Professional Documents
Culture Documents
Inggris 7.1 Dan 7.5
Inggris 7.1 Dan 7.5
Uploaded by
Rizkymubarok0 ratings0% found this document useful (0 votes)
18 views3 pages1) The Chernobyl nuclear power plant disaster in 1986 was the worst nuclear accident in history. During a safety test, operators deliberately interrupted cooling water flow to the reactor core, causing temperatures and pressure to rise dangerously. A series of explosions destroyed the reactor and released large amounts of radioactive material into the atmosphere.
2) Over a 10 day period, the burning reactor core continued to spew radioactive fission products into the air, exposing around 250 million people across Europe. Nearly 150,000 people within 60 km of the plant were permanently evacuated.
3) The human toll was immediate - several workers were killed, and 31 firefighters later died of acute radiation sickness. Long term effects included increased thyroid cancer in children near
Original Description:
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document1) The Chernobyl nuclear power plant disaster in 1986 was the worst nuclear accident in history. During a safety test, operators deliberately interrupted cooling water flow to the reactor core, causing temperatures and pressure to rise dangerously. A series of explosions destroyed the reactor and released large amounts of radioactive material into the atmosphere.
2) Over a 10 day period, the burning reactor core continued to spew radioactive fission products into the air, exposing around 250 million people across Europe. Nearly 150,000 people within 60 km of the plant were permanently evacuated.
3) The human toll was immediate - several workers were killed, and 31 firefighters later died of acute radiation sickness. Long term effects included increased thyroid cancer in children near
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
0 ratings0% found this document useful (0 votes)
18 views3 pagesInggris 7.1 Dan 7.5
Inggris 7.1 Dan 7.5
Uploaded by
Rizkymubarok1) The Chernobyl nuclear power plant disaster in 1986 was the worst nuclear accident in history. During a safety test, operators deliberately interrupted cooling water flow to the reactor core, causing temperatures and pressure to rise dangerously. A series of explosions destroyed the reactor and released large amounts of radioactive material into the atmosphere.
2) Over a 10 day period, the burning reactor core continued to spew radioactive fission products into the air, exposing around 250 million people across Europe. Nearly 150,000 people within 60 km of the plant were permanently evacuated.
3) The human toll was immediate - several workers were killed, and 31 firefighters later died of acute radiation sickness. Long term effects included increased thyroid cancer in children near
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
Download as docx, pdf, or txt
You are on page 1of 3
7.
1 | Nuclear Power Worldwide
Most people fl ip a light switch without thinking of the energy source that caused the bulb to
glow. But others, especially those who previously have lost electric power because of a storm or
a power blackout, may not as easily take electricity for granted. All too well they know the
sensation of hearing the switch click, but remaining in the dark.
Let’s assume that the power is working and that you switch on your coffee pot. If you live in the
United States, the odds are about 1 in 5 that a nuclear power plant is providing the electricity to
brew your coffee. But if you live in France, the odds are better than even. Nations of the world
differ in the extent to which they employ nuclear power commercially. For example, in the
United States, about 20% of the electric power is produced from just over 100 nuclear reactors,
all licensed by the Nuclear Regulatory Commission (NRC). These reactors operate at 65 sites in
31 states. As you can see by Figure 7.1, the electricity generated by these nuclear plants has
increased over the years,despite the drop in the number of operating reactors from its 1990 peak
of 112. Nonetheless,
in the United States, nuclear power remains a relatively steady 20% of the total production
because the other sources have increased as well.
When you brew your coffee a decade from now, from where will the electricity come? No new
nuclear plants have been built in the United States since 1978. Furthermore, nine nuclear plants
ceased their operations, some of them before their licenses expired. These include what was once
the nation’s largest, the Zion nuclear power station on the shores of Lake Michigan. Reasons
cited for plant closings included the competition of natural gas and the competitive pressures of
energy deregulation.
7.5 | Looking Backward to Go Forward
We need to examine the legacy of nuclear power—what has worked and what has not.
All nuclear plants use the process of fi ssion to produce energy; all produce radioactive fission
products. Have these radioactive products posed a danger in the past? Are they likely to in the
future? In this section, we consider a signifi cant part of the legacy; that is, the scenario of an
accidental release of radioisotopes into the environment. In 1979, a fi lm called The China
Syndrome portrayed a near-disaster in a fi ctitious nuclear power plant. The heat-generating fi
ssion reaction went critical and a meltdown of the reactor was imminent. Supposedly, the heat
was capable of melting the underlying rock all the way to China. But in the nick of time, the
safety features of the system prevailed and no meltdown occurred.
