Professional Documents
Culture Documents
Allow Answer in J If (B) (I) Expressed in Joule (ECF), Otherwise Award (1 Max)
Allow Answer in J If (B) (I) Expressed in Joule (ECF), Otherwise Award (1 Max)
Uploaded by
superpooh-1Copyright:
Available Formats
You might also like
- PDFDocument50 pagesPDFk_gh22No ratings yet
- Physics 2P - Specimen PaperDocument20 pagesPhysics 2P - Specimen PaperHan Thi Win KoNo ratings yet
- 1000 Solved Problem in Modern Physics-104Document1 page1000 Solved Problem in Modern Physics-104Sano ManjiroNo ratings yet
- Dual Nature ExemplarDocument7 pagesDual Nature Exemplarvj jNo ratings yet
- Physics: Chapter - Dual Nature of Radiation and Matter Chapterwise Practise Problems (CPP) For NEETDocument12 pagesPhysics: Chapter - Dual Nature of Radiation and Matter Chapterwise Practise Problems (CPP) For NEETsoyel afridiNo ratings yet
- QM Ts 01 2024Document2 pagesQM Ts 01 2024Aviraj KhareNo ratings yet
- 14 15 H2 Quantum Physics1 NotesDocument29 pages14 15 H2 Quantum Physics1 NotesAgus LeonardiNo ratings yet
- Modern Physics EXERCISE-1 Qs. + Sol..pmdDocument18 pagesModern Physics EXERCISE-1 Qs. + Sol..pmdSubhangi MohantyNo ratings yet
- Modern Physics IDocument2 pagesModern Physics IAnik GhoshNo ratings yet
- Physics: DPP - Daily Practice ProblemsDocument8 pagesPhysics: DPP - Daily Practice ProblemsAnonymous 9uu04elNo ratings yet
- 2019-Key Physics Equations and Experiments Explained and Derived by Energy Wave EquationsDocument29 pages2019-Key Physics Equations and Experiments Explained and Derived by Energy Wave Equationsjoao pedro GomesNo ratings yet
- Physics Modern Physics IDocument15 pagesPhysics Modern Physics Ianuveshr13No ratings yet
- Assignment 2&3Document4 pagesAssignment 2&3Shakun Kumar Padhy100% (1)
- As Wet 6Document8 pagesAs Wet 6Rsrao JNo ratings yet
- DUAL NATURE TestDocument2 pagesDUAL NATURE TestChitesh MarmatNo ratings yet
- Pyl101 QM L2Document15 pagesPyl101 QM L2kamakshi gargNo ratings yet
- Atoms PYQ SDocument2 pagesAtoms PYQ Spatiminati2020No ratings yet
- Photoelectric Effect & Atomic Spectra 2 MSDocument8 pagesPhotoelectric Effect & Atomic Spectra 2 MSGulab RayNo ratings yet
- VG FRJ CIr YTa DUMz Y69 VMDocument6 pagesVG FRJ CIr YTa DUMz Y69 VMAyisha AfraNo ratings yet
- 03-Electron, Photon, PEE-Critical Question + SolutionDocument32 pages03-Electron, Photon, PEE-Critical Question + SolutionRNo ratings yet
- HL MSDocument12 pagesHL MSChinmay BhaveNo ratings yet
- Exercise: Violet: Iitians Spectrum EdutechDocument4 pagesExercise: Violet: Iitians Spectrum EdutechAarav ShahNo ratings yet
- Wave-Particle DualityDocument3 pagesWave-Particle DualityNyak Perera50% (2)
- Part One Geometrical Optics: Light RaysDocument13 pagesPart One Geometrical Optics: Light Raysgoran physyaNo ratings yet
- Duarte PhotoelectricDocument15 pagesDuarte PhotoelectricKonata OzumiNo ratings yet
- Tutorial 18A: Photoelectric Effect Photoelectric EffectDocument2 pagesTutorial 18A: Photoelectric Effect Photoelectric EffectAgus LeonardiNo ratings yet
- PhysicsDocument4 pagesPhysicsmathsbykeshavNo ratings yet
- Dual Nature of Radiation & Matter DPPDocument4 pagesDual Nature of Radiation & Matter DPPChristopher NolanNo ratings yet
- IITBHU Physics Assignmnet2Document2 pagesIITBHU Physics Assignmnet2Yash BhosaleNo ratings yet
- Topic No. 1 - Introduction To Atomic Structure-1-1Document92 pagesTopic No. 1 - Introduction To Atomic Structure-1-1Chand HiraniNo ratings yet
- QP ch3Document13 pagesQP ch3phuctranlvNo ratings yet
