproportionation of nitri¢cation, was cultivated in an acetate-limited
hydrazine into dinitrogen gas and ammonium [9]. chemostat at 30³C and pH 8.0 with a dilution rate
Recently a novel process was discovered in which of 0.05 h31 [13]. Saccharomyces cerevisiae T23D was
hydrazine occurred as an intermediate. In this proc- grown aerobically in a glucose-limited chemostat
ess, called anaerobic ammonium oxidation (Anam- with a dilution rate of 0.1 h31 at 30³C and pH 5.0
mox), nitrite is reduced to dinitrogen gas with am- [14]. SHARON sludge was obtained from a labora-
monium as the electron donor [10]. Inhibition studies tory-scale-nitrifying reactor fed with synthetic me-
showed that this process had a biological nature [11]. dium [15].
It was possible to enrich the anaerobic ammonium
oxidizing microorganisms in an medium containing 2.2. Anaerobic batch culture experiments
ammonium, nitrite and bicarbonate as the sole car-
bon source. Based on results from 15 N-labelling stud- Anaerobic batch cultures were grown in 50 ml
ies a novel pathway for the Anammox process has serum bottles at 30³C. Each bottle contained 10 ml
been proposed [12]. In this reaction mechanism am- of 20 mM KHCO3 bu¡er (pH 7.5) and approxi-
monium and hydroxylamine are combined to form mately 20 mg volatile suspended solids (VSS) of
hydrazine. Hydrazine itself is then converted to dini- Anammox biomass, except for the incubation with
trogen gas, generating four reduction equivalents. It hydrazine alone, when approximately 75 mg VSS
is postulated that these reduction equivalents are of Anammox was used. The bottles sealed with
used for the reduction of nitrite to hydroxylamine, butyl-rubber stoppers were made anaerobic with
as shown in Fig. 1 [12]. an Argon/CO2 (95/5%) mixture. Hydrazine
Since hydrazine is a strong reductant and was ob- (H4 N2 WH2 SO4 ), ammonium ((NH4 )2 SO4 ), nitrite
served as an intermediate in the Anammox process, (NaNO2 ), and nitrate (NaNO3 ) solutions were sub-
we investigated whether the Anammox culture is sequently added with a syringe. N2 O was added in
able to use hydrazine as a substrate for growth. In the headspace. The initial NH 3
4 , N2 H4 , NO2 , NO3
3
order to gain more knowledge about the physiology and N2 O concentrations were approximately 3 mM.
of the hydrazine metabolism in Anammox, the re- The pH was adjusted to 7.5 with 1 M Na2 CO3 . Con-
search was focussed on three topics. The ¢rst aim trol experiments without biomass were performed
was to con¢rm that the hydrazine conversions in simultaneously.
Anammox were of a biological nature. The second For the experiments with gamma-irradiated cells,
topic was to investigate the hydrazine conversions by 50 ml serum bottles with Anammox sludge, nitrify-
the Anammox culture in batch experiments with and ing sludge, A. faecalis and S. cerevisiae cells were
without additional electron acceptors. Finally, the exposed to 25 kGy of radiation for 7 h using a
57
capability of Anammox to grow on hydrazine in Co source (Gammaster, Ede, The Netherlands),
bio¢lm reactors was investigated. before use [11].
FEMSLE 7929 5-1-98
J. Schalk et al. / FEMS Microbiology Letters 158 (1998) 61^67 63
2.3. Bio¢lm reactors with hydrazine Instruments, Interscience, Breda, The Netherlands)
equipped with a thermal conductivity detector and
2.3.1. Operation of the bio¢lm reactors an electron capture detector [13]. Hydrazine was de-
Two small bio¢lm reactors (height 25 cm, volume termined colorimetrically by means of the method
330 ml) were operated at 30³C for 10 weeks using an described by Watt and Crisp [17]. Dry weight was
anaerobic autotrophic mineral medium [10]. The pH determined as previously described [16].
of the medium was kept constant at pH 7.5 by £ush-
ing continuously with an Argon/CO2 (95/5%) mix-
ture during the experiment [16]. Both reactors were 3. Results
inoculated with approximately 0.25 g VSS of Anam-
mox biomass. To establish a bio¢lm the feed of am- 3.1. Determination of the biological nature of
monium and nitrite was increased in a stepwise man- hydrazine conversion in Anammox
ner from 2 to 10 mM at a £ow rate of 0.16 ml min31 .
After 3 weeks, 1 mM of ammonium was replaced by Batch experiments with active cells and cells inac-
0.1 mM hydrazine in one of the reactors. The hydra- tivated by Q-radiation from four di¡erent microor-
zine was supplied from a separate bottle to avoid ganisms with hydrazine and nitrite as substrates
chemical decomposition. When the hydrazine con- were performed to determine if hydrazine conversion
centration fell below the detection limit (1 WM), the was of a biological nature. No signi¢cant conversion
in£owing concentration was increased stepwise, of hydrazine was observed in three batch experi-
reaching 0.9 mM at day 70. The second bio¢lm re- ments with cells inactivated by Q-radiation. The in-
actor was used as a control, and was fed with 10 mM activated Anammox sludge showed low activity com-
of ammonium and nitrite. pared to active Anammox cells. Active A. faecalis
and S. cerevisiae were also not able to convert hy-
2.3.2. Activity measurements in the bio¢lm reactors drazine and nitrite (Table 1). On the other hand,
To determine the activity of the Anammox bio- Anammox showed high conversion of hydrazine
mass in both reactors, the reactors were run in batch and nitrite, and the nitrifying sludge showed low
mode for 6 h with ammonium and nitrite. The initial conversion of hydrazine and nitrite under anaerobic
concentration of both ammonium and nitrite was conditions. The hydrazine conversion rate of the
6 mM. Samples were collected at appropriate time Anammox culture was approximately 10-fold higher
intervals and analyzed for the di¡erent nitrogen than the nitrifying sludge.
compounds.
