Professional Documents
Culture Documents
(CC Lab) Calcium & Magnesium
(CC Lab) Calcium & Magnesium
Uploaded by
Dennisse San JoseOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
(CC Lab) Calcium & Magnesium
(CC Lab) Calcium & Magnesium
Uploaded by
Dennisse San JoseCopyright:
Available Formats
CLINICAL CHEMISTRY 2 (MT 6324) LABORATORY
nd
MS. Canellie Canlas & Asst. Prof Vivian Asuncion | 2 Shifting
d
CALCIUM & MAGNESIUM
CALCIUM
● 5th most common element
● Most prevalent cation in the body
FUNCTIONS
● It is an essential element because it is involved
in several processes such as:
○ Bone mineralization
○ Blood coagulation
○ Neutral transmission
○ Muscle contraction
○ Cardiac contractility & conduction
○ Hormonal secretion
DISTRIBUTION ○ Vitamin D is obtained from sunlight in
the form of 7-dehydrocholesterol it
● Calcium is distributed in the following: would be converted to Pre Vitamin D3
○ 99% in the bone and the remaining 1% and then into Vitamin D. This Vitamin D
is found in the blood and ECF. will be hydroxylated by the
○ Of this 1%, calcium has three forms: 25-hydroxylase enzyme from the liver
■ 45% - Ionized form of calcium into 25-hydroxycholecalciferol. This
or free form of calcium- most 25-hydroxycholecalciferol is
important; physiologically active hydroxylated by 1-α hydroxylase into
form 1,25-dihydroxycholecalciferol which is
■ 40% bound to protein primarily the active form of vitamin D.
albumin ○ 1-α hydroxylase is being influenced by
■ 15% bound to anions such as the release of parathyroid hormone
citrate, bicarbonate and lactate. (PTH); when PTH is released, this will
activate the kidneys to secrete 1-α
hydroxylase to convert the inactive of
vitamin D into its active form
1,25-dihydroxycholecalciferol, and this is
the one responsible for the absorption of
calcium ions in the kidneys and intestine
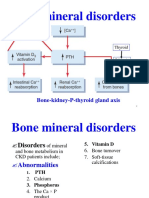
REGULATION
● Calcium is regulated by the following hormones:
● Promote Ca++ absorption
○ PTH (Parathyroid Hormone)
▪ Kidneys
▪ Bone
○ Vitamin D ○ As you can see in this image here, when
▪ Kidneys there is a low calcium concentration in
▪ Intestines the blood, this would trigger the release
of PTH and this hormone will act on the
bone and kidneys to increase Ca
absorption.
BERON • BORILLO • BUGARIN • CHENG • CO • DE LEON • GALLOSA • PABON | 1
MT 6324: CLINICAL CHEMISTRY 2 | LABORATORY | 2 ND SHIFTING
○ Take note that the form of Ca that is
detected by the glands is the ionized
form.