- Time Averaged Total Force On A Dipolar Sphere in An Electromagnetic FieldDocument3 pagesTime Averaged Total Force On A Dipolar Sphere in An Electromagnetic Fieldbagus putraNo ratings yet
- Pellegrini 2001 0119Document5 pagesPellegrini 2001 0119Particle Beam Physics LabNo ratings yet
- Dosimetric Principles, Quantities and UnitsDocument26 pagesDosimetric Principles, Quantities and UnitsRajesh Padiyaar SinghNo ratings yet
- 1modern Physics 1 - AnsDocument24 pages1modern Physics 1 - Ansrineeth22745No ratings yet
- 1 Basics of Optical Emission and AbsorptionDocument10 pages1 Basics of Optical Emission and AbsorptionChuxuan SunNo ratings yet
- Tsubono Calculation of Helium Ground State Energy by BohrDocument10 pagesTsubono Calculation of Helium Ground State Energy by BohrDr. John BeveridgeNo ratings yet
- The Magnetic Field Induced by A Lightning Strike's Indirect Effect Double Exponential Current WaveformDocument5 pagesThe Magnetic Field Induced by A Lightning Strike's Indirect Effect Double Exponential Current WaveformVijay KumarNo ratings yet
- EMG Theory of The PhotonDocument22 pagesEMG Theory of The Photondiablo441No ratings yet
- ch17 PDFDocument14 pagesch17 PDFRodrigo S QuirinoNo ratings yet
- Chapter 40Document53 pagesChapter 40ArthurNo ratings yet
- 126Document18 pages126rabinNo ratings yet
- The Particle Properties of Waves: Applied Modern Physics - Eceg 241Document5 pagesThe Particle Properties of Waves: Applied Modern Physics - Eceg 241YITBAREKNo ratings yet
- Sachi SPECTROSCOPY NOTES PDFDocument17 pagesSachi SPECTROSCOPY NOTES PDFarchana giriNo ratings yet
- 29 Particles and WavesDocument14 pages29 Particles and WavesMikaela Rome BigayNo ratings yet
- Compton ScatteringDocument6 pagesCompton Scatteringffr.gimenezNo ratings yet
- Two-Dimensional Numerical Simulation of A Planar Radio-Frequency Atmospheric Pressure Plasma SourceDocument5 pagesTwo-Dimensional Numerical Simulation of A Planar Radio-Frequency Atmospheric Pressure Plasma SourceMilica IvanovicNo ratings yet
- MIT8 04S16 LecNotes3 PDFDocument8 pagesMIT8 04S16 LecNotes3 PDFJefersonNo ratings yet
- Prac Que MP Dual NatureDocument5 pagesPrac Que MP Dual Naturetasneemkirana900No ratings yet
- Atomic StructureDocument4 pagesAtomic StructurePriyanshu PopNo ratings yet
- Lecture 07 - Introduction To Quantum MechanicsDocument26 pagesLecture 07 - Introduction To Quantum MechanicsArc ZeroNo ratings yet
- Lecture 07 - Introduction To Quantum MechanicsDocument26 pagesLecture 07 - Introduction To Quantum MechanicsArc ZeroNo ratings yet
- Wave Properties of ParticlesDocument4 pagesWave Properties of ParticlesPRIYAA A/P JAYASANKAR / UPMNo ratings yet
- 136 Sabi2007Document7 pages136 Sabi2007Jose M. MassaNo ratings yet
- Unit - 7 (Dual-Nature)Document2 pagesUnit - 7 (Dual-Nature)garvit1223No ratings yet
- A Heitler Model of Extensive Air ShowersDocument11 pagesA Heitler Model of Extensive Air ShowersRafaelawNo ratings yet
- M40 Knig2461 04 Ism C40Document20 pagesM40 Knig2461 04 Ism C40kymm7827No ratings yet
- Topic 3 Waves and The Particle Nature of Light 3D Quantum PhysicsDocument3 pagesTopic 3 Waves and The Particle Nature of Light 3D Quantum Physicsmaram shbairNo ratings yet
- Compton - Scattering (7) CorrectedDocument11 pagesCompton - Scattering (7) CorrectedLim Zheng LiangNo ratings yet
- Electron Beam-Specimen Interactions and Simulation Methods in MicroscopyFrom EverandElectron Beam-Specimen Interactions and Simulation Methods in MicroscopyNo ratings yet
- 4ph1 - 1p - Que - 20190523 (Q10 Series Circuit)Document2 pages4ph1 - 1p - Que - 20190523 (Q10 Series Circuit)superpooh-1No ratings yet