3.2. Anaerobic batch culture experiments with
2.4. Analytical procedures hydrazine
Nitrate, nitrite, hydroxylamine and ammonium Since Anammox showed a high hydrazine conver-
were determined colorimetrically as previously de- sion, the oxidation of hydrazine with and without
scribed [11]. Nitrous oxide and dinitrogen gas forma- nitrite, nitrate and nitrous oxide as electron accept-
tion were quanti¢ed using a GC 8340 model (Fisons ors was investigated in anaerobic batch experiments.
Table 1
Conversion rates (nmol min31 (mg VSS)31 ) of hydrazine and nitrite by active and Q-radiated inactive cells from di¡erent sources in batch
cultures
Inactive cells after Q-radiation Active cells
N2 H4 NO3
2 N2 H4 NO3
2
A. faecalis 6 0.01 6 0.01 6 0.01 6 0.01
S. cerevisiae 6 0.01 6 0.01 6 0.01 6 0.01
Nitrifying sludge 6 0.10 6 0.01 0.47 1.80
Anammox sludge 0.39 0.19 4.23 5.46
FEMSLE 7929 5-1-98
64 J. Schalk et al. / FEMS Microbiology Letters 158 (1998) 61^67
Fig. 2. The conversion of hydrazine in a batch culture with an Fig. 3. The conversion of hydrazine and nitrite in a batch culture
Anammox culture (closed symbols) and without Anammox (open by the Anammox culture. Symbols : (b) N2 H4 ; (R) NO3 2 ; (F)
symbols). Symbols : (b and a) N2 H4 ; (F and E) NH
4. NH4.
Each experiment was repeated at least twice. The any compound was observed during such experi-
standard deviation was not more than 25%. When ments.
the culture was provided with hydrazine alone, am-
monium was formed (Fig. 2). The conversion rate 3.3. Bio¢lm reactors with hydrazine
for hydrazine was 1.6 nmol min31 (mg VSS)31 and
for the ammonium formation a rate of 1.8 nmol After the short-term batch experiments, the long-
min31 (mg VSS)31 was calculated. Incubations with term e¡ect of hydrazine on the Anammox culture
hydrazine and nitrite as the electron acceptor showed was studied. For this purpose anaerobic bio¢lm re-
a rapid conversion of both nitrite and hydrazine actors were operated for 80 days with the Anammox
(Fig. 3). When all the nitrite was consumed, hydra- culture. The reactors were ¢rst fed with ammonium
zine was disproportionated into ammonium and di- and nitrite to produce su¤cient biomass as bio¢lm.
nitrogen gas. No hydroxylamine, nitrous oxide or In£owing NH 3
4 and NO2 concentrations were in-
nitrate was detected. The rates of nitrite and hydra- creased from 2 to 10 mM in 20 days. At that time
zine conversion were 4.2 and 5.5 nmol min31 (mg the NH 3
4 and NO2 removal rates of the Anammox
VSS)31 , respectively. Nitrate and nitrous oxide could bio¢lm was 9.0 and 9.5 WM min31 at day 20, respec-
also serve as electron acceptors (not shown). Since it tively (Table 2), leaving some residual ammonium,
was shown that hydrazine reacts easily with metal but no nitrite (Fig. 4). At day 23, 1 mM NH 4 was
ions [2], control experiments without sludge were replaced by 0.1 mM N2 H4 and increased in a step-
performed. No spontaneous chemical conversion of wise manner up to 0.9 mM in 10 days. In the ¢rst 20
Table 2
Anammox activity (WM min31 ) in a bio¢lm reactor, fed with 10 mM NH 3
4 and 10 mM NO2 before and after the addition of N2 H4
Day N2 H4 in (mM) N2 H4 out (mM) Activity (WM min31 )
NH
4 conversion NO3
2 conversion NO3
3 formation
20 0 0 9.0 9.5 1.5
43 0.8 0 N.D. N.D N.D
70 0.9 0.1 6.0 11.0 0.7
80 0.9 0.2 1.2 1.7 ^*
The Anammox activity in the bio¢lm reactors was measured in batch mode for 6 h with ammonium and nitrite. The initial concentration of
both ammonium and nitrite was 6 mM. Hydrazine was added at day 23.
N.D., not determined.
*Below detection limit.