○ In the bone it promotes bone resorption ●
Calcium binds to the
while in the kidneys it promotes tubular dye to form a colored
reabsorption of Ca. Therefore, leading complex
to an increased Ca levels. This is where (Calcium-oCPC
the Vitamin D comes in. complex) and this will
● Decrease Ca++ levels be the one that is
○ Calcitonin measured
▪ total effect of calcitonin is to spectrophotometrically
have a decrease in calcium ● 8-hydroxyquinoline is
levels used to prevent or
▪ inhibitory to PTH and Vitamin D removed any
▪ when there is an increase in the interference from
ionized calcium levels, this will magnesium ions
trigger the release of calcitonin ■ Arsenazo III dye
from the thyroid gland, and fill ● The same principle
have the following actions: applies when the
1. decrease in intestinal Arsenazo III dye is
absorption of calcium used. The calcium will
ions bind to this dye to form
2. decrease in bone a colored complex
resorption ● Atomic absorption spectrophotometer
3. a decrease in calcium ○ reference method
reabsorption in the
kidneys Ionized Ca++ determination
● Ion Selective Electrode
○ use membranes impregnated with
special molecules that selectively, but
reversibly, bind Ca++ ions
○ an electric potential develops that is
proportional to the ionized Ca++
concentration
METHODS OF DETERMINATION
Total Ca++ determination
● Colorimetric Method
○ Calcium + R → AR complex (calcium
binds with the reagent/dye to form a ● As you can see in this image here, this electrode
colored complex, this will be the one (right portion) is the reference electrode while
measured spectrophotometrically; an this one (left portion) is selective for calcium ions
increase in absorbance is directly and within the electrodes, there are membranes
proportional with the ion concentration) inside which are incorporated in the silver
■ Ortho-cresolphthalein chloride and this one will selectively bind to
complexone (CPC) → in the calcium ions
experiment, this is the dye being ● And then an electro-potential will develop
used inside which is directly proportional to the
calcium ions
BERON • BORILLO • BUGARIN • CHENG • CO • DE LEON • GALLOSA • PABON | 2
MT 6324: CLINICAL CHEMISTRY 2 | LABORATORY | 2 ND SHIFTING
MATERIALS/INSTRUMENTATION PROCEDURES
● Automatic pipettor 1. Prepare and label cuvettes properly
● Pipette tips 2. Prepare the working reagent by mixing equal
● Tissue volumes of color reagent and buffer
● Parafilm 3. Follow the micropipetting scheme in the table
● Specimen (controls & unknowns - serum or below:
plasma)
● Spectrophotometer set at 570 nm and 37C Pipette into RB S CN CP U
cuvettes
(body temperature)
Standard - 0.02 mL - - -
● Cuvettes solution
● Centrifuge machine Control - - 0.02 mL - -
● Reagents normal
○ Calcium reagent kit supernatant
Control - - - 0.02 mL -
■ 8-hydroxyquinoline pathologic
● to remove any supernatant
interference from Unknown - - - - 0.02 mL
magnesium ions supernatant
■ O-cresolphthalein complexone Working 1.0 1.0 mL 1.0 mL 1.0 mL 1.0 mL
reagent mL
● dye to be used
Mix well. Measure absorbance of sample and standard
■ Hydrochloric acid against reagent blank within 5 to 50 minutes
● used to release the
calcium ions from the
CALCULATION FOR CALCIUM DETERMINATION
protein carriers
● So before the reaction (𝐴 𝑠𝑎𝑚𝑝𝑙𝑒)
to proceed, the calcium 𝐶 = 𝐶𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 𝑠𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝑥 (𝐴 𝑠𝑡𝑎𝑛𝑑𝑎𝑟𝑑)
𝑖𝑛 𝑚𝑔/𝑑𝐿 𝑜𝑟 𝑚𝑚𝑜𝑙/𝐿
ions must be released
from the protein carriers Conversion factor: multiply by 0.25 by mg/dL = mmol
by acidification of the
sample REFERENCE RANGES
○ Buffer & stabilizer
■ Lysine buffer (pH 11.1) Reference Range for Calcium
● alkaline pH of the buffer Serum Child 2.20-2.70 mmol/L
because the reaction or < 12 years (8.8-10.8 mg/dL)
will proceed in an Total plasma Adults 2.15 - 2.50 mmol/L
Calcium (8.6 - 10.0 mg/dL)
alkaline pH
Urine 2.50-7.50 mmol/day (100-300 mg/day)
■ Sodium azide (24h) varies with diet
○ Standard: Calcium (2mmol/L or 8 Child 1.20-1.38 mmol/L
mg/dL) (4.8-5.5 mg/dL)
Ionized Serum Adult 1.16 - 1.23 mmol/dL
calcium (4.6-5.3 mg/dL)
SPECIMEN
Plasma Adult 1.03 - 1.23 mmol/L
(4.1-4.9 mg/dL)
Total Ca++ determination Whole Adult 1.15 - 1.27 mmol/L
blood (4.6-5.1 mg/dL)
● Serum or lithium heparin plasma - preferred
● EDTA, oxalate
○ bind Ca++ CLINICAL SIGNIFICANCE
○ Anticoagulant such as EDTA and
oxalate will bind Ca++, therefore leading HYPERCALCEMIA
to a falsely decreased ion ● Primary hyperthyroidism
concentration ○ As mentioned earlier, parathyroid
hormone increases calcium absorption in
the kidneys and in the bone.
