Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
540 viewsIdeal Gases Exam-6636
Ideal Gases Exam-6636
Uploaded by
Jewel Lim1. The entropy change (ΔS) of an ideal gas undergoing a constant-volume process is determined from its internal energy change and specific heat.
2. Two vessels containing gases are connected and cooled, and the final common pressure is calculated.
3. The entropy change of an ideal gas undergoing an isobaric process is determined from its initial and final enthalpies and the temperature change.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
You might also like
- Refrigeration and Air Conditioning Technology 8th Edition Tomczyk Silberstein Whitman Johnson Solution ManualDocument5 pagesRefrigeration and Air Conditioning Technology 8th Edition Tomczyk Silberstein Whitman Johnson Solution Manualrachel100% (32)
- 6 - Nitrogen PumpingDocument36 pages6 - Nitrogen PumpingJesus Sullivan73% (11)
- Assignment Open and Closed Thermodynamic SystemDocument1 pageAssignment Open and Closed Thermodynamic SystemJenellie BahintingNo ratings yet
- PS4Document1 pagePS4Friend ANo ratings yet
- Centrifugal Pumps & Fluid Flow Practical Calculations: Pdhonline Course M388 (3 PDH)Document53 pagesCentrifugal Pumps & Fluid Flow Practical Calculations: Pdhonline Course M388 (3 PDH)Prad1979100% (7)
- Busch 0872-900-823 R 5 0025-0101 E & 0250 C 0511 PDFDocument20 pagesBusch 0872-900-823 R 5 0025-0101 E & 0250 C 0511 PDFRodolfo M. PortoNo ratings yet
- ProblemsDocument3 pagesProblemsimPERFECTme09No ratings yet
- Topic 7 Cascade Refrigeration SystemsDocument6 pagesTopic 7 Cascade Refrigeration SystemsJanelle D. Puti-anNo ratings yet
- Hea RansferDocument40 pagesHea RansferHikki KunNo ratings yet
- EE21L Experiment 7Document4 pagesEE21L Experiment 7Filbert SaavedraNo ratings yet
- Constant Volume, Temperature Enthalpy1Document14 pagesConstant Volume, Temperature Enthalpy1Bae.J GAMINGNo ratings yet
- Heat and Mass Transfer: "Solved Problems"Document16 pagesHeat and Mass Transfer: "Solved Problems"qiritical99No ratings yet
- CO Assign#2 BSEE-2ADocument3 pagesCO Assign#2 BSEE-2AEisen JaylordNo ratings yet
- Mikee EncodedDocument2 pagesMikee EncodedjaysonNo ratings yet
- RankineDocument12 pagesRankineMeej AustriaNo ratings yet
- Fluid Mechanics Problem Set 1Document3 pagesFluid Mechanics Problem Set 1Kathleen RafananNo ratings yet
- Solved Problems in Mechanics 2016Document28 pagesSolved Problems in Mechanics 2016Debbie TonogNo ratings yet
- Refrigeration Problem 1-2 SolvedDocument4 pagesRefrigeration Problem 1-2 SolvedNorma FrancoNo ratings yet
- P4-10 ThermodynamicsDocument1 pageP4-10 ThermodynamicsjimrNo ratings yet
- 311 ThermoDynamics ThermoDynamicsDocument5 pages311 ThermoDynamics ThermoDynamicsmozam haqNo ratings yet
- ME REVIEW ThermodynamicsDocument65 pagesME REVIEW ThermodynamicsKhate ÜüNo ratings yet
- Me6301 QBDocument46 pagesMe6301 QBNaveen Dhanuraj100% (1)
- Thermo Solutions - Part67 PDFDocument1 pageThermo Solutions - Part67 PDFLiz ArfinNo ratings yet
- Phychem BasicsDocument104 pagesPhychem BasicsDanice LunaNo ratings yet
- Chapter 4Document14 pagesChapter 4Anthony Leire MontealtoNo ratings yet
- Problem Set: Strain and Thermal StressesDocument5 pagesProblem Set: Strain and Thermal StressesClint Charles P. BrutasNo ratings yet
- Properties of FluidsDocument57 pagesProperties of FluidsPatricia SorianoNo ratings yet