- 4ph1 - 1p - Que - 20190523 (Q5 Hooke's Law and Elastic Limit)Document2 pages4ph1 - 1p - Que - 20190523 (Q5 Hooke's Law and Elastic Limit)superpooh-1No ratings yet
- Physics: Pearson Edexcel International GCSE (9-1)Document36 pagesPhysics: Pearson Edexcel International GCSE (9-1)superpooh-1100% (2)
- Pages From s19-8421-03 (Q7 Quantum)Document4 pagesPages From s19-8421-03 (Q7 Quantum)superpooh-1No ratings yet
- @gmail - Co: 17 The Diagram Shows A Simplified Version of The Apparatus Used in An Experiment To DetermineDocument3 pages@gmail - Co: 17 The Diagram Shows A Simplified Version of The Apparatus Used in An Experiment To Determinesuperpooh-1No ratings yet
- @gmail: Answer All QuestionsDocument3 pages@gmail: Answer All Questionssuperpooh-1No ratings yet
- 4PH0 - 1P - Que - 20170112 (Q5 I-V Graph, Circuit)Document3 pages4PH0 - 1P - Que - 20170112 (Q5 I-V Graph, Circuit)superpooh-1No ratings yet
- Hong Kong Examinations and Assessment Authority: Pp-Dse-Cs (Phy) - 1 MsDocument8 pagesHong Kong Examinations and Assessment Authority: Pp-Dse-Cs (Phy) - 1 Mssuperpooh-1No ratings yet
- Pages From s19-8421-03 (Q2 Wave)Document3 pagesPages From s19-8421-03 (Q2 Wave)superpooh-1No ratings yet
- 2015 MC AnsDocument13 pages2015 MC Anssuperpooh-1No ratings yet
- 2015 DSE Phy 2-MS (E)Document8 pages2015 DSE Phy 2-MS (E)superpooh-1No ratings yet
- Suggested Answers and Marking Schemes: Physics Sample Mock Paper For HkdseeDocument9 pagesSuggested Answers and Marking Schemes: Physics Sample Mock Paper For Hkdseesuperpooh-1No ratings yet
- Physics Paper 1: (Sample Mock Paper) Section B: Question-Answer Book BDocument16 pagesPhysics Paper 1: (Sample Mock Paper) Section B: Question-Answer Book Bsuperpooh-1No ratings yet
- N12 PhotoelectricDocument1 pageN12 Photoelectricsuperpooh-1No ratings yet
- Phy - HKDSE2013 - Mock - 1A - EEDocument17 pagesPhy - HKDSE2013 - Mock - 1A - EEsuperpooh-1No ratings yet
- Hong Kong Examinations and Assessment Authority: Pp-Dse-Phy 1-1 MsDocument17 pagesHong Kong Examinations and Assessment Authority: Pp-Dse-Phy 1-1 Mssuperpooh-1No ratings yet
- Physics Paper 1 DR Ken ChanDocument19 pagesPhysics Paper 1 DR Ken Chansuperpooh-1No ratings yet
- 2015 May 20 Q9 (Edexcel 4PH0 FBI)Document2 pages2015 May 20 Q9 (Edexcel 4PH0 FBI)superpooh-1No ratings yet
- Physics Answer Key DR Ken ChanDocument7 pagesPhysics Answer Key DR Ken Chansuperpooh-1No ratings yet
- Energy Stores and TransfersDocument4 pagesEnergy Stores and Transferssuperpooh-1No ratings yet
- BBMP 1103 Matematik PengurusanDocument24 pagesBBMP 1103 Matematik PengurusanSean GomezNo ratings yet
- Gas Laws: Sagana National High SchoolDocument17 pagesGas Laws: Sagana National High SchoolHelma Jabello AriolaNo ratings yet
- Jackson 1 7 Homework SolutionDocument3 pagesJackson 1 7 Homework SolutionAna MariaNo ratings yet
- Final Exam Spectroscopy 2022 23 RepeatDocument17 pagesFinal Exam Spectroscopy 2022 23 RepeatIris BenardeteNo ratings yet
- Physics - 1 - LESSON 1 (Final Term - Summer 23)Document18 pagesPhysics - 1 - LESSON 1 (Final Term - Summer 23)fardinmojumdar123No ratings yet
- Van Der Heijden A.M.A. (Ed.) W. T. Koiter's Elastic Stability of Solids and Structures (CUP, 2009) (ISBN 0521515289) (O) (240s) - EMDocument240 pagesVan Der Heijden A.M.A. (Ed.) W. T. Koiter's Elastic Stability of Solids and Structures (CUP, 2009) (ISBN 0521515289) (O) (240s) - EMviovinNo ratings yet
- Classifying Deviation From Standard Quantum Behavior Using Kullback-Leibler DivergenceDocument13 pagesClassifying Deviation From Standard Quantum Behavior Using Kullback-Leibler DivergenceMohammad zaid ZazNo ratings yet