Seven years later on April 26, 1986, the engineers of the very real Chernobyl power plant in
Ukraine, then part of the Soviet Union, were less fortunate (Figure 7.13). This plant had four
reactors—two built in the 1970s and two more in the 1980s—all near the town of Chernobyl
(pop. 12,500). Water from the nearby Pripyat River was used to cool the reactors. Although the
surrounding region was not heavily populated, nonetheless approximately 120,000 people lived
within a 30-km radius.
Chernobyl stands as the world’s worst nuclear power plant accident. What went wrong? During
an electric power safety test at the Chernobyl Unit 4 reactor, operators deliberately interrupted
the fl ow of cooling water to the core as part of the test. The temperature of the reactor rose
rapidly. In addition, the operators had left an insuffi cient number of control rods in the reactor
(that couldn’t be reinserted quickly enough), and the steam pressure was too low to provide
coolant (due to both operator error and faulty design). A chain of events quickly produced a
disaster. An overwhelming power surge produced heat, rupturing the fuel elements, and releasing
hot reactor fuel particles. These, in turn, exploded on contact with the coolant water, and the
reactor core was
destroyed in seconds. The graphite used to slow neutrons in the reactor caught fi re in the heat.
When water was sprayed on the burning graphite, the water and graphite reacted chemically to
produce hydrogen gas, which exploded when it reacted with oxygen in the air.
2 H2O(l) _ C(graphite) 2 H2(g) _ CO2(g) [7.7]
2 H2(g) _ O2(g) 2 H2O(g) [7.8]
The explosion blasted off the 4000-ton steel plate covering the reactor (Figure 7.14).
Although a “nuclear” explosion never occurred, the fi re and explosions of hydrogen blew vast
quantities of radioactive material out of the reactor core and into the atmosphere. Fires started in
what remained of the building. In a short time, the plant lay in ruins. The head of the crew on
duty at the time of the accident wrote: “It seemed as if the world was coming to an end . . . I
could not believe my eyes; I saw the reactor ruined by the explosion. I was the fi rst man in the
world to see this. As a nuclear engineer I realized the consequences of what had happened. It was
a nuclear hell. I was gripped with fear.” ( Scientifi c American, April 1996, p. 44)
The disaster continued. As the reactor burned, for 10 days it continued to spew large quantities of
radioactive fi ssion products into the atmosphere. Nearly 150,000 people living within 60 km of
the power plant were permanently evacuated after the meltdown. People in nearby regions
reported an odd, bitter, and metallic taste as they inhaled the invisible particles. The release of
radioactivity was estimated to be on the order of 100 of the atomic bombs dropped on Hiroshima
and Nagasaki. The radioactive dust cut a swath across Ukraine, Belarus, and up into Scandinavia,
affecting some who never had benefi ted from the power plant but nonetheless shared in its risks.
The human toll was immediate. Several people working at the plant were killed outright, and
another 31 fi refi ghters died in the cleanup process from acute radiation sickness, a topic we
examine in a later section. An estimated 250 million people were exposed to levels of radiation
that ultimately may shorten their lives. Included in this figure are 200,000 “liquidators,” people
who buried the most hazardous wastes and constructed a 10-story concrete structure (“the
sarcophagus”) to surround the failed reactor. One of the particularly hazardous radioisotopes
released was iodine-131. It is a
beta emitter with an accompanying gamma ray.
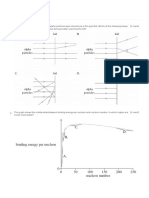
If ingested, I-131 can cause thyroid cancer. In the contaminated area near Chernobyl, the
incidence of thyroid cancer increased sharply, especially for those younger than age 15 (Figure
7.15). As of 2001, more than 700 children in Belarus, a neighboring country, were treated for
thyroid cancer. Fortunately, with treatment, the survival rate for thyroid cancer is high and most
have survived. As Dr. Akira Sugenoya, a Japanese physician who volunteered his expertise in
Belarus to treat the children suffering from thyroid cancer, remarked “The last chapter of the
terrible accident is far
from written.”