- Heat Transfer Problem SetDocument6 pagesHeat Transfer Problem Setkim dianonNo ratings yet
- EXP. 2-Voltage MultiplierDocument3 pagesEXP. 2-Voltage MultiplierJohn Ratilla100% (1)
- Abraham F. Ramos IPE M 3 Activity 6Document3 pagesAbraham F. Ramos IPE M 3 Activity 6Leyzer MalumayNo ratings yet
- Variable Stresses With Stress ConcentrationsDocument16 pagesVariable Stresses With Stress ConcentrationsBryan GounzoNo ratings yet
- ModuleDocument37 pagesModuleAlvin RazoNo ratings yet
- ME301 Paper ADocument2 pagesME301 Paper AMitesh KumarNo ratings yet
- ThermoproblemDocument20 pagesThermoproblemmark anthony tutorNo ratings yet
- 3 Properties of Refrigerants On P-H DiagramDocument7 pages3 Properties of Refrigerants On P-H DiagramJustin MercadoNo ratings yet
- REFSYS - 3ME-8 (2ND SEM/ A.Y. 2020-2021) Midterm ExaminationDocument3 pagesREFSYS - 3ME-8 (2ND SEM/ A.Y. 2020-2021) Midterm ExaminationRobert GarlandNo ratings yet
- ALGEBRA Worded ProblemDocument2 pagesALGEBRA Worded ProblemanthonyNo ratings yet
- BSABE2 - Blasquez - Lab Report 2Document7 pagesBSABE2 - Blasquez - Lab Report 2Lorenzo Niño BlasquezNo ratings yet
- MARTINEZ Ideal Gas and Polytropic ProblemDocument3 pagesMARTINEZ Ideal Gas and Polytropic Problemyeng botzNo ratings yet
- PASSDocument11 pagesPASSMakobasaNo ratings yet
- Fluid-Mechanics-Problems 3Document5 pagesFluid-Mechanics-Problems 3Rhea EnriquezNo ratings yet
- Reviewer in Heat TransferDocument12 pagesReviewer in Heat TransferKristian Allen B. DomingoNo ratings yet
- Ideal Gas Law Simulation: PV NRTDocument3 pagesIdeal Gas Law Simulation: PV NRTElla GreenNo ratings yet
- Thermodynamics Lesson 1-5Document43 pagesThermodynamics Lesson 1-5Cristel Shane DuranNo ratings yet
- Properties of Fluids: 1.1. Fluid Mechanics and HydraulicsDocument16 pagesProperties of Fluids: 1.1. Fluid Mechanics and HydraulicsJoshua FactorNo ratings yet
- Day 02 - Algebra 2Document26 pagesDay 02 - Algebra 2Ironfalcon101100% (2)
- Gmas Plane and Solid GeometryDocument11 pagesGmas Plane and Solid Geometryjonnel batuigasNo ratings yet
- Refrigeration-Systems Part 1Document11 pagesRefrigeration-Systems Part 1Sean GuanzonNo ratings yet
- Laboratory Manual For Exercise No 2Document7 pagesLaboratory Manual For Exercise No 2Cabagnot Piolo JuliusNo ratings yet
- Adamson UniversityDocument3 pagesAdamson UniversityVanessa Elaine CaoNo ratings yet
- Thermodynamics 1: Prepared byDocument105 pagesThermodynamics 1: Prepared byReyven Recon100% (1)
- MIDTERMSDocument1 pageMIDTERMSMelindaNo ratings yet
- Electrical Engineering SolutionsDocument14 pagesElectrical Engineering SolutionsJustine Kyla OlivarNo ratings yet
- FluidsDocument1 pageFluidsnico aspraNo ratings yet
- Fixed Capacitors - Characteristics and ApplicationsDocument72 pagesFixed Capacitors - Characteristics and ApplicationsPratisha Padishalwar0% (1)
- HW Static FluidDocument5 pagesHW Static FluidMobile LegendNo ratings yet
- CHE 2202 Module1 - Evaluation of Enthalpy Diference Part 1 - StudentDocument18 pagesCHE 2202 Module1 - Evaluation of Enthalpy Diference Part 1 - StudentKing Antonio AbellaNo ratings yet
- Sample ProblemsDocument8 pagesSample ProblemsKenn Earl Bringino VillanuevaNo ratings yet
- 101 ThermoDynamics ThermoDynamicsDocument5 pages101 ThermoDynamics ThermoDynamicsmozam haqNo ratings yet
- ThermoDocument6 pagesThermoE.G Boy GudaNo ratings yet
- Problem Set - Thermodynamics & ICEDocument2 pagesProblem Set - Thermodynamics & ICEBea Therese RadubanNo ratings yet