FEMSLE 7929 5-1-98
J. Schalk et al. / FEMS Microbiology Letters 158 (1998) 61^67 65
Fig. 4. The e¡ect of hydrazine on the anaerobic ammonium oxidation in a bio¢lm reactor. The bio¢lm reactor was fed with 10 mM NH
4
and NO3 3
2 . N2 H4 was added at day 23. Symbols : (b) N2 H4 in; (a) N2 H4 out; (O) NO2 out; (E) NH4 out.
days after the hydrazine addition, the bio¢lm was ferent microorganisms with hydrazine and nitrite
able to convert and tolerate increasing amounts of showed that Anammox was able to convert hydra-
hydrazine (100% conversion, Fig. 4). Conversions of zine biologically (Table 1). A. faecalis and S. cerevi-
ammonium and nitrite did not change signi¢cantly. siae did not show any conversion of hydrazine and
From day 45 the hydrazine and ammonium conver- nitrite. The nitrifying sludge, on the other hand,
sion rate decreased. The ammonium removal, which showed some hydrazine conversion. This could be
is correlated to the Anammox activity, dropped to explained by the presence of about 70% of Nitroso-
6.0 WM min31 at day 70, while the nitrite conversion monas bacteria in the nitrifying sludge (Jetten, un-
increased up to 11.0 WM min31 (Table 2). A very published results). Nitrosomonas contains a large
small amount of nitrous oxide was detected. Activity amount of hydroxylamine oxidoreductase (HAO),
measurements at day 80 showed a greatly reduced an enzyme catalyzing the oxidation of hydroxyl-
capacity for both ammonium and nitrite removal. amine to nitrite. This enzyme can also convert hy-
Apparently, in spite of its capability to convert hy- drazine to dinitrogen gas [7,8]. However, the conver-
drazine, the (mixed) Anammox culture cannot be sion rate of hydrazine by the nitrifying sludge was
grown on hydrazine alone. A second attempt to about ten times lower than the rate observed in the
grow Anammox in a bio¢lm reactor on hydrazine Anammox culture (Table 1). A. faecalis, being a het-
also failed. The control reactor, which was fed with erotrophic nitri¢er with HAO activity, seems not
10 mM NH 3
4 and NO2 performed constantly during capable of oxidizing hydrazine, in contrast to the
the whole experiment. autotrophic nitri¢er N. europaea. The low conversion
of hydrazine observed in the batch experiment with
Q-radiated inactive Anammox culture may be caused
4. Discussion by incomplete inactivation of the hydrazine-convert-
ing enzyme, since a small amount of ammonium was
In microbial nitrogen conversions hydrazine is produced. Chemical decomposition of hydrazine was
rarely observed as an intermediate. Previously, batch not observed in the batch experiments [1], because
experiments with Anammox sludge showed that hy- the hydrazine concentration in the batch experiments
drazine accumulated when the culture was fed with with A. faecalis and S. cerevisiae and in the batch
hydroxylamine [12]. However, it was not known if experiments without cells did not decrease (Table 1).
this phenomenon had a biological nature. The ex- The Anammox culture was also capable to convert
periments described in this article with active cells hydrazine in the absence of other electron acceptors
and cells inactivated with Q-radiation from four dif- (Fig. 2). These results con¢rmed observations made
FEMSLE 7929 5-1-98
66 J. Schalk et al. / FEMS Microbiology Letters 158 (1998) 61^67
earlier in our laboratory [12]. According to the con- possible that an enzyme similar to that observed in
version rate of hydrazine and formation rate of am- the R2 subunit of ribonucleotide reductase from E.
monium the following equation could be derived: coli with a dinuclear iron centre is present in Anam-
3N2 H4 +4H C4NH4 +N2 . The disproportionation mox, or an enzyme similar to that of HAO from N.
of hydrazine to ammonium and dinitrogen was europaea. More studies need to be performed to
also found for the R2 subunit of the enzyme ribonu- solve the mechanism of hydrazine conversion in
cleotide reductase from E. coli [9]. In batch experi- Anammox.
ments with hydrazine and nitrite a diauxic substrate
conversion was observed (Fig. 3). Nitrite was ¢rst
reduced with hydrazine as the electron donor. After References
all the nitrite was converted, disproportionation of
hydrazine to ammonium and dinitrogen was ob- [1] Moliner, A.M. and Street, J.J. (1989) Decomposition of hy-
served, as shown in Fig. 2. According to the conver- drazine in aqueous solutions. J. Environ. Qual. 18, 483^
sion rates of hydrazine and nitrite during the ¢rst 487.
[2] Demadis, K.D., Malinak, S.M. and Coucouvanis, D. (1996)
5 h the following equation could be derived:
Catalytic reduction of hydrazine to ammonia with MoFe3 S4 -
4NO3
2 4H 3N2 H4 !5N2 8H2 O. To resolve polycarboxylate clusters. Possible relevance regarding the
the possible mechanism by which hydrazine is con- function of the molybdenum-coordinated homocitrate in ni-
verted with and without additional electron accept- trogenase. Inorg. Chem. 35, 4038^4046.
ors, 15 N-labelling studies should provide more infor- [3] Sykes, A.G. (1970) Some reactions of ions and molecules of
non-metallic elements in aqueous solutions. In: Kinetics of
mation.
Inorganic Reactions, 2nd Edn., pp. 195^197. Pergamon, Ox-
The disproportionation of hydrazine to ammo- ford, UK.
nium and dinitrogen, as well as the conversion of [4] Dilworth, M.J. and Eady, R.R. (1991) Hydrazine is a product
hydrazine and nitrite are thermodynamically favour- of dinitrogen reduction by the vanadium-nitrogenase from
able reactions (vGo P = 3181 kJ and 3311 kJ per mol Azotobacter chroococcum. Biochem. J. 277, 465^468.