- To Estimate Charge On Pith Ball Class XIIDocument19 pagesTo Estimate Charge On Pith Ball Class XIIDDDPPNo ratings yet
- chpt2 Vector Analysis Part 2Document25 pageschpt2 Vector Analysis Part 2Ahmet imanlıNo ratings yet
- Modul 2.3 Physics Form 4 - AnswerDocument6 pagesModul 2.3 Physics Form 4 - AnswerNURSHAFAZLEEN BINTI AK DAMIT KPM-GuruNo ratings yet
- Quan TenDocument218 pagesQuan TenAngelo MartinezNo ratings yet
- JEE Overview TejaswiDocument6 pagesJEE Overview Tejaswig6sb94mgkwNo ratings yet
- Ch.4 DifferentiationDocument25 pagesCh.4 DifferentiationZanfalawy BashaNo ratings yet
- Input and Output in Damped Quantum Systems by Gardiner Collett PRA 1985 Paper ID V Imp Quantum Core PhysRevA.31.3761Document14 pagesInput and Output in Damped Quantum Systems by Gardiner Collett PRA 1985 Paper ID V Imp Quantum Core PhysRevA.31.3761Raktim HalderNo ratings yet
- Permutation Symmetry For Many ParticlesDocument16 pagesPermutation Symmetry For Many Particlesseda öztürkNo ratings yet
- CHEM1100 - Module 1 - SEM2 - 2019 - Combined Student Notes 1pp - 2.pd PDFDocument297 pagesCHEM1100 - Module 1 - SEM2 - 2019 - Combined Student Notes 1pp - 2.pd PDFNicholas BahNo ratings yet
- Design Considerations For Quantum Radar ImplementationDocument9 pagesDesign Considerations For Quantum Radar ImplementationAlex YangNo ratings yet
- Electron Spin Resonance Spectroscopy: Calculating The Land e G-Factor For A Free ElectronDocument5 pagesElectron Spin Resonance Spectroscopy: Calculating The Land e G-Factor For A Free ElectronRahul DevarakondaNo ratings yet
- Tudent Worksheet Ubble Chamber Pictures: Main Body of The 2 M Bubble Chamber © CERNDocument15 pagesTudent Worksheet Ubble Chamber Pictures: Main Body of The 2 M Bubble Chamber © CERNChai Min HiungNo ratings yet
- IITBHU Physics Assignmnet2Document2 pagesIITBHU Physics Assignmnet2Yash BhosaleNo ratings yet
- Chapter 4Document47 pagesChapter 4biniyam kefyalewNo ratings yet
- Lec 4 Equation of PlaneDocument6 pagesLec 4 Equation of PlaneMahad ElahiNo ratings yet
- Matti Pitkanen - Magnetospheric ConsciousnessDocument388 pagesMatti Pitkanen - Magnetospheric ConsciousnessJoaokaa100% (1)
- AE01 M1 SolutionsDocument154 pagesAE01 M1 Solutionshemanthindia19No ratings yet
- Chemistry Presentation Report - MT2Document7 pagesChemistry Presentation Report - MT2Nisarg BediyaNo ratings yet
- Comparison Between Geometric Quantisation and Covariant Quantum MechanicsDocument21 pagesComparison Between Geometric Quantisation and Covariant Quantum MechanicsresbickrayNo ratings yet
- Regarding The Formalism of Quantum MechanicsDocument2 pagesRegarding The Formalism of Quantum MechanicsΚουταντου ΕφηNo ratings yet
- TRM 2 15Document143 pagesTRM 2 15tabilinNo ratings yet
- CSEC Physics - The AtomDocument5 pagesCSEC Physics - The AtomCornflakes ToastedNo ratings yet
Allow Answer in J If (B) (I) Expressed in Joule (ECF), Otherwise Award (1 Max)
Allow Answer in J If (B) (I) Expressed in Joule (ECF), Otherwise Award (1 Max)
Uploaded by
superpooh-1Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Allow Answer in J If (B) (I) Expressed in Joule (ECF), Otherwise Award (1 Max)
Allow Answer in J If (B) (I) Expressed in Joule (ECF), Otherwise Award (1 Max)
Uploaded by
superpooh-1Copyright:
Available Formats
al
op
– 14 – M11/4/PHYSI/HP2/ENG/TZ2/XX/M
hy
s@
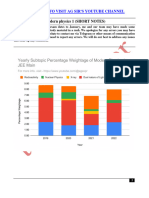
Part 2 Photoelectric effect and de Broglie wavelength
gm
(a) Look for these main points.