- Assignment No. 4Document2 pagesAssignment No. 4Charie EralinoNo ratings yet
- Assignment 1 First Law 2016Document8 pagesAssignment 1 First Law 2016PabitraBadhuk0% (1)
- 3rd Q L3 - HORTICULTURE Farm Tools and Their UsesDocument32 pages3rd Q L3 - HORTICULTURE Farm Tools and Their UsesJewel LimNo ratings yet
- STORYLINEDocument2 pagesSTORYLINEJewel LimNo ratings yet
- Homeroom Guidance: My Road To SuccessDocument9 pagesHomeroom Guidance: My Road To SuccessJewel LimNo ratings yet
- Homeroom Guidance: Quarter 2 - Module 6Document9 pagesHomeroom Guidance: Quarter 2 - Module 6Jewel Lim91% (11)
- The Rational Choice TheoryDocument5 pagesThe Rational Choice TheoryJewel LimNo ratings yet
- Reviewer in Understanding The Self Quiz 2 PDFDocument7 pagesReviewer in Understanding The Self Quiz 2 PDFJewel LimNo ratings yet
- CyberbullyingDocument2 pagesCyberbullyingJewel LimNo ratings yet
- 8-The Chemistry of The Environment PDFDocument52 pages8-The Chemistry of The Environment PDFJewel LimNo ratings yet
- 5 - Intro To Nuclear Chemistry PDFDocument41 pages5 - Intro To Nuclear Chemistry PDFJewel LimNo ratings yet
- Reflection Paper On Chosen Historical PlaceDocument1 pageReflection Paper On Chosen Historical PlaceJewel LimNo ratings yet
- 5 Band Resistor Color Code Chart PDFDocument3 pages5 Band Resistor Color Code Chart PDFJewel LimNo ratings yet
- Electronics Engineering Program - PUPSTB ECEN 30012 Basic Electronics 1 Experiment #4: Parallel Circuit - Voltage and Current Measurement PrelabDocument4 pagesElectronics Engineering Program - PUPSTB ECEN 30012 Basic Electronics 1 Experiment #4: Parallel Circuit - Voltage and Current Measurement PrelabJewel Lim100% (1)
- Summary of Factors That Affect The HUMSS Students Absenteeism in Mataasnakahoy Senior High SchoolDocument6 pagesSummary of Factors That Affect The HUMSS Students Absenteeism in Mataasnakahoy Senior High SchoolJewel Lim100% (1)
- A Concept Paper About LoveDocument1 pageA Concept Paper About LoveJewel Lim67% (3)
- Philosophical Self ReviewerDocument4 pagesPhilosophical Self ReviewerJewel LimNo ratings yet
- Amca 222-16Document34 pagesAmca 222-16Hany RifaatNo ratings yet
- Flare System Design TipsDocument5 pagesFlare System Design TipsbalajikrishnanNo ratings yet
- CHPT 2 U Nyi Hla Nge Singly Doubly T BeamDocument27 pagesCHPT 2 U Nyi Hla Nge Singly Doubly T BeamMartin NaingNo ratings yet
- Hydraulic Press Machine PartsDocument12 pagesHydraulic Press Machine PartsKereme JulienNo ratings yet
- PHYS 1421-11 (Nov) - 08Document14 pagesPHYS 1421-11 (Nov) - 08yassin mashalyNo ratings yet
- Cold Tapping HydrotestDocument4 pagesCold Tapping Hydrotestninju1100% (1)
- Deliverable Report IngasDocument54 pagesDeliverable Report Ingaseko handoyoNo ratings yet
- 08 - 2061 USTR 2222a (1) Supor EKVDocument24 pages08 - 2061 USTR 2222a (1) Supor EKVHassan Houdoud0% (1)
- Corken ButanoDocument48 pagesCorken ButanoCristobal CherigoNo ratings yet
- PVT ExperimentDocument23 pagesPVT ExperimentAbdullah FarhanNo ratings yet
- Design of Pressure VesselDocument136 pagesDesign of Pressure VesselHarshal JadhavNo ratings yet
- Physics PressureDocument35 pagesPhysics Pressuresai yadaNo ratings yet
- Tutorial No.5-Applications of Bernoulli's Equation: AE 225 Fluid DynamicsDocument2 pagesTutorial No.5-Applications of Bernoulli's Equation: AE 225 Fluid DynamicssoumyaNo ratings yet