Ionized Ca++ determination ○ Increase in parathyroid hormone
● Samples must be collected anaerobically secretion → increase in calcium levels
● Heparinized whole blood - preferred ○ Adenoma of the gland
● Serum collected in sealed evacuated blood ○ Glandular hyperplasia
● Hyperthyroidism
tubes at room temperature ○ Because of the proximity or anatomical
○ may be used if clotting and location of the parathyroid gland to the
centrifugation is done quickly thyroid gland, an increase in the activity
BERON • BORILLO • BUGARIN • CHENG • CO • DE LEON • GALLOSA • PABON | 3
MT 6324: CLINICAL CHEMISTRY 2 | LABORATORY | 2 ND SHIFTING
of the thyroid gland will also lead to an ○ No active form of vitamin D that will be
increase in the activity of the parathyroid formed
gland. ● Rhabdomyolysis
● Benign familial hypocalciuria ○ An increase in the release of phosphate
○ Calcium sensing receptor in the from the cells binding with the calcium
parathyroid gland and in the kidneys, ions in the circulation
normally this calcium sensing receptor ● Pseudohypoparathyroidism
influence the secretion of PTH and the ○ End-organ resistance so the target
renal calcium secretion, an increase in tissues do not response to the PTH
the Ca ions, these calcium sensing
receptors inhibits PTH and the renal MAGNESIUM
absorption of Ca. Benign familial
● 4th most abundant cation in the body
hypocalciuria, defect in the calcium
sensing receptor therefore increases in ● 2nd most abundant intracellular ion
Ca levels and to hypercalcemia.
● Malignancy (PTH-rp) FUNCTIONS
○ tumor cells PTH related peptide, acts like ● Cofactor of cellular enzymes
PTH, leading to hypercalcemia ● DNA replication and transcription
● Multiple myeloma and prolonged immobilization ● Cellular energy metabolism
○ increase in bone resorption ● Membrane stabilization
● Increased Vitamin D
● Ion transport
○ more absorption of Ca ions
● Thiazide diuretics ● Nerve conduction
○ Inc tubular reabsorption of Ca
● Prolonged immobilization DISTRIBUTION
● 53% = bone
● 46% = muscle, other organs and soft tissues
● <1% = serum, RBCs
○ ⅓ bound to proteins (albumin)
○ ⅔
▪ 61% free/ionized state
(physiologically active)
▪ 5% complexed to other ions
(PO4-, citrate)
REGULATION
● Intestinal absorption
● Kidneys
○ Major organ that is responsible for the
overall regulation of Mg++ (reabsorb or
excrete)
○ Renal absorption:
▪ 25-50% in PCT(Proximal
Convoluted Tubule)
HYPOCALCEMIA ▪ 50-60% in Ascending limb
● Primary Hypoparathyroidism ▪ 2-5% in DCT(Distal Convoluted
○ Decrease in PTH, decrease absorption of Tubule)
Ca ions Note: Majority are present in the ascending limb in
● Hypomagnesemia
○ Inhibition of PTH secretion
contrast to other ions which are absorbed in the PCT
● Hypermagnesemia ● PTH increases in renal and intestinal Mg++
● Hypoalbuminemia reabsorption
○ Primarily bound to albumin, decrease in ○ but changes in ionized Ca++ have a
albumin decreases Ca greater effect on PTH secretion
● Acute pancreatitis ● Aldosterone and Thyroxine increase renal
○ Increase of lipase, lipase release Free excretion of Mg++
FA, binds to Ca, leading to hypocalcemia
● Vitamin D deficiency
○ Lead to a decrease in absorption of
calcium ions
● Renal disease
BERON • BORILLO • BUGARIN • CHENG • CO • DE LEON • GALLOSA • PABON | 4
MT 6324: CLINICAL CHEMISTRY 2 | LABORATORY | 2 ND SHIFTING
METHODS OF DETERMINATION SPECIMEN
● Non hemolyzed serum or lithium heparin
Total Ca++ determination plasma - preferred
● EDTA, oxalate, and citrate
● Colorimetric method
○ Not recommended because it will bind
○ The principle is the same as with
Mg++ causing falsely decreased ion
calcium determination. Magnesium ion
concentration.