ai
light consists of photons whose energy depends on the frequency/hf;
l.c
hence the energy available to the (photo)electrons will depend on f;
om
the potentials VA and VB correspond to/are a measure of the maximum kinetic of
the emitted electrons;
the work function (of metal)/energy to emit electron is same for both light sources;
as electrons in A have more kinetic energy available, this frequency must be
higher;
(so A) [4 max]
(b) (i) 1.6 eV; (answer must be expressed in eV) [1]
6.6 ×10−34 × 8.8 ×1014
(ii)=
energy of photons = 3.6 (eV) ;
1.6 ×10−19
work function = (3.6 − 1.6 = ) 2.0 eV ; [2]
Allow answer in J if (b)(i) expressed in joule (ECF), otherwise award [1 max].
(c) photon energy increases (because frequency increases);
so for same intensity fewer photons per second;
so current reduced / fewer electrons emitted per second; [3]
(d) all particles/electrons exhibit wave properties/have an associated wavelength
(called the de Broglie wavelength);
the wavelength is equal to the Planck constant divided by the
h (terms must be
momentum of the particle/electron/ λ = with terms defined; defined for mark) [2]
p
You might also like
- PDFDocument50 pagesPDFk_gh22No ratings yet
- Physics 2P - Specimen PaperDocument20 pagesPhysics 2P - Specimen PaperHan Thi Win KoNo ratings yet
- 1000 Solved Problem in Modern Physics-104Document1 page1000 Solved Problem in Modern Physics-104Sano ManjiroNo ratings yet
- Dual Nature ExemplarDocument7 pagesDual Nature Exemplarvj jNo ratings yet
- Physics: Chapter - Dual Nature of Radiation and Matter Chapterwise Practise Problems (CPP) For NEETDocument12 pagesPhysics: Chapter - Dual Nature of Radiation and Matter Chapterwise Practise Problems (CPP) For NEETsoyel afridiNo ratings yet
- QM Ts 01 2024Document2 pagesQM Ts 01 2024Aviraj KhareNo ratings yet
- 14 15 H2 Quantum Physics1 NotesDocument29 pages14 15 H2 Quantum Physics1 NotesAgus LeonardiNo ratings yet
- Modern Physics EXERCISE-1 Qs. + Sol..pmdDocument18 pagesModern Physics EXERCISE-1 Qs. + Sol..pmdSubhangi MohantyNo ratings yet
- Modern Physics IDocument2 pagesModern Physics IAnik GhoshNo ratings yet
- Physics: DPP - Daily Practice ProblemsDocument8 pagesPhysics: DPP - Daily Practice ProblemsAnonymous 9uu04elNo ratings yet
- 2019-Key Physics Equations and Experiments Explained and Derived by Energy Wave EquationsDocument29 pages2019-Key Physics Equations and Experiments Explained and Derived by Energy Wave Equationsjoao pedro GomesNo ratings yet
- Physics Modern Physics IDocument15 pagesPhysics Modern Physics Ianuveshr13No ratings yet
- Assignment 2&3Document4 pagesAssignment 2&3Shakun Kumar Padhy100% (1)
- As Wet 6Document8 pagesAs Wet 6Rsrao JNo ratings yet
- DUAL NATURE TestDocument2 pagesDUAL NATURE TestChitesh MarmatNo ratings yet
- Pyl101 QM L2Document15 pagesPyl101 QM L2kamakshi gargNo ratings yet
- Atoms PYQ SDocument2 pagesAtoms PYQ Spatiminati2020No ratings yet
- Photoelectric Effect & Atomic Spectra 2 MSDocument8 pagesPhotoelectric Effect & Atomic Spectra 2 MSGulab RayNo ratings yet
- VG FRJ CIr YTa DUMz Y69 VMDocument6 pagesVG FRJ CIr YTa DUMz Y69 VMAyisha AfraNo ratings yet
- 03-Electron, Photon, PEE-Critical Question + SolutionDocument32 pages03-Electron, Photon, PEE-Critical Question + SolutionRNo ratings yet