- Preliminary Exam in FuildsDocument6 pagesPreliminary Exam in FuildsJojimar JulianNo ratings yet
- Axis Mechanical DesignDocument1 pageAxis Mechanical DesignHenno TrautNo ratings yet
- Duo Safe Double Cont InstallDocument8 pagesDuo Safe Double Cont InstallahmedNo ratings yet
- Volume BoostersDocument12 pagesVolume BoosterswindoutNo ratings yet
- Technical Sheet (Model ILVA) PDFDocument2 pagesTechnical Sheet (Model ILVA) PDFMuhammad Ahsan KhanNo ratings yet
- Product Data Sheet Metco 6A Air Control Unit: 2 Features and Benefits N N NDocument2 pagesProduct Data Sheet Metco 6A Air Control Unit: 2 Features and Benefits N N Nlhphong021191No ratings yet
- Stress 1Document6 pagesStress 1azhagu durai100% (1)
- Prediction of Wave Impact Loads On Ship-Type Offshore Structures in Steep Fronted Waves PDFDocument9 pagesPrediction of Wave Impact Loads On Ship-Type Offshore Structures in Steep Fronted Waves PDFNguyen ThangNo ratings yet
- Unit 1 Concept and TheoryDocument57 pagesUnit 1 Concept and TheoryRahaf HammadNo ratings yet
- Paper-2 Set B (Final)Document15 pagesPaper-2 Set B (Final)Dewan Olin ChotepadaeNo ratings yet
- 35391B RevaDocument234 pages35391B RevaFelipe FloresNo ratings yet
- Steps To Completing Sprinkler Layout 2012Document24 pagesSteps To Completing Sprinkler Layout 2012Anonymous qrSsYuxyoYNo ratings yet
- Cooling TowerDocument16 pagesCooling Towerbakhtyar21No ratings yet
Ideal Gases Exam-6636
Ideal Gases Exam-6636
Uploaded by
Jewel Lim0 ratings0% found this document useful (0 votes)
540 views12 pages1. The entropy change (ΔS) of an ideal gas undergoing a constant-volume process is determined from its internal energy change and specific heat.
2. Two vessels containing gases are connected and cooled, and the final common pressure is calculated.
3. The entropy change of an ideal gas undergoing an isobaric process is determined from its initial and final enthalpies and the temperature change.
Original Description:
Copyright
© © All Rights Reserved
Available Formats
PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1. The entropy change (ΔS) of an ideal gas undergoing a constant-volume process is determined from its internal energy change and specific heat.
2. Two vessels containing gases are connected and cooled, and the final common pressure is calculated.
3. The entropy change of an ideal gas undergoing an isobaric process is determined from its initial and final enthalpies and the temperature change.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
0 ratings0% found this document useful (0 votes)
540 views12 pagesIdeal Gases Exam-6636
Ideal Gases Exam-6636
Uploaded by
Jewel Lim1. The entropy change (ΔS) of an ideal gas undergoing a constant-volume process is determined from its internal energy change and specific heat.
2. Two vessels containing gases are connected and cooled, and the final common pressure is calculated.
3. The entropy change of an ideal gas undergoing an isobaric process is determined from its initial and final enthalpies and the temperature change.
Copyright:
© All Rights Reserved
Available Formats
Download as PDF, TXT or read online from Scribd
Download as pdf or txt
You are on page 1of 12
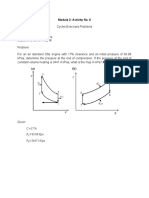
1.
The decrease in internal energy of
3lb of an ideal gas is 325BTU when the
pressure decreases from 100psia to 20
psia and volume increases from 1.5ft3
to 4.5ft3. Cv = 0.25BTU/lb°R.
Determine ΔS (BTU/°R).