[5] Hyman, M.R. and Arp, D.J. (1995) E¡ects of ammonia on the
N2 H4 , respectively). Therefore the capability of the
de novo synthesis of polypeptides in cells of Nitrosomonas
Anammox culture to grow on hydrazine was tested. europaea denied ammonia as an energy source. J. Bacteriol.
This long-term e¡ect was studied with bio¢lm reac- 177, 4974^4979.
tors. During the ¢rst 20 days after the addition of [6] Remde, A. and Conrad, R. (1990) Production of nitric oxide
hydrazine it was observed that the Anammox culture in Nitrosomonas europaea by reduction of nitrite. Arch. Mi-
crobiol. 154, 187^191.
was able to convert and tolerate 1 mM hydrazine
[7] Nicholas, D.J.D. and Jones, O.T.G. (1960) Oxidation of hy-
(Fig. 4). The results from the activity measurements droxylamine in cell-free extracts of Nitrosomonas europaea.
(Table 2) showed that the Anammox activity de- Nature 185, 512^514.
creased according to the rate of ammonium removal, [8] Hooper, A.B. and Terry, K.R. (1977) Hydroxylamine oxido-
while the rate of nitrite conversion increased. The reductase from Nitrosomonas: Inactivation by hydrogen per-
oxide. Biochemistry 16, 455^459.
increase in nitrite conversion is probably due to the
[9] Han, J.Y., Swarts, J.C. and Sykes, A.G. (1996) Kinetic studies
increase of denitri¢cation at the expense of decaying on the hydrazine and phenylhydrazine reductions of the Es-
biomass. Since denitri¢cation is a faster process than cherichia coli R2 subunit of ribonucleotide reductase. Inorg.
the Anammox process [18], this will result in a lower Chem. 35, 4629^4634.
Anammox activity, as was observed after 80 days [10] van de Graaf, A.A., de Bruijn, P., Robertson, L.A., Jetten,
M.S.M. and Kuenen, J.G. (1996) Autotrophic growth of anae-
(Table 2 and Fig. 4). Thus it is concluded that in
robic, ammonium-oxidizing micro-organisms in a £uidized
the long run hydrazine was toxic for the Anammox bed reactor. Microbiology 142, 2187^2196.
culture, as was observed for most forms of life [1]. [11] van de Graaf, A.A., Mulder, A., de Bruijn, P., Jetten, M.S.M.,
Although Anammox could not grow on hydrazine, Robertson, L.A. and Kuenen, J.G. (1995) Anaerobic oxida-
the enzyme responsible for the conversion of hydra- tion of ammonium is a biologically mediated process. Appl.
Environ. Microbiol. 61, 1246^1251.
zine is still very interesting. Whether one enzyme is
[12] van de Graaf, A.A., de Bruijn, P., Robertson, L.A., Jetten,
responsible for the disproportionation of hydrazine M.S.M. and Kuenen, J.G. (1997) Metabolic pathway of anae-
into dinitrogen gas and ammonium or two di¡erent robic ammonium oxidation on basis of 15 N-studies in a £uid-
enzymes are involved needs to be investigated. It is ized bed reactor. Microbiology 143, 2415^2421.
FEMSLE 7929 5-1-98
J. Schalk et al. / FEMS Microbiology Letters 158 (1998) 61^67 67
[13] Otte, S., Grobben, N.G., Robertson, L.A., Jetten, M.S.M. M.S.M. (1997) Ammonium removal from concentrated waste
and Kuenen, J.G. (1996) Nitrous oxide production by Alcali- streams with the Anaerobic Ammonium Oxidation (Anam-
genes faecalis under transient and dynamic aerobic and anae- mox) process in di¡erent reactor con¢gurations. Water Res.
robic conditions. Appl. Environ. Microbiol. 62, 2421^2426. 31, 1955^1962.
[14] de Jong-Gubbels, P., Vanrolleghem, P., Heijnen, S., van Dij- [17] Watt, G.W. and Chrisp, J.D. (1952) A spectrophotometric
ken, J.P. and Pronk, J.T. (1995) Regulation of carbon metab- method for the determination of hydrazine. Anal. Chem. 24,
olism in chemostat cultures of Saccharomyces cerevisiae grown 2006^2008.
on mixtures of glucose and ethanol. Yeast 11, 407^418. [18] Jetten, M.S.M., Logemann, S., Muyzer, G., Robertson, L.A.,
[15] Jetten, M.S.M., Horn, S.J. and van Loosdrecht, M.C.M. de Vries, S., van Loosdrecht, M.C.M. and Kuenen, J.G.
(1997) Towards a more sustainable municipal wastewater (1996) Novel principles in the microbial conversion of nitro-
treatment system. Water Sci. Tech. 35, 171^180. gen compounds. Antonie van Leeuwenhoek Int. J. Gen. Mol.
[16] Strous, M., van Gerven, E., Ping, Z., Kuenen, J.G. and Jetten, Microbiol. 71, 75^93.