- HL MSDocument12 pagesHL MSChinmay BhaveNo ratings yet
- Exercise: Violet: Iitians Spectrum EdutechDocument4 pagesExercise: Violet: Iitians Spectrum EdutechAarav ShahNo ratings yet
- Wave-Particle DualityDocument3 pagesWave-Particle DualityNyak Perera50% (2)
- Part One Geometrical Optics: Light RaysDocument13 pagesPart One Geometrical Optics: Light Raysgoran physyaNo ratings yet
- Duarte PhotoelectricDocument15 pagesDuarte PhotoelectricKonata OzumiNo ratings yet
- Tutorial 18A: Photoelectric Effect Photoelectric EffectDocument2 pagesTutorial 18A: Photoelectric Effect Photoelectric EffectAgus LeonardiNo ratings yet
- PhysicsDocument4 pagesPhysicsmathsbykeshavNo ratings yet
- Dual Nature of Radiation & Matter DPPDocument4 pagesDual Nature of Radiation & Matter DPPChristopher NolanNo ratings yet
- IITBHU Physics Assignmnet2Document2 pagesIITBHU Physics Assignmnet2Yash BhosaleNo ratings yet
- Topic No. 1 - Introduction To Atomic Structure-1-1Document92 pagesTopic No. 1 - Introduction To Atomic Structure-1-1Chand HiraniNo ratings yet
- QP ch3Document13 pagesQP ch3phuctranlvNo ratings yet
- Time Averaged Total Force On A Dipolar Sphere in An Electromagnetic FieldDocument3 pagesTime Averaged Total Force On A Dipolar Sphere in An Electromagnetic Fieldbagus putraNo ratings yet
- Pellegrini 2001 0119Document5 pagesPellegrini 2001 0119Particle Beam Physics LabNo ratings yet
- Dosimetric Principles, Quantities and UnitsDocument26 pagesDosimetric Principles, Quantities and UnitsRajesh Padiyaar SinghNo ratings yet
- 1modern Physics 1 - AnsDocument24 pages1modern Physics 1 - Ansrineeth22745No ratings yet
- 1 Basics of Optical Emission and AbsorptionDocument10 pages1 Basics of Optical Emission and AbsorptionChuxuan SunNo ratings yet
- Tsubono Calculation of Helium Ground State Energy by BohrDocument10 pagesTsubono Calculation of Helium Ground State Energy by BohrDr. John BeveridgeNo ratings yet
- The Magnetic Field Induced by A Lightning Strike's Indirect Effect Double Exponential Current WaveformDocument5 pagesThe Magnetic Field Induced by A Lightning Strike's Indirect Effect Double Exponential Current WaveformVijay KumarNo ratings yet
- EMG Theory of The PhotonDocument22 pagesEMG Theory of The Photondiablo441No ratings yet
- ch17 PDFDocument14 pagesch17 PDFRodrigo S QuirinoNo ratings yet
- Chapter 40Document53 pagesChapter 40ArthurNo ratings yet
- 126Document18 pages126rabinNo ratings yet
- The Particle Properties of Waves: Applied Modern Physics - Eceg 241Document5 pagesThe Particle Properties of Waves: Applied Modern Physics - Eceg 241YITBAREKNo ratings yet
- Sachi SPECTROSCOPY NOTES PDFDocument17 pagesSachi SPECTROSCOPY NOTES PDFarchana giriNo ratings yet
- 29 Particles and WavesDocument14 pages29 Particles and WavesMikaela Rome BigayNo ratings yet
- Compton ScatteringDocument6 pagesCompton Scatteringffr.gimenezNo ratings yet
- Two-Dimensional Numerical Simulation of A Planar Radio-Frequency Atmospheric Pressure Plasma SourceDocument5 pagesTwo-Dimensional Numerical Simulation of A Planar Radio-Frequency Atmospheric Pressure Plasma SourceMilica IvanovicNo ratings yet
- MIT8 04S16 LecNotes3 PDFDocument8 pagesMIT8 04S16 LecNotes3 PDFJefersonNo ratings yet