2. A rigid vessel of volume 0.5m3 (Vessel X)
containing H2 at 200K and a pressure of
60kPa is connected to a second rigid vessel
of volume 0.8m3 (Vessel Y) containing Ar at
300K at a pressure of 40kPa. A valve
separating the two vessels is opened and
both are cooled to a temperature of 150K.
What is the final pressure (kPa) in the
vessels?
3. A certain gas with a value of k = 1.3
has an initial enthalpy of 61.34BTU
undergoes an isobaric process until its
enthalpy becomes 184.03BTU. The
temperature change of the gas is
1080°R. Determine ΔS (BTU/°R).
4. An unknown ideal gas is cooled
under constant pressure from 200°C to
50°C. Assuming cp = 1 kJ/kgK,
determine the change of entropy
(kJ/kgK) of the gas.
5. A gas whose composition is not
known has 42.2 KJ of work input at
constant volume of 566 liters. Initially,
P1 = 138 KPaa, T1 = 26.7°C, T2 = 82.2°C.
What is the change in internal energy
(KJ) if cp = 1.21?
6. A 1m3 rigid tank with air at 1 MPa, 400K
is connected to an air line. The valve is
opened and air flows into the tank until the
pressure reaches 5 MPa at which point the
valve is closed and the temperature inside is
450K. The tank eventually cools to room
temperature of 300K. What is the pressure
(MPa) inside the tank?
7. Why there’s no constant
temperature specific heat?
Explain.
8. A 1lit gas is allowed to expand at
constant temperature until it reaches its
final volume of 3lit and its pressure is 1atm.
It is then heated at constant volume until it
reaches a pressure of 3atm. The increase in
internal energy is 456J. Find the heat (J)
absorbed by the gas during this process.
9. Why is cp should be
greater than cv?
10. A cylindrical storage tank having an
internal volume of 0.465m3 contains
methane at 20°C with a pressure of 137bar.
If the tank outlet is opened until the
pressure in the cylinder is halved,
determine the mass of gas which escapes
from the tank assuming the tank
temperature remains constant.
11. A rigid tank of 20ft3 contains air at
100psia and 70°F. The tank is equipped with
a relief valve that opens at a pressure of
125psia and remains open until the
pressure drops to 120psia. If a fire causes
the valve to operate, what is the quantity
(lbm) of air lost due to the fire?
You might also like
- Refrigeration and Air Conditioning Technology 8th Edition Tomczyk Silberstein Whitman Johnson Solution ManualDocument5 pagesRefrigeration and Air Conditioning Technology 8th Edition Tomczyk Silberstein Whitman Johnson Solution Manualrachel100% (32)
- 6 - Nitrogen PumpingDocument36 pages6 - Nitrogen PumpingJesus Sullivan73% (11)
- Assignment Open and Closed Thermodynamic SystemDocument1 pageAssignment Open and Closed Thermodynamic SystemJenellie BahintingNo ratings yet
- PS4Document1 pagePS4Friend ANo ratings yet
- Centrifugal Pumps & Fluid Flow Practical Calculations: Pdhonline Course M388 (3 PDH)Document53 pagesCentrifugal Pumps & Fluid Flow Practical Calculations: Pdhonline Course M388 (3 PDH)Prad1979100% (7)
- Busch 0872-900-823 R 5 0025-0101 E & 0250 C 0511 PDFDocument20 pagesBusch 0872-900-823 R 5 0025-0101 E & 0250 C 0511 PDFRodolfo M. PortoNo ratings yet
- ProblemsDocument3 pagesProblemsimPERFECTme09No ratings yet
- Topic 7 Cascade Refrigeration SystemsDocument6 pagesTopic 7 Cascade Refrigeration SystemsJanelle D. Puti-anNo ratings yet