FEMSLE 7929 5-1-98
You might also like
- What A Waste Rubbish Recycling and Protecting Our Planet - Jess French PDFDocument72 pagesWhat A Waste Rubbish Recycling and Protecting Our Planet - Jess French PDFEverything About Youtube100% (6)
- Chapter 12 - Sh. Based Payments (Part 1)Document6 pagesChapter 12 - Sh. Based Payments (Part 1)Xiena100% (1)
- Exegetical Dictionary of The New TestamentDocument4 pagesExegetical Dictionary of The New Testamentmung_khat0% (2)
- Eco ChicDocument19 pagesEco Chicoananichi7386No ratings yet
- Artículo DHPDocument10 pagesArtículo DHPEfraínNo ratings yet
- Journal of Molecular Catalysis A: ChemicalDocument7 pagesJournal of Molecular Catalysis A: ChemicalHuỳnh JKesorNo ratings yet
- Catalysis Letters 10.1007s10562-012-0829-xDocument8 pagesCatalysis Letters 10.1007s10562-012-0829-xyokeshNo ratings yet
- Kinetic Analysis of Nitrophenol Reduction and Colourimetric Detection ofDocument12 pagesKinetic Analysis of Nitrophenol Reduction and Colourimetric Detection ofcalormdpNo ratings yet
- Homoacetogenesis As The Alternative Pathway For HDocument7 pagesHomoacetogenesis As The Alternative Pathway For HProfessor Douglas TorresNo ratings yet
- Colorimetric Analysis of Captopril On The Basis of Its Free Radical Scavenger Character With Carbon Nanozymes As CatalystDocument8 pagesColorimetric Analysis of Captopril On The Basis of Its Free Radical Scavenger Character With Carbon Nanozymes As CatalystEl Idrissi MoulayNo ratings yet
- Completely Autotrophic Nitrogen Removal Over Nitrite in One Single Reactor - 2002 - Water ResearchDocument8 pagesCompletely Autotrophic Nitrogen Removal Over Nitrite in One Single Reactor - 2002 - Water ResearchAlvaro HueteNo ratings yet
- Changiz Karami, Keivan Ghodrati, Mina Izadi, Azita Farrokh, Sedigheh Jafari, Maryam Mahmoudiyani, and Nahid HaghnazariDocument4 pagesChangiz Karami, Keivan Ghodrati, Mina Izadi, Azita Farrokh, Sedigheh Jafari, Maryam Mahmoudiyani, and Nahid HaghnazariMiriam GarciaNo ratings yet
- MoradiDocument6 pagesMoradiDarian HerascuNo ratings yet
- 1 Methylimidazolium Di2 Ethylhexyl Phosphate As Ionic Liquid in Dysprosium Extraction From Nitrate MediumDocument10 pages1 Methylimidazolium Di2 Ethylhexyl Phosphate As Ionic Liquid in Dysprosium Extraction From Nitrate MediumHaseeb ChaudharyNo ratings yet
- Fed-Batch Fermentation For Production of Nitrile Hydratase by Rhodococcus Rhodochrous M33Document7 pagesFed-Batch Fermentation For Production of Nitrile Hydratase by Rhodococcus Rhodochrous M33Nòng Nọc Cô ĐơnNo ratings yet
- (Fe) - Hydrogenases in Green AlgaeDocument9 pages(Fe) - Hydrogenases in Green AlgaejafallavollitaNo ratings yet
- Subhedar 2018Document10 pagesSubhedar 2018Angélica Andrea SalinasNo ratings yet
- Baybars Ali Fil, and Mustafa Korkmaz, Cengiz ÖzmetinDocument6 pagesBaybars Ali Fil, and Mustafa Korkmaz, Cengiz ÖzmetinAmmr MahmoodNo ratings yet
- 10 1002@ejoc 202001132Document20 pages10 1002@ejoc 202001132Zina ZinaNo ratings yet
- Adsorption2 PDFDocument8 pagesAdsorption2 PDFJaberNo ratings yet
- Article 8 JSCIENCES - Volume 27 - Issue 2 - Pages 119-127Document9 pagesArticle 8 JSCIENCES - Volume 27 - Issue 2 - Pages 119-127fatimazahraNo ratings yet
- Efficient and Convenient Oxidation of AlcoholsDocument5 pagesEfficient and Convenient Oxidation of AlcoholsMedNo ratings yet
- Ereprint OCAIJ 338Document5 pagesEreprint OCAIJ 338Noemi LopezNo ratings yet
- Islam2011 PDFDocument8 pagesIslam2011 PDFOussamaNeharNo ratings yet
- Drozdov LimoneneDocument7 pagesDrozdov LimoneneElizaveta SeleznevaNo ratings yet
- Solvent Free Green Synthesis of 5-Arylidine Barbituric Acid Derivatives Catalyzed by Copper Oxide NanoparticlesDocument6 pagesSolvent Free Green Synthesis of 5-Arylidine Barbituric Acid Derivatives Catalyzed by Copper Oxide NanoparticlesbaskhemNo ratings yet
- Phenanthrene HydrogenationDocument7 pagesPhenanthrene HydrogenationVishal GoswamiNo ratings yet
- Patsula 2016Document10 pagesPatsula 2016Nandya AristaNo ratings yet
- ZrO2 Paper PDFDocument6 pagesZrO2 Paper PDFANANDAKUMAR B.SNo ratings yet
- MiniRevOrgChem2015 127 PDFDocument23 pagesMiniRevOrgChem2015 127 PDFShashank SinghNo ratings yet
- Environmental Microbiology - 4Document22 pagesEnvironmental Microbiology - 4Cathe RodriguezNo ratings yet