You might also like
- The Control Room Operator PDFDocument38 pagesThe Control Room Operator PDFMatthew100% (2)
- Jeremy Clawson - Rough DraftDocument9 pagesJeremy Clawson - Rough Draftapi-546692450No ratings yet
- Devastating Nuclear Accidents throughout History: Causes and Results - Science Book for Kids 9-12 | Children's Science & Nature BooksFrom EverandDevastating Nuclear Accidents throughout History: Causes and Results - Science Book for Kids 9-12 | Children's Science & Nature BooksNo ratings yet
- Nuclear PowerDocument8 pagesNuclear PowerStefaniaNo ratings yet
- By: Grade:: Mariam Sayyar 12Document4 pagesBy: Grade:: Mariam Sayyar 12Maryoom SalmanNo ratings yet
- Nuclear AccidentDocument8 pagesNuclear AccidentMD. MAHFUGE RAHMAN TAMIMNo ratings yet
- Forbidden Energy PaperDocument15 pagesForbidden Energy Paperapi-231969725No ratings yet
- Final Report On Nuclear ReactionDocument13 pagesFinal Report On Nuclear ReactionhelperforeuNo ratings yet
- Nuclear (Good or Bad)Document22 pagesNuclear (Good or Bad)Adnan Zaki BunyaminNo ratings yet
- Rhodes 2018 Why Nuclear Power Must Be Part of The Energy Solution - Yale E360Document7 pagesRhodes 2018 Why Nuclear Power Must Be Part of The Energy Solution - Yale E360May Myat Noe KhineNo ratings yet
- Introduction To Nuclear Energy For Civilian PurposesDocument5 pagesIntroduction To Nuclear Energy For Civilian PurposesDeen ZakariaNo ratings yet
- Joyee Basu: Nuclear Energy: The Peaceful Atom?Document4 pagesJoyee Basu: Nuclear Energy: The Peaceful Atom?Joyee BasuNo ratings yet
- Nuclear EnergyDocument60 pagesNuclear EnergyArun KumarNo ratings yet
- WP 3 GerchicknuclearpowerfinalDocument17 pagesWP 3 Gerchicknuclearpowerfinalapi-218717967No ratings yet
- Redilla Semester PaperDocument12 pagesRedilla Semester Paperapi-316657763No ratings yet
- Meltdown 1Document6 pagesMeltdown 1Randy BrockmannNo ratings yet
- On The Implications of Nuclear Power Final 1Document30 pagesOn The Implications of Nuclear Power Final 1api-623860027No ratings yet
- English Thesis - Nuclear Power PlantDocument14 pagesEnglish Thesis - Nuclear Power PlantHaiqal Halim ハイカル0% (1)
- Myp Chemistry EssayDocument3 pagesMyp Chemistry Essayapi-318867868No ratings yet
- Literature Review FinalDocument10 pagesLiterature Review Finalapi-459981181No ratings yet
- TAUFIA ALIYAR - Attachment: PDF: WW - Lev.1 - No.1Document7 pagesTAUFIA ALIYAR - Attachment: PDF: WW - Lev.1 - No.1TAUFIA ALIYARNo ratings yet
- Julia R. Larsen English 120.040 08 February 2015 Coal For The FutureDocument5 pagesJulia R. Larsen English 120.040 08 February 2015 Coal For The Futureapi-285193441No ratings yet
- The Legacy of Three Mile Island: "What's Up With The Weather?"Document3 pagesThe Legacy of Three Mile Island: "What's Up With The Weather?"gayneNo ratings yet
- Nuclear EnergyDocument4 pagesNuclear EnergyVasil GeorgijevskiNo ratings yet
- Nuclear Power HistolgyDocument40 pagesNuclear Power HistolgyPodremos llegar a los 10000 sin ningun videoNo ratings yet
- Nuclear Accidents and HolocaustDocument3 pagesNuclear Accidents and HolocaustShiju R Nair50% (4)
- Pros and Cons of Nuclear Energy-10 ExplanationsDocument5 pagesPros and Cons of Nuclear Energy-10 ExplanationsAgieLazarefNo ratings yet
- MEE22 GRP-3 The Chernobyl AccidentDocument18 pagesMEE22 GRP-3 The Chernobyl AccidentNelia BrionesNo ratings yet
- Nuclear Energy EssayDocument8 pagesNuclear Energy Essay3813561577No ratings yet