- Hea RansferDocument40 pagesHea RansferHikki KunNo ratings yet
- EE21L Experiment 7Document4 pagesEE21L Experiment 7Filbert SaavedraNo ratings yet
- Constant Volume, Temperature Enthalpy1Document14 pagesConstant Volume, Temperature Enthalpy1Bae.J GAMINGNo ratings yet
- Heat and Mass Transfer: "Solved Problems"Document16 pagesHeat and Mass Transfer: "Solved Problems"qiritical99No ratings yet
- CO Assign#2 BSEE-2ADocument3 pagesCO Assign#2 BSEE-2AEisen JaylordNo ratings yet
- Mikee EncodedDocument2 pagesMikee EncodedjaysonNo ratings yet
- RankineDocument12 pagesRankineMeej AustriaNo ratings yet
- Fluid Mechanics Problem Set 1Document3 pagesFluid Mechanics Problem Set 1Kathleen RafananNo ratings yet
- Solved Problems in Mechanics 2016Document28 pagesSolved Problems in Mechanics 2016Debbie TonogNo ratings yet
- Refrigeration Problem 1-2 SolvedDocument4 pagesRefrigeration Problem 1-2 SolvedNorma FrancoNo ratings yet
- P4-10 ThermodynamicsDocument1 pageP4-10 ThermodynamicsjimrNo ratings yet
- 311 ThermoDynamics ThermoDynamicsDocument5 pages311 ThermoDynamics ThermoDynamicsmozam haqNo ratings yet
- ME REVIEW ThermodynamicsDocument65 pagesME REVIEW ThermodynamicsKhate ÜüNo ratings yet
- Me6301 QBDocument46 pagesMe6301 QBNaveen Dhanuraj100% (1)
- Thermo Solutions - Part67 PDFDocument1 pageThermo Solutions - Part67 PDFLiz ArfinNo ratings yet
- Phychem BasicsDocument104 pagesPhychem BasicsDanice LunaNo ratings yet
- Chapter 4Document14 pagesChapter 4Anthony Leire MontealtoNo ratings yet
- Problem Set: Strain and Thermal StressesDocument5 pagesProblem Set: Strain and Thermal StressesClint Charles P. BrutasNo ratings yet
- Properties of FluidsDocument57 pagesProperties of FluidsPatricia SorianoNo ratings yet
- Heat Transfer Problem SetDocument6 pagesHeat Transfer Problem Setkim dianonNo ratings yet
- EXP. 2-Voltage MultiplierDocument3 pagesEXP. 2-Voltage MultiplierJohn Ratilla100% (1)
- Abraham F. Ramos IPE M 3 Activity 6Document3 pagesAbraham F. Ramos IPE M 3 Activity 6Leyzer MalumayNo ratings yet
- Variable Stresses With Stress ConcentrationsDocument16 pagesVariable Stresses With Stress ConcentrationsBryan GounzoNo ratings yet
- ModuleDocument37 pagesModuleAlvin RazoNo ratings yet
- ME301 Paper ADocument2 pagesME301 Paper AMitesh KumarNo ratings yet
- ThermoproblemDocument20 pagesThermoproblemmark anthony tutorNo ratings yet
- 3 Properties of Refrigerants On P-H DiagramDocument7 pages3 Properties of Refrigerants On P-H DiagramJustin MercadoNo ratings yet
- REFSYS - 3ME-8 (2ND SEM/ A.Y. 2020-2021) Midterm ExaminationDocument3 pagesREFSYS - 3ME-8 (2ND SEM/ A.Y. 2020-2021) Midterm ExaminationRobert GarlandNo ratings yet
- ALGEBRA Worded ProblemDocument2 pagesALGEBRA Worded ProblemanthonyNo ratings yet
- BSABE2 - Blasquez - Lab Report 2Document7 pagesBSABE2 - Blasquez - Lab Report 2Lorenzo Niño BlasquezNo ratings yet
- MARTINEZ Ideal Gas and Polytropic ProblemDocument3 pagesMARTINEZ Ideal Gas and Polytropic Problemyeng botzNo ratings yet
- PASSDocument11 pagesPASSMakobasaNo ratings yet
- Fluid-Mechanics-Problems 3Document5 pagesFluid-Mechanics-Problems 3Rhea EnriquezNo ratings yet
- Reviewer in Heat TransferDocument12 pagesReviewer in Heat TransferKristian Allen B. DomingoNo ratings yet
- Ideal Gas Law Simulation: PV NRTDocument3 pagesIdeal Gas Law Simulation: PV NRTElla GreenNo ratings yet