- Prac Que MP Dual NatureDocument5 pagesPrac Que MP Dual Naturetasneemkirana900No ratings yet
- Atomic StructureDocument4 pagesAtomic StructurePriyanshu PopNo ratings yet
- Lecture 07 - Introduction To Quantum MechanicsDocument26 pagesLecture 07 - Introduction To Quantum MechanicsArc ZeroNo ratings yet
- Lecture 07 - Introduction To Quantum MechanicsDocument26 pagesLecture 07 - Introduction To Quantum MechanicsArc ZeroNo ratings yet
- Wave Properties of ParticlesDocument4 pagesWave Properties of ParticlesPRIYAA A/P JAYASANKAR / UPMNo ratings yet
- 136 Sabi2007Document7 pages136 Sabi2007Jose M. MassaNo ratings yet
- Unit - 7 (Dual-Nature)Document2 pagesUnit - 7 (Dual-Nature)garvit1223No ratings yet
- A Heitler Model of Extensive Air ShowersDocument11 pagesA Heitler Model of Extensive Air ShowersRafaelawNo ratings yet
- M40 Knig2461 04 Ism C40Document20 pagesM40 Knig2461 04 Ism C40kymm7827No ratings yet
- Topic 3 Waves and The Particle Nature of Light 3D Quantum PhysicsDocument3 pagesTopic 3 Waves and The Particle Nature of Light 3D Quantum Physicsmaram shbairNo ratings yet
- Compton - Scattering (7) CorrectedDocument11 pagesCompton - Scattering (7) CorrectedLim Zheng LiangNo ratings yet
- Electron Beam-Specimen Interactions and Simulation Methods in MicroscopyFrom EverandElectron Beam-Specimen Interactions and Simulation Methods in MicroscopyNo ratings yet
- 4ph1 - 1p - Que - 20190523 (Q10 Series Circuit)Document2 pages4ph1 - 1p - Que - 20190523 (Q10 Series Circuit)superpooh-1No ratings yet
- 4ph1 - 1p - Que - 20190523 (Q5 Hooke's Law and Elastic Limit)Document2 pages4ph1 - 1p - Que - 20190523 (Q5 Hooke's Law and Elastic Limit)superpooh-1No ratings yet
- Physics: Pearson Edexcel International GCSE (9-1)Document36 pagesPhysics: Pearson Edexcel International GCSE (9-1)superpooh-1100% (2)
- Pages From s19-8421-03 (Q7 Quantum)Document4 pagesPages From s19-8421-03 (Q7 Quantum)superpooh-1No ratings yet
- @gmail - Co: 17 The Diagram Shows A Simplified Version of The Apparatus Used in An Experiment To DetermineDocument3 pages@gmail - Co: 17 The Diagram Shows A Simplified Version of The Apparatus Used in An Experiment To Determinesuperpooh-1No ratings yet
- @gmail: Answer All QuestionsDocument3 pages@gmail: Answer All Questionssuperpooh-1No ratings yet
- 4PH0 - 1P - Que - 20170112 (Q5 I-V Graph, Circuit)Document3 pages4PH0 - 1P - Que - 20170112 (Q5 I-V Graph, Circuit)superpooh-1No ratings yet
- Hong Kong Examinations and Assessment Authority: Pp-Dse-Cs (Phy) - 1 MsDocument8 pagesHong Kong Examinations and Assessment Authority: Pp-Dse-Cs (Phy) - 1 Mssuperpooh-1No ratings yet
- Pages From s19-8421-03 (Q2 Wave)Document3 pagesPages From s19-8421-03 (Q2 Wave)superpooh-1No ratings yet
- 2015 MC AnsDocument13 pages2015 MC Anssuperpooh-1No ratings yet
- 2015 DSE Phy 2-MS (E)Document8 pages2015 DSE Phy 2-MS (E)superpooh-1No ratings yet
- Suggested Answers and Marking Schemes: Physics Sample Mock Paper For HkdseeDocument9 pagesSuggested Answers and Marking Schemes: Physics Sample Mock Paper For Hkdseesuperpooh-1No ratings yet
- Physics Paper 1: (Sample Mock Paper) Section B: Question-Answer Book BDocument16 pagesPhysics Paper 1: (Sample Mock Paper) Section B: Question-Answer Book Bsuperpooh-1No ratings yet
- N12 PhotoelectricDocument1 pageN12 Photoelectricsuperpooh-1No ratings yet