binds with a dye to form a colored
● 24-hour urine
complex and the colored complex is the
○ Preferred because of diurnal variation
one read spectrophotometrically.
○ The increase in absorbance is directly
proportional with the added PROCEDURES
concentration 1. Prepare and label cuvettes properly
○ Calmagite method 2. Prepare the working reagent by mixing equal
▪ Mg++ + Calmagite → reddish volumes of color reagent and buffer
violet complex (read at 532 nm) 3. Follow the micropipetting scheme in the table
○ Formazan dye below:
▪ Mg++ + Formazan dye →
Pipette into RB S CN CP U
colored complex (read at 660 cuvettes
nm) Standard - 0.01 mL - - -
○ Methylthymol blue or Magon or solution
Xylidyl blue Control - - 0.01 mL - -
normal
▪ Mg++ + Methylthymol blue → supernatant
colored complex Control - - - 0.01 mL -
▪ This method is used in the pathologic
experiment in the laboratory supernatant
manual. Unknown - - - - 0.01 mL
supernatant
● Atomic Absorption Spectrophotometer Working 1.0 1.0 mL 1.0 mL 1.0 mL 1.0 mL
○ reference method reagent mL
Mix and incubate for 10mins at 37C. Measure the
Ionized Mg++ determination absorbance of the sample and the standard against the
reagent blank within 60 minutes
● Ion Selective Electrode
CALCULATION FOR MAGNESIUM
MATERIALS/INSTRUMENTATION
● Automatic pipettor DETERMINATION
● Pipette tips
(𝐴 𝑠𝑎𝑚𝑝𝑙𝑒)
● Tissue 𝐶 = 𝐶𝑜𝑛𝑐𝑒𝑛𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝑜𝑓 𝑠𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝑥 (𝐴 𝑠𝑡𝑎𝑛𝑑𝑎𝑟𝑑)
𝑖𝑛 𝑚𝐸𝑞/𝐿 𝑜𝑟 𝑚𝑚𝑜𝑙/𝐿
● Parafilm
● Specimen (controls & unknowns - serum or Conversion factor: multiply by 0.5 by mEq/L = mmol
plasma) ● Reference Range:
● Spectrophotometer set at 520 nm and 37C ○ Serum
● Cuvettes ■ 0.63-1.0 mmol/L
● Centrifuge machine ■ 1.26-2.10 mEq/L
● Reagents
○ Magnesium Reagent Kit CLINICAL SIGNIFICANCE
■ potassium carbonate
■ GEDTA (Glycoletherdiamine -
HYPERMAGNESEMIA
N, N, N’. N’ - tetraacetic acid)
● Acute/chronic renal failure
■ xylidyl blue ● Hypothyroidism
■ activators ● Hypoaldosteronism
○ Buffer & Stabilizer ● Hypopituitarism
■ TRIS buffer (pH 11.0) ○ There’s a decrease in the
■ Sodium azide thyroid stimulating
○ Standard: Magnesium (1.03 Decreased hormone therefore leading
mmol/L or 2.5 mg/dL; may vary) excretion to hyperthyroidism
Thyroxine and aldosterone increases
renal excretion of magnesium ions.