- Thermodynamics Lesson 1-5Document43 pagesThermodynamics Lesson 1-5Cristel Shane DuranNo ratings yet
- Properties of Fluids: 1.1. Fluid Mechanics and HydraulicsDocument16 pagesProperties of Fluids: 1.1. Fluid Mechanics and HydraulicsJoshua FactorNo ratings yet
- Day 02 - Algebra 2Document26 pagesDay 02 - Algebra 2Ironfalcon101100% (2)
- Gmas Plane and Solid GeometryDocument11 pagesGmas Plane and Solid Geometryjonnel batuigasNo ratings yet
- Refrigeration-Systems Part 1Document11 pagesRefrigeration-Systems Part 1Sean GuanzonNo ratings yet
- Laboratory Manual For Exercise No 2Document7 pagesLaboratory Manual For Exercise No 2Cabagnot Piolo JuliusNo ratings yet
- Adamson UniversityDocument3 pagesAdamson UniversityVanessa Elaine CaoNo ratings yet
- Thermodynamics 1: Prepared byDocument105 pagesThermodynamics 1: Prepared byReyven Recon100% (1)
- MIDTERMSDocument1 pageMIDTERMSMelindaNo ratings yet
- Electrical Engineering SolutionsDocument14 pagesElectrical Engineering SolutionsJustine Kyla OlivarNo ratings yet
- FluidsDocument1 pageFluidsnico aspraNo ratings yet
- Fixed Capacitors - Characteristics and ApplicationsDocument72 pagesFixed Capacitors - Characteristics and ApplicationsPratisha Padishalwar0% (1)
- HW Static FluidDocument5 pagesHW Static FluidMobile LegendNo ratings yet
- CHE 2202 Module1 - Evaluation of Enthalpy Diference Part 1 - StudentDocument18 pagesCHE 2202 Module1 - Evaluation of Enthalpy Diference Part 1 - StudentKing Antonio AbellaNo ratings yet
- Sample ProblemsDocument8 pagesSample ProblemsKenn Earl Bringino VillanuevaNo ratings yet
- 101 ThermoDynamics ThermoDynamicsDocument5 pages101 ThermoDynamics ThermoDynamicsmozam haqNo ratings yet
- ThermoDocument6 pagesThermoE.G Boy GudaNo ratings yet
- Problem Set - Thermodynamics & ICEDocument2 pagesProblem Set - Thermodynamics & ICEBea Therese RadubanNo ratings yet
- Assignment No. 4Document2 pagesAssignment No. 4Charie EralinoNo ratings yet
- Assignment 1 First Law 2016Document8 pagesAssignment 1 First Law 2016PabitraBadhuk0% (1)
- 3rd Q L3 - HORTICULTURE Farm Tools and Their UsesDocument32 pages3rd Q L3 - HORTICULTURE Farm Tools and Their UsesJewel LimNo ratings yet
- STORYLINEDocument2 pagesSTORYLINEJewel LimNo ratings yet
- Homeroom Guidance: My Road To SuccessDocument9 pagesHomeroom Guidance: My Road To SuccessJewel LimNo ratings yet
- Homeroom Guidance: Quarter 2 - Module 6Document9 pagesHomeroom Guidance: Quarter 2 - Module 6Jewel Lim91% (11)
- The Rational Choice TheoryDocument5 pagesThe Rational Choice TheoryJewel LimNo ratings yet
- Reviewer in Understanding The Self Quiz 2 PDFDocument7 pagesReviewer in Understanding The Self Quiz 2 PDFJewel LimNo ratings yet
- CyberbullyingDocument2 pagesCyberbullyingJewel LimNo ratings yet
- 8-The Chemistry of The Environment PDFDocument52 pages8-The Chemistry of The Environment PDFJewel LimNo ratings yet
- 5 - Intro To Nuclear Chemistry PDFDocument41 pages5 - Intro To Nuclear Chemistry PDFJewel LimNo ratings yet
- Reflection Paper On Chosen Historical PlaceDocument1 pageReflection Paper On Chosen Historical PlaceJewel LimNo ratings yet
- 5 Band Resistor Color Code Chart PDFDocument3 pages5 Band Resistor Color Code Chart PDFJewel LimNo ratings yet