- Degradation of Metronidazole From Aqueous Environment Using Hydrothermally Synthesized ZN o N Doped ZN o and ZN o Ac NanoparticlesDocument13 pagesDegradation of Metronidazole From Aqueous Environment Using Hydrothermally Synthesized ZN o N Doped ZN o and ZN o Ac NanoparticlesHadi jameel hadiNo ratings yet
- Production of Aldehyde Oxidases by Microorganisms and Their Enzymatic PropertiesDocument6 pagesProduction of Aldehyde Oxidases by Microorganisms and Their Enzymatic Propertiesapi-3743140No ratings yet
- Research Article: Trifluoromethanesulfonic Anhydride in Amide Activation: A Powerful Tool To Forge Heterocyclic CoresDocument9 pagesResearch Article: Trifluoromethanesulfonic Anhydride in Amide Activation: A Powerful Tool To Forge Heterocyclic Coressinxcosx75No ratings yet
- Competitive Biosorptive Removal of A Basic Dye From Ternary Dye Mixture Using SawdustDocument9 pagesCompetitive Biosorptive Removal of A Basic Dye From Ternary Dye Mixture Using Sawdustsagar dasguptaNo ratings yet
- 1 ST Published PaperDocument13 pages1 ST Published PaperVikram PanditNo ratings yet
- NN 101400013 20111220 055413Document9 pagesNN 101400013 20111220 055413edvin2012No ratings yet
- Jhet 44 1223Document8 pagesJhet 44 1223jason fiasonNo ratings yet
- Dimethylformamide: Purification, Tests For Purity and Physical ProperliesDocument7 pagesDimethylformamide: Purification, Tests For Purity and Physical ProperliesAttique AlviNo ratings yet
- Alizadeh Et Al. - 2012 - Biguanide-Functionalized Fe3O4SiO2 Magnetic Nanoparticles An Efficient Heterogeneous Organosuperbase CatalystDocument7 pagesAlizadeh Et Al. - 2012 - Biguanide-Functionalized Fe3O4SiO2 Magnetic Nanoparticles An Efficient Heterogeneous Organosuperbase Catalystcukaasam123456No ratings yet
- Report 4Document36 pagesReport 4gurumeetNo ratings yet
- Jbacter00357 0034Document9 pagesJbacter00357 0034Zineb SkaliNo ratings yet
- Global Impact - AnammoxDocument5 pagesGlobal Impact - AnammoxPhuNo ratings yet
- New Microsoft Word DocumentDocument6 pagesNew Microsoft Word Documentlubna84No ratings yet
- Solventless Green ApproachDocument6 pagesSolventless Green Approachintata 24No ratings yet
- Review On: Synthesis, Chemistry and Therapeutic Approaches of Imidazole DerivativesDocument17 pagesReview On: Synthesis, Chemistry and Therapeutic Approaches of Imidazole DerivativesMustafa KhatabiNo ratings yet
- (Doi 10.1055 - s-0029-1218827) H. Veisi - Direct Oxidative Conversion of Alcohols, Amines, Aldehydes, and Benzyl Halides Into The Corresponding Nitriles With TrichloroisoDocument5 pages(Doi 10.1055 - s-0029-1218827) H. Veisi - Direct Oxidative Conversion of Alcohols, Amines, Aldehydes, and Benzyl Halides Into The Corresponding Nitriles With TrichloroisoDarian HerascuNo ratings yet
- H2 BatchDocument6 pagesH2 BatchAlejandra LopezNo ratings yet
- JPST - Volume 4 - Issue 2 - Pages 111-117Document7 pagesJPST - Volume 4 - Issue 2 - Pages 111-117Utibe basseyNo ratings yet
- Wang 2017Document9 pagesWang 2017Кирило КириченкоNo ratings yet
- Research Article Electrochemical Determination of Triclosan Using ZIF-11/Activated Carbon Derived From The Rice Husk Modified ElectrodeDocument14 pagesResearch Article Electrochemical Determination of Triclosan Using ZIF-11/Activated Carbon Derived From The Rice Husk Modified ElectrodeVõ Thắng NguyênNo ratings yet
- Journal of Molecular StructureDocument7 pagesJournal of Molecular StructureMiaHusnaNo ratings yet
- s10562 019 02678 XDocument13 pagess10562 019 02678 XYoki YulizarNo ratings yet
- Enantiopure Drug Synthesis: From Methyldopa To Imipenem To EfavirenzDocument11 pagesEnantiopure Drug Synthesis: From Methyldopa To Imipenem To EfavirenzNelson Daniel Guio PatiñoNo ratings yet
- An Efficient Conversion of Alcohols To Alkyl Bromides Using Pyridinium Based Ionic Liquids: A Green Alternative To Appel ReactionDocument5 pagesAn Efficient Conversion of Alcohols To Alkyl Bromides Using Pyridinium Based Ionic Liquids: A Green Alternative To Appel ReactionananNo ratings yet
- Synthesis & Characterization of Quinoxalines Chapter-4Document29 pagesSynthesis & Characterization of Quinoxalines Chapter-4Prasada Rao Ch MMNo ratings yet