BERON • BORILLO • BUGARIN • CHENG • CO • DE LEON • GALLOSA • PABON | 5
MT 6324: CLINICAL CHEMISTRY 2 | LABORATORY | 2 ND SHIFTING
When there is a decrease of these ● Digitalis (interferes with Mg
hormones, these will lead to a decrease reabsorption)
in the excretion of magnesium ions Miscellaneous ● Excess lactation
leading to hypermagnesemia. ● Pregnancy
Increased ● Antacids
intake ● Enemas
(Medications ● Cathartics
that contain ● Therapeutics
magnesium ○ eclampsia
ions) ○ cardiac arrhythmia
● Dehydration
○ Reversible
Miscellaneous ● Bone carcinoma
● Bone metastases
○ both causing increased
bone loss leading to
increased Mg
HYPOMAGNESEMIA
Reduce intake ● Poor diet
● Prolonged magnesium
○ deficient IV therapy
○ chronic alcoholism
Decreased ● Malabsorption syndrome
absorption ● Surgical resection of small
intestine
● Nasogastric suction
● Pancreatitis
● Vomiting
● Diarrhea
● Laxative abuse
● Neonatal hypomagnesemia
● Primary Deficiency
○ selective
malabsorption of the
Mg ion in the intestine
● Chronic congenital
hypomagnesemia
○ defect in the transport
protein for magnesium
Renal
● Tubular disorder
● Glomerulonephritis
● Pyelonephritis
Leads to increased excretion of Mg ions
Endocrine
● Hyperparathyroidism
● Hyperaldosteronism
● Hyperthyroidism
● Hypercalcemia
Increased Leads to increased renal excretion of Mg
excretion ions
● Diabetic ketoacidosis
○ Mg ions are lost in the
urine along w/
glucosuria
Drug Induced
● Diuretics
● Antibiotics
● Cyclosporin (inhibits renal
absorption of Mg ions)
BERON • BORILLO • BUGARIN • CHENG • CO • DE LEON • GALLOSA • PABON | 6
You might also like
- KidneyDocument19 pagesKidneySridharNo ratings yet
- Drugs Affecting Calcium BalanceDocument63 pagesDrugs Affecting Calcium BalanceRd Chandane100% (1)
- Calcium, Phosphate and MagnesiumDocument46 pagesCalcium, Phosphate and Magnesiumkiedd_04100% (3)
- Calcium MetabolismDocument51 pagesCalcium MetabolismAlan ThomasNo ratings yet
- 3.2 Renal Physiology 6Document4 pages3.2 Renal Physiology 6Carmela MarianoNo ratings yet
- 16-K-Ca ImbalanceDocument11 pages16-K-Ca Imbalanceمصطفى محمد جواد كاظمNo ratings yet
- Calcium HomeostasisDocument38 pagesCalcium Homeostasiskelvinmaina9993No ratings yet
- Drugs Affecting Calcium Balance: Harsh Vinayak Roll No. 39 Mbbs 2 Yr Student BATCH 2019 Rdasmc, AyodhyaDocument24 pagesDrugs Affecting Calcium Balance: Harsh Vinayak Roll No. 39 Mbbs 2 Yr Student BATCH 2019 Rdasmc, AyodhyaA2Z GyanNo ratings yet
- Parathormone, CalcitoninDocument14 pagesParathormone, CalcitoninIbrahimNo ratings yet
- Calcium Phosphorus Metabolic DisordersDocument102 pagesCalcium Phosphorus Metabolic DisordersAME DENTAL COLLEGE RAICHUR, KARNATAKANo ratings yet
- Calcium HomeostasisDocument23 pagesCalcium HomeostasisBivek Singh RathoreNo ratings yet
- Calcium PPT BSDocument58 pagesCalcium PPT BSMedical NotesNo ratings yet
- Disorders of Calcium, Phosphorus, and Magnesium - Core CurriculumDocument6 pagesDisorders of Calcium, Phosphorus, and Magnesium - Core CurriculumSri PutrianaNo ratings yet