- Electronics Engineering Program - PUPSTB ECEN 30012 Basic Electronics 1 Experiment #4: Parallel Circuit - Voltage and Current Measurement PrelabDocument4 pagesElectronics Engineering Program - PUPSTB ECEN 30012 Basic Electronics 1 Experiment #4: Parallel Circuit - Voltage and Current Measurement PrelabJewel Lim100% (1)
- Summary of Factors That Affect The HUMSS Students Absenteeism in Mataasnakahoy Senior High SchoolDocument6 pagesSummary of Factors That Affect The HUMSS Students Absenteeism in Mataasnakahoy Senior High SchoolJewel Lim100% (1)
- A Concept Paper About LoveDocument1 pageA Concept Paper About LoveJewel Lim67% (3)
- Philosophical Self ReviewerDocument4 pagesPhilosophical Self ReviewerJewel LimNo ratings yet
- Amca 222-16Document34 pagesAmca 222-16Hany RifaatNo ratings yet
- Flare System Design TipsDocument5 pagesFlare System Design TipsbalajikrishnanNo ratings yet
- CHPT 2 U Nyi Hla Nge Singly Doubly T BeamDocument27 pagesCHPT 2 U Nyi Hla Nge Singly Doubly T BeamMartin NaingNo ratings yet
- Hydraulic Press Machine PartsDocument12 pagesHydraulic Press Machine PartsKereme JulienNo ratings yet
- PHYS 1421-11 (Nov) - 08Document14 pagesPHYS 1421-11 (Nov) - 08yassin mashalyNo ratings yet
- Cold Tapping HydrotestDocument4 pagesCold Tapping Hydrotestninju1100% (1)
- Deliverable Report IngasDocument54 pagesDeliverable Report Ingaseko handoyoNo ratings yet
- 08 - 2061 USTR 2222a (1) Supor EKVDocument24 pages08 - 2061 USTR 2222a (1) Supor EKVHassan Houdoud0% (1)
- Corken ButanoDocument48 pagesCorken ButanoCristobal CherigoNo ratings yet
- PVT ExperimentDocument23 pagesPVT ExperimentAbdullah FarhanNo ratings yet
- Design of Pressure VesselDocument136 pagesDesign of Pressure VesselHarshal JadhavNo ratings yet
- Physics PressureDocument35 pagesPhysics Pressuresai yadaNo ratings yet
- Tutorial No.5-Applications of Bernoulli's Equation: AE 225 Fluid DynamicsDocument2 pagesTutorial No.5-Applications of Bernoulli's Equation: AE 225 Fluid DynamicssoumyaNo ratings yet
- Preliminary Exam in FuildsDocument6 pagesPreliminary Exam in FuildsJojimar JulianNo ratings yet
- Axis Mechanical DesignDocument1 pageAxis Mechanical DesignHenno TrautNo ratings yet
- Duo Safe Double Cont InstallDocument8 pagesDuo Safe Double Cont InstallahmedNo ratings yet
- Volume BoostersDocument12 pagesVolume BoosterswindoutNo ratings yet
- Technical Sheet (Model ILVA) PDFDocument2 pagesTechnical Sheet (Model ILVA) PDFMuhammad Ahsan KhanNo ratings yet
- Product Data Sheet Metco 6A Air Control Unit: 2 Features and Benefits N N NDocument2 pagesProduct Data Sheet Metco 6A Air Control Unit: 2 Features and Benefits N N Nlhphong021191No ratings yet
- Stress 1Document6 pagesStress 1azhagu durai100% (1)
- Prediction of Wave Impact Loads On Ship-Type Offshore Structures in Steep Fronted Waves PDFDocument9 pagesPrediction of Wave Impact Loads On Ship-Type Offshore Structures in Steep Fronted Waves PDFNguyen ThangNo ratings yet
- Unit 1 Concept and TheoryDocument57 pagesUnit 1 Concept and TheoryRahaf HammadNo ratings yet
- Paper-2 Set B (Final)Document15 pagesPaper-2 Set B (Final)Dewan Olin ChotepadaeNo ratings yet
- 35391B RevaDocument234 pages35391B RevaFelipe FloresNo ratings yet
- Steps To Completing Sprinkler Layout 2012Document24 pagesSteps To Completing Sprinkler Layout 2012Anonymous qrSsYuxyoYNo ratings yet
- Cooling TowerDocument16 pagesCooling Towerbakhtyar21No ratings yet