- Nghiên Cứu Sử Dụng Zif-8 Làm Xúc Tác Cho Phản Ứng Alkyl HóaDocument11 pagesNghiên Cứu Sử Dụng Zif-8 Làm Xúc Tác Cho Phản Ứng Alkyl Hóathanhsieu92No ratings yet
- A New Reagent To Access Methyl SulfonesDocument10 pagesA New Reagent To Access Methyl Sulfonessaravana kumar.cNo ratings yet
- Complex Formation Between Iron (III) and Isonicotinohydroxamic Acid and Its Microbial Studies A.O. Aliyu and J.N. NwabuezeDocument8 pagesComplex Formation Between Iron (III) and Isonicotinohydroxamic Acid and Its Microbial Studies A.O. Aliyu and J.N. NwabuezeGINA PAOLA BERRÍO JULIONo ratings yet
- Naeimi 2015Document22 pagesNaeimi 2015Rushikesh G ParitNo ratings yet
- J. Biol. Chem.-1916-Harding-319-35 PDFDocument18 pagesJ. Biol. Chem.-1916-Harding-319-35 PDFDev AgarwalNo ratings yet
- 10 Chapter2Document36 pages10 Chapter2gurumeetNo ratings yet
- The Direct Catalytic Asymmetric Mannich Reaction: Armando Co Ä RdovaDocument11 pagesThe Direct Catalytic Asymmetric Mannich Reaction: Armando Co Ä RdovaiqbalardiwibowoNo ratings yet
- Iridium Complexes in Organic SynthesisFrom EverandIridium Complexes in Organic SynthesisLuis A. OroNo ratings yet
- Cost and Benefit in HSE: A Model For Calculation of Cost-Benefit Using Incident PotentialDocument8 pagesCost and Benefit in HSE: A Model For Calculation of Cost-Benefit Using Incident PotentialLuqmanNo ratings yet
- Ace The Interview - Candidate Interview PrepDocument1 pageAce The Interview - Candidate Interview PrepLuqmanNo ratings yet
- Sayler's Suncoast Water: Your One-Stop Water Treatment Shop in St. PetersburgDocument2 pagesSayler's Suncoast Water: Your One-Stop Water Treatment Shop in St. PetersburgLuqmanNo ratings yet
- Chlor Alkali Process: Membrane ElectrolysisDocument18 pagesChlor Alkali Process: Membrane ElectrolysisLuqmanNo ratings yet
- Visual Models of Plants Interacting With Their EnvironmentDocument15 pagesVisual Models of Plants Interacting With Their EnvironmentLuqmanNo ratings yet
- LAWM Prairies 5 ENDocument60 pagesLAWM Prairies 5 ENLuqmanNo ratings yet
- Spatial Temporal Models For Ambient Hourly PM in VancouverDocument18 pagesSpatial Temporal Models For Ambient Hourly PM in VancouverLuqmanNo ratings yet
- Sustainability 10 04668Document22 pagesSustainability 10 04668LuqmanNo ratings yet
- Environmental Modelling: (ENGO 583/ENEN 635)Document4 pagesEnvironmental Modelling: (ENGO 583/ENEN 635)LuqmanNo ratings yet
- ENGO 583/ENEN 635 Environmental Modelling: Modelling Project Presentation Evaluation FormDocument1 pageENGO 583/ENEN 635 Environmental Modelling: Modelling Project Presentation Evaluation FormLuqmanNo ratings yet
- Using Species Distribution Models To Infer Potential Climate Change-Induced Range Shifts of Freshwater Fish in South-Eastern AustraliaDocument19 pagesUsing Species Distribution Models To Infer Potential Climate Change-Induced Range Shifts of Freshwater Fish in South-Eastern AustraliaLuqmanNo ratings yet
- Microchemical Journal: Roman M. Balabin, Ravilya Z. Sa Fieva, Ekaterina I. LomakinaDocument8 pagesMicrochemical Journal: Roman M. Balabin, Ravilya Z. Sa Fieva, Ekaterina I. LomakinaLuqmanNo ratings yet
- Community-Based Model For Bioenergy Production Coupled To Forest Land Management For Wildfire Control Using Combined Heat and PowerDocument9 pagesCommunity-Based Model For Bioenergy Production Coupled To Forest Land Management For Wildfire Control Using Combined Heat and PowerLuqmanNo ratings yet
- A Statistical Analysis of Wind Energy Potential at The Eastern Region of Saudi ArabiaDocument9 pagesA Statistical Analysis of Wind Energy Potential at The Eastern Region of Saudi ArabiaLuqmanNo ratings yet
- Ational Petroleum Conference - Calgary, Alberta (2005-06-07) ) Canadian InternationaDocument13 pagesAtional Petroleum Conference - Calgary, Alberta (2005-06-07) ) Canadian InternationaLuqmanNo ratings yet
- Bioenergy Plantations or Long-Term Carbon Sinks? - A Model Based AnalysisDocument10 pagesBioenergy Plantations or Long-Term Carbon Sinks? - A Model Based AnalysisLuqmanNo ratings yet
- Journal of Unconventional Oil and Gas Resources: Nesreen A. Elsayed, Maria A. Barrufet, Mahmoud M. El-HalwagiDocument9 pagesJournal of Unconventional Oil and Gas Resources: Nesreen A. Elsayed, Maria A. Barrufet, Mahmoud M. El-HalwagiLuqmanNo ratings yet