- Nuclear Power FDocument10 pagesNuclear Power Fsik160100% (1)
- Production: Nuclear Energy Is Released by The Splitting (Fission) or Merging Together (Fusion) of TheDocument10 pagesProduction: Nuclear Energy Is Released by The Splitting (Fission) or Merging Together (Fusion) of TheFatin Farhanah NazarudinNo ratings yet
- The Nuclear Option: Is Atomic Energy Clean and Green?Document2 pagesThe Nuclear Option: Is Atomic Energy Clean and Green?fernandosanzy_366552No ratings yet
- 5 Worst Nuclear Disasters in The World - HowStuffWorksDocument8 pages5 Worst Nuclear Disasters in The World - HowStuffWorksMenona AnconaNo ratings yet
- The Nuclear Debate - PhysicsDocument2 pagesThe Nuclear Debate - PhysicsroshansdecostaNo ratings yet
- Impact of Nuclear Power To The WorldDocument15 pagesImpact of Nuclear Power To The WorldTaniya WijesinghaNo ratings yet
- Chernobyl 1Document21 pagesChernobyl 1Abhishek RayangoudarNo ratings yet
- Nuclear EnergyThe Sun and Stars Are Seemingly Inexhaustible Sources of EnergyDocument4 pagesNuclear EnergyThe Sun and Stars Are Seemingly Inexhaustible Sources of EnergyMohammad AhsanNo ratings yet
- Research Paper in PHYSICSDocument8 pagesResearch Paper in PHYSICSAngelica Rico100% (1)
- Class 12Document64 pagesClass 12vishwaNo ratings yet
- Chernobyl Nuclear Power Plant Case Study Roselle Marie D. Azucena, MAN, MBADocument12 pagesChernobyl Nuclear Power Plant Case Study Roselle Marie D. Azucena, MAN, MBAroselle azucenaNo ratings yet
- Tml#Ixzz3O2Up9Iro: Read More: Follow UsDocument3 pagesTml#Ixzz3O2Up9Iro: Read More: Follow UsLuana BarretoNo ratings yet
- 12 Physics Project 1Document21 pages12 Physics Project 1Suresh kumar AgrawalNo ratings yet
- Meded 15Document7 pagesMeded 15api-553659197No ratings yet
- Nuclear EnergyDocument4 pagesNuclear Energymaria yvonne camilotesNo ratings yet
- Nuclear EnergyDocument35 pagesNuclear Energypaulhoff7No ratings yet
- MEE22 GRP-3 The Chernobyl AccidentDocument18 pagesMEE22 GRP-3 The Chernobyl AccidentNelia BrionesNo ratings yet
- 50 Years of Nuclear Energy1 PDFDocument9 pages50 Years of Nuclear Energy1 PDFRomly Tri TjahjantoNo ratings yet
- A Brief History of Nuclear Accidents Worldwide - Union of Concerned ScientistsDocument4 pagesA Brief History of Nuclear Accidents Worldwide - Union of Concerned ScientistsAjay SharmaNo ratings yet
- Nuclear Meltdown AlertDocument3 pagesNuclear Meltdown AlertInqueNo ratings yet
- Chem DebateDocument16 pagesChem Debateapi-513510848No ratings yet
- Nuclear EnergyDocument6 pagesNuclear EnergyMuhammad Rafianur ZulmiNo ratings yet
- Chernobyl Case Study PDFDocument10 pagesChernobyl Case Study PDFPranav Balsara100% (1)
- NuclearpowerDocument3 pagesNuclearpowerapi-250652876No ratings yet
- Nuclear Power StudyDocument5 pagesNuclear Power StudySaniru JayasingheNo ratings yet
- Chernobyl Disaster - WikipediaDocument62 pagesChernobyl Disaster - WikipediaIrtiza HussainNo ratings yet
- Fukushima Nuclear Disaster: Mark HoltDocument15 pagesFukushima Nuclear Disaster: Mark HoltIranurarbaatuljannah100% (1)
- Nuclear 2Document7 pagesNuclear 2api-302936360100% (1)
- Nuclear HazardsDocument18 pagesNuclear HazardsNavya Shree100% (2)
- Meitnerium in Honor of This Extraordinary Physicist.: U Caused by Neutron BombardmentDocument5 pagesMeitnerium in Honor of This Extraordinary Physicist.: U Caused by Neutron BombardmentMilescent Rose Juguilon PadillaNo ratings yet