- Disorders of Calcium, Inorganic Phosphate and Magnesium Metabolism 1Document62 pagesDisorders of Calcium, Inorganic Phosphate and Magnesium Metabolism 1IiiNo ratings yet
- Calcium Homeostasis: Parathyroid Hormone, Calcitonin and Vitamin D3Document31 pagesCalcium Homeostasis: Parathyroid Hormone, Calcitonin and Vitamin D3Noval FebriNo ratings yet
- Calcium and Phosphate MetabolismDocument27 pagesCalcium and Phosphate MetabolismIshaqNo ratings yet
- Parathyroid-Hormone Calcium HomeostasisDocument58 pagesParathyroid-Hormone Calcium HomeostasisSudhakar LakavathNo ratings yet
- Learning Objectives Biochemistry of BonesDocument7 pagesLearning Objectives Biochemistry of BonesRaissa GabriellaNo ratings yet
- Calcium Balance I 2020Document35 pagesCalcium Balance I 2020Nadun MethwadaneNo ratings yet
- BMD and DialysisDocument30 pagesBMD and DialysisThe AbyssinicansNo ratings yet
- Mineral Metabolism.Document26 pagesMineral Metabolism.Shivanand MaliNo ratings yet
- 5675932Document89 pages5675932JuhiJahan AmanullahNo ratings yet
- Calcium Salts Calcitonin and CA RegulatorsDocument40 pagesCalcium Salts Calcitonin and CA RegulatorsSudhakar LakavathNo ratings yet
- Vitamin D MetabolismDocument16 pagesVitamin D MetabolismZille HumaNo ratings yet
- Calcium, Phosphate and Vitamin D MetabolismDocument25 pagesCalcium, Phosphate and Vitamin D MetabolismRendy LectusNo ratings yet
- Endo Lect - Vit D and CalcitoninDocument34 pagesEndo Lect - Vit D and CalcitonindoctorrfarrukhNo ratings yet
- Calcium MetabolismDocument41 pagesCalcium MetabolismadhillaagniNo ratings yet
- Calcium MetabolismDocument53 pagesCalcium MetabolismTanu ShreyaNo ratings yet
- Regulation of Calcium Parathyroid, Vitamin D & CalcitoninDocument29 pagesRegulation of Calcium Parathyroid, Vitamin D & CalcitoninJessica StewartNo ratings yet
- Alex Yartsev - Calcium Metabolism and HypercalcemiaDocument31 pagesAlex Yartsev - Calcium Metabolism and Hypercalcemiaahmedradwan2005No ratings yet
- Calcium and PhosporusDocument29 pagesCalcium and PhosporusJoe AjibadeNo ratings yet
- Parathyroid GlandDocument3 pagesParathyroid GlandElla OrtegaNo ratings yet
- 287 FullDocument4 pages287 FullRye CalderonNo ratings yet
- Hormonal Control of Calcium Homeostasis Chapter 9Document8 pagesHormonal Control of Calcium Homeostasis Chapter 9Roua SafwatNo ratings yet
- What Is The Most Abundant Mineral in The Body?Document33 pagesWhat Is The Most Abundant Mineral in The Body?Niño Española BenedictoNo ratings yet
- Renal & Electrolyted Physiology FinalDocument21 pagesRenal & Electrolyted Physiology FinalVondNo ratings yet
- Biochem 11Document5 pagesBiochem 11Abdullah RaufNo ratings yet
- Vitamin D Metabolism and Associated DiseasesDocument13 pagesVitamin D Metabolism and Associated DiseasesRiman LemechaNo ratings yet
- Endocrine Physiology: Dale Buchanan Hales, PHD Department of Physiology & BiophysicsDocument74 pagesEndocrine Physiology: Dale Buchanan Hales, PHD Department of Physiology & BiophysicsOngen AchillesNo ratings yet