- Lfc3-Ld: Specified PerformanceDocument1 pageLfc3-Ld: Specified PerformanceLuqmanNo ratings yet
- R. Albion, S. - World&apos S First SAGD Facility Using Evaporators, Drum Boilers, and ZDocument7 pagesR. Albion, S. - World&apos S First SAGD Facility Using Evaporators, Drum Boilers, and ZLuqmanNo ratings yet
- Icient Treatment of Oil Sands PDocument36 pagesIcient Treatment of Oil Sands PLuqmanNo ratings yet
- Control of A Distillation Column With A Heat PumpDocument6 pagesControl of A Distillation Column With A Heat PumpLuqmanNo ratings yet
- G Zero Liquid Discharge in SAGD Heavy Oil RecoveryDocument6 pagesG Zero Liquid Discharge in SAGD Heavy Oil RecoveryLuqmanNo ratings yet
- LCA Residential BuildingDocument8 pagesLCA Residential BuildingLuqmanNo ratings yet
- National Petroleum Conference - Calgary, Alberta (2002-06-11) ) Canadian InternationaDocument10 pagesNational Petroleum Conference - Calgary, Alberta (2002-06-11) ) Canadian InternationaLuqmanNo ratings yet
- Energy Use Pullen2000Document9 pagesEnergy Use Pullen2000LuqmanNo ratings yet
- Building and Environment: Kevin Van Ooteghem, Lei XuDocument15 pagesBuilding and Environment: Kevin Van Ooteghem, Lei XuLuqmanNo ratings yet
- Pvc-Final Report LcaDocument325 pagesPvc-Final Report LcaLuqmanNo ratings yet
- LCA Passive Housej - Enbuild.2012.07.029Document10 pagesLCA Passive Housej - Enbuild.2012.07.029LuqmanNo ratings yet
- Life Cycle ToolsDocument5 pagesLife Cycle ToolsLuqmanNo ratings yet
- Aavani AvittamDocument2 pagesAavani AvittamUmashakti PeethNo ratings yet
- Presentación Argentina NegociaciónDocument33 pagesPresentación Argentina NegociaciónLeandro Bustamante TrujilloNo ratings yet
- Unit 5Document17 pagesUnit 5Jai PrasannaNo ratings yet
- Philosophy of Economics A Heterodox Introduction (Oliver Schlaudt)Document181 pagesPhilosophy of Economics A Heterodox Introduction (Oliver Schlaudt)Monseñior Melcacho AramburuzabalaNo ratings yet
- Driver Packaging Utility User Guide MinoltaDocument54 pagesDriver Packaging Utility User Guide MinoltagedangboyNo ratings yet
- Mahindra CV 2019Document3 pagesMahindra CV 2019mahindra boradeNo ratings yet
- Assess 311 WEEK1Document8 pagesAssess 311 WEEK1cloe reginaldoNo ratings yet
- Analytical Exposition Text - Weather Related ProblemDocument3 pagesAnalytical Exposition Text - Weather Related ProblemDilla Amalia Hamdi NafilahNo ratings yet
- Teodora Alonzo Was A Woman of Steel: So That's Where Jose Rizal Got His FireDocument13 pagesTeodora Alonzo Was A Woman of Steel: So That's Where Jose Rizal Got His FireAJ Grean EscobidoNo ratings yet
- Conclusion For Romeo and Juliet EssayDocument8 pagesConclusion For Romeo and Juliet EssayaerftuwhdNo ratings yet
- ELA Continuum Y1 To Y7Document7 pagesELA Continuum Y1 To Y7meryem rhimNo ratings yet
- Anatomy Compre Exams 2004 2005Document7 pagesAnatomy Compre Exams 2004 2005GLeen Rose Onguda AguiLarNo ratings yet
- Atlantic BlueDocument70 pagesAtlantic BlueLyubomir IvanovNo ratings yet
- Howto Become A NudistDocument5 pagesHowto Become A NudistLerche85WichmannNo ratings yet
- Announcer: Good Morning, Philippines. I Am Eva Trinidad and This 4 Music: StingerDocument2 pagesAnnouncer: Good Morning, Philippines. I Am Eva Trinidad and This 4 Music: StingerEva TrinidadNo ratings yet
- Boppps DetailDocument10 pagesBoppps DetailMUHAMMAD SAYYIDNo ratings yet
- The Socio-Economic Significance of Food DesertsDocument1 pageThe Socio-Economic Significance of Food Desertsapi-466415791No ratings yet
- Gold Sluice Box LegsDocument3 pagesGold Sluice Box LegsEricNo ratings yet
- Primary Sources of ShariahDocument15 pagesPrimary Sources of ShariahAeina FaiiziNo ratings yet
- Ibf AssignmentDocument55 pagesIbf AssignmentrashiNo ratings yet
- 3d Panel SystemDocument25 pages3d Panel Systemprobir100% (3)
- Apple Iphone XR Price - Google SearchDocument1 pageApple Iphone XR Price - Google Searchkamarie DavisNo ratings yet
- Math 6 q2 Week 6 Day 4Document15 pagesMath 6 q2 Week 6 Day 4Rowel CasoneteNo ratings yet
- EAPP LectureDocument11 pagesEAPP LectureDe Juan, Angela Marie G.No ratings yet
- Karma by Khushwant SinghDocument13 pagesKarma by Khushwant SinghHazelClaveNo ratings yet