- Fukushima: The Story of a Nuclear DisasterFrom EverandFukushima: The Story of a Nuclear DisasterRating: 3.5 out of 5 stars3.5/5 (21)
- 12-0308 ACRS XCRPT ML12080A125Document386 pages12-0308 ACRS XCRPT ML12080A125Sam HobbsNo ratings yet
- Topic 8.1 - Energy SourcesDocument92 pagesTopic 8.1 - Energy SourcesAnshulNo ratings yet
- Errata, Lamarsh Intro To Nuc EngDocument4 pagesErrata, Lamarsh Intro To Nuc EngBen ClemensNo ratings yet
- Muhammad Farooq, C.V.Document4 pagesMuhammad Farooq, C.V.taha88No ratings yet
- B353-OL3-057-Part-1 - Pres - RevC - Core Related - EGEDocument78 pagesB353-OL3-057-Part-1 - Pres - RevC - Core Related - EGEvrajakisoriDasiNo ratings yet
- Sop-100 Replacement of Radiaactive SourceDocument3 pagesSop-100 Replacement of Radiaactive SourceOSAMANo ratings yet
- PSV BasicsDocument24 pagesPSV BasicsBobby VonnNo ratings yet
- The Power Plants and Their Capacity in TurkeyDocument4 pagesThe Power Plants and Their Capacity in TurkeyElif SalmanNo ratings yet
- Crane in ChinaDocument25 pagesCrane in Chinasabah8800No ratings yet
- Modeling of Emergency Diesel Generators PDFDocument9 pagesModeling of Emergency Diesel Generators PDFyaoNo ratings yet
- The Stony Brook Press - Volume 4, Issue 25Document11 pagesThe Stony Brook Press - Volume 4, Issue 25The Stony Brook PressNo ratings yet
- Notice: Alaska Native Claims Selection: Garfield and Rio Blanco Counties, CODocument2 pagesNotice: Alaska Native Claims Selection: Garfield and Rio Blanco Counties, COJustia.comNo ratings yet
- Impersonal Pasive E9Document3 pagesImpersonal Pasive E9Hoàng Ngân100% (1)
- LNG, CNG, LPG, NGLDocument6 pagesLNG, CNG, LPG, NGLshuvo134No ratings yet
- BOMAFADocument19 pagesBOMAFAConcept -CEPLNo ratings yet
- 1.-T7-1 T-CuestionesDocument48 pages1.-T7-1 T-CuestionesAnonymous zP1ek3ya5nNo ratings yet
- J PowerDocument1 pageJ PowerJagabanta NingthoujamNo ratings yet
- 200 Top Electrical Q&ans PDFDocument26 pages200 Top Electrical Q&ans PDFRajesh MachchaNo ratings yet
- Hydrocracking Catalyst and Processing DevelopmentsDocument7 pagesHydrocracking Catalyst and Processing DevelopmentsLindsey BondNo ratings yet
- Lecture 9Document53 pagesLecture 9plyx xyNo ratings yet
- P1298 PostersDocument433 pagesP1298 PostersAli SharafNo ratings yet
- ML12044A208 Human Reliability Analysis (HRA) NRC TRAININGDocument298 pagesML12044A208 Human Reliability Analysis (HRA) NRC TRAININGnukafridiNo ratings yet
- Astm A 691 .98Document6 pagesAstm A 691 .98FrengkiNo ratings yet
- Comsy - SoftwareDocument9 pagesComsy - SoftwareAlberto Carel SimanjuntakNo ratings yet
- Nuclear Power Corporation of India LTDDocument7 pagesNuclear Power Corporation of India LTDDEBARSHEECHAKRABARTI4245No ratings yet
- Safety Practices in Chemical and Nuclear IndustriesDocument91 pagesSafety Practices in Chemical and Nuclear IndustriesJohn Harvey Siriban LozanoNo ratings yet
- Roll No./: Question Booklet Number/ Question Booklet SeriesDocument24 pagesRoll No./: Question Booklet Number/ Question Booklet SeriesMohit RanaNo ratings yet
- Development of A New Monte Carlo Reactor Physics Code VTTDocument241 pagesDevelopment of A New Monte Carlo Reactor Physics Code VTTtarek el zayatNo ratings yet
- Linear Piston Actuators: by Sekhar Samy, CCI, and Dave Stemler, CCIDocument18 pagesLinear Piston Actuators: by Sekhar Samy, CCI, and Dave Stemler, CCIapi-3854910No ratings yet