- Bone Markers - FinalDocument79 pagesBone Markers - FinalSparrowNo ratings yet
- Vitamin D Synthesis.Document7 pagesVitamin D Synthesis.Iender LegjonNo ratings yet
- 001 Drugs Affecting Calcium RegulationDocument2 pages001 Drugs Affecting Calcium RegulationReddy Mohan100% (1)
- Endocrine 3Document115 pagesEndocrine 3Dessalegn LemmaNo ratings yet
- Parathyroid: Calcium and Vitamin DDocument135 pagesParathyroid: Calcium and Vitamin DPhysiology by Dr RaghuveerNo ratings yet
- Calcium and PhosphorusDocument34 pagesCalcium and Phosphorus075 Keerthighaa SNo ratings yet
- Parathyroid Hormone and Calcium Regulation: By: Abebe TDocument29 pagesParathyroid Hormone and Calcium Regulation: By: Abebe TLidiya TeshomeNo ratings yet
- Treatment of Hypo and Hypercalcemia FC For StudentsDocument20 pagesTreatment of Hypo and Hypercalcemia FC For Studentsjamil aoudeNo ratings yet
- Calcium HomeostasisDocument37 pagesCalcium Homeostasispolog.jm610No ratings yet
- Calcium and PhosphateDocument35 pagesCalcium and PhosphateSULEIMAN OMARNo ratings yet
- 15th Week Minerals 2023Document40 pages15th Week Minerals 2023VizhiNo ratings yet
- Calcium and Phosphate MetabolismDocument70 pagesCalcium and Phosphate MetabolismHariprasad L0% (1)
- LN CardioPart1 2023Document65 pagesLN CardioPart1 2023farahafiqahNo ratings yet
- Calcium MetabolismDocument28 pagesCalcium MetabolismAhmedkhaed100% (1)
- Calcium Metabolism PraveenDocument42 pagesCalcium Metabolism PraveenDr PraveenNo ratings yet
- Calcium and Phosphorus Metabolism in Health and Disease-1Document44 pagesCalcium and Phosphorus Metabolism in Health and Disease-1Igwe SolomonNo ratings yet
- Diuretics 160418020107Document21 pagesDiuretics 160418020107P meruguNo ratings yet
- Hormonal Control of Plasma CalciumDocument6 pagesHormonal Control of Plasma CalciumNaveen KumarNo ratings yet
- CA Vit D MetabolismDocument41 pagesCA Vit D MetabolismPramod N KNo ratings yet
- (Ih Lec) 1ST ShiftDocument73 pages(Ih Lec) 1ST ShiftDennisse San JoseNo ratings yet
- (CC Lec) Fluid & ElectrolytesDocument30 pages(CC Lec) Fluid & ElectrolytesDennisse San JoseNo ratings yet
- (CC Lab) Sodium and PotassiumDocument8 pages(CC Lab) Sodium and PotassiumDennisse San JoseNo ratings yet
- 3F Hema 1 Lab 1ST Shift Ola PDFDocument5 pages3F Hema 1 Lab 1ST Shift Ola PDFDennisse San JoseNo ratings yet
- Vent Silencer Data SheetDocument1 pageVent Silencer Data SheetRamazan YaşarNo ratings yet
- Straight Twist JointDocument3 pagesStraight Twist JointavanifbNo ratings yet
- I Want It AllDocument25 pagesI Want It AllEmanuelle OliveiraNo ratings yet
- GLXXMobilgrease FM SeriesDocument4 pagesGLXXMobilgrease FM SeriesMufti AhmadNo ratings yet
- Synthesis EssayDocument4 pagesSynthesis Essayapi-284842143No ratings yet
- Step by Step Lihation of The Internal Iliac ArteryDocument6 pagesStep by Step Lihation of The Internal Iliac ArteryAkbar PurnadiputraNo ratings yet
- Uzbekistan Pharma IndustryDocument50 pagesUzbekistan Pharma IndustryAnjum MushtaqNo ratings yet
- EREC-43 Load ScheduleDocument61 pagesEREC-43 Load SchedulePartha SundarNo ratings yet
- NDT Certi JaydeepDocument5 pagesNDT Certi JaydeepDarshanRavalNo ratings yet
- Dass42 PDFDocument2 pagesDass42 PDFJohaima HaronNo ratings yet
- General Management Project On PDFDocument29 pagesGeneral Management Project On PDFNishit KanchanNo ratings yet
- Automotive Diesel Fuel Specs.Document17 pagesAutomotive Diesel Fuel Specs.narendradounde143No ratings yet
- Functional Anatomy-RespirationDocument37 pagesFunctional Anatomy-RespirationASIIMWE WINNIE CATHERINENo ratings yet
- Skull Atlas: Anatomy & PhysiologyDocument6 pagesSkull Atlas: Anatomy & PhysiologyFrancheska Kyla GomezNo ratings yet
- ZMI Battery 7800-EnDocument2 pagesZMI Battery 7800-EnAbiNo ratings yet
- 3RD Quarter Learning TaskDocument6 pages3RD Quarter Learning TaskHazel marie LazoNo ratings yet
- Basic Formulas - Mini ChallengeDocument6 pagesBasic Formulas - Mini ChallengeMuktadur RahmanNo ratings yet
- 1725S1TKCE60132018 - Operasi Teknik Kimia III - Pertemuan 8 - TugasDocument2 pages1725S1TKCE60132018 - Operasi Teknik Kimia III - Pertemuan 8 - TugasPaulus Sampe LambiNo ratings yet
- Chapter 23 - SensorsDocument18 pagesChapter 23 - SensorsnikolasthermosolutionsNo ratings yet
- RTI Act Now Applicable in Private Schools - Chief Information Commission PDFDocument4 pagesRTI Act Now Applicable in Private Schools - Chief Information Commission PDFLive Law86% (7)
- 11 Soriano Et Al V Secretary of Finance and The CIRDocument1 page11 Soriano Et Al V Secretary of Finance and The CIRAnn QuebecNo ratings yet
- Specification For Fire Water HydrantDocument5 pagesSpecification For Fire Water HydrantThiru AnanthNo ratings yet
- LW EthericAcupunctureDocument3 pagesLW EthericAcupuncturethiago.msnbr259150% (2)
- Class Test 2 31 JULY 2021: Process Fluid FlowDocument6 pagesClass Test 2 31 JULY 2021: Process Fluid FlowEnabewhkom OhpmNo ratings yet
- DNR Duct Detector Installation DetailsDocument6 pagesDNR Duct Detector Installation DetailsBinu SulochananNo ratings yet
- Hospital Management System Project Proposal Hospital Management System Project ProposalDocument24 pagesHospital Management System Project Proposal Hospital Management System Project ProposalBimadraj Sharan SinhaNo ratings yet
- Soal PAT B.ING Kelas 4Document4 pagesSoal PAT B.ING Kelas 4NurmaAnggeliaNo ratings yet
- If Installing A New Clutch Go To Step 19. 2. Install The Special Tool in A ViseDocument18 pagesIf Installing A New Clutch Go To Step 19. 2. Install The Special Tool in A Viserolly abantoNo ratings yet
- HDFC Cashback Redemption FormDocument1 pageHDFC Cashback Redemption Formswapnilgadekar30% (1)
- AWS D1 1 D1 1M 2015 Structural Welding Code Steel Errata PDFDocument2 pagesAWS D1 1 D1 1M 2015 Structural Welding Code Steel Errata PDFbilling cbi housingNo ratings yet