- Phy - HKDSE2013 - Mock - 1A - EEDocument17 pagesPhy - HKDSE2013 - Mock - 1A - EEsuperpooh-1No ratings yet
- Hong Kong Examinations and Assessment Authority: Pp-Dse-Phy 1-1 MsDocument17 pagesHong Kong Examinations and Assessment Authority: Pp-Dse-Phy 1-1 Mssuperpooh-1No ratings yet
- Physics Paper 1 DR Ken ChanDocument19 pagesPhysics Paper 1 DR Ken Chansuperpooh-1No ratings yet
- 2015 May 20 Q9 (Edexcel 4PH0 FBI)Document2 pages2015 May 20 Q9 (Edexcel 4PH0 FBI)superpooh-1No ratings yet
- Physics Answer Key DR Ken ChanDocument7 pagesPhysics Answer Key DR Ken Chansuperpooh-1No ratings yet
- Energy Stores and TransfersDocument4 pagesEnergy Stores and Transferssuperpooh-1No ratings yet
- BBMP 1103 Matematik PengurusanDocument24 pagesBBMP 1103 Matematik PengurusanSean GomezNo ratings yet
- Gas Laws: Sagana National High SchoolDocument17 pagesGas Laws: Sagana National High SchoolHelma Jabello AriolaNo ratings yet
- Jackson 1 7 Homework SolutionDocument3 pagesJackson 1 7 Homework SolutionAna MariaNo ratings yet
- Final Exam Spectroscopy 2022 23 RepeatDocument17 pagesFinal Exam Spectroscopy 2022 23 RepeatIris BenardeteNo ratings yet
- Physics - 1 - LESSON 1 (Final Term - Summer 23)Document18 pagesPhysics - 1 - LESSON 1 (Final Term - Summer 23)fardinmojumdar123No ratings yet
- Van Der Heijden A.M.A. (Ed.) W. T. Koiter's Elastic Stability of Solids and Structures (CUP, 2009) (ISBN 0521515289) (O) (240s) - EMDocument240 pagesVan Der Heijden A.M.A. (Ed.) W. T. Koiter's Elastic Stability of Solids and Structures (CUP, 2009) (ISBN 0521515289) (O) (240s) - EMviovinNo ratings yet
- Classifying Deviation From Standard Quantum Behavior Using Kullback-Leibler DivergenceDocument13 pagesClassifying Deviation From Standard Quantum Behavior Using Kullback-Leibler DivergenceMohammad zaid ZazNo ratings yet
- To Estimate Charge On Pith Ball Class XIIDocument19 pagesTo Estimate Charge On Pith Ball Class XIIDDDPPNo ratings yet
- chpt2 Vector Analysis Part 2Document25 pageschpt2 Vector Analysis Part 2Ahmet imanlıNo ratings yet
- Modul 2.3 Physics Form 4 - AnswerDocument6 pagesModul 2.3 Physics Form 4 - AnswerNURSHAFAZLEEN BINTI AK DAMIT KPM-GuruNo ratings yet
- Quan TenDocument218 pagesQuan TenAngelo MartinezNo ratings yet
- JEE Overview TejaswiDocument6 pagesJEE Overview Tejaswig6sb94mgkwNo ratings yet
- Ch.4 DifferentiationDocument25 pagesCh.4 DifferentiationZanfalawy BashaNo ratings yet
- Input and Output in Damped Quantum Systems by Gardiner Collett PRA 1985 Paper ID V Imp Quantum Core PhysRevA.31.3761Document14 pagesInput and Output in Damped Quantum Systems by Gardiner Collett PRA 1985 Paper ID V Imp Quantum Core PhysRevA.31.3761Raktim HalderNo ratings yet
- Permutation Symmetry For Many ParticlesDocument16 pagesPermutation Symmetry For Many Particlesseda öztürkNo ratings yet
- CHEM1100 - Module 1 - SEM2 - 2019 - Combined Student Notes 1pp - 2.pd PDFDocument297 pagesCHEM1100 - Module 1 - SEM2 - 2019 - Combined Student Notes 1pp - 2.pd PDFNicholas BahNo ratings yet
- Design Considerations For Quantum Radar ImplementationDocument9 pagesDesign Considerations For Quantum Radar ImplementationAlex YangNo ratings yet
- Electron Spin Resonance Spectroscopy: Calculating The Land e G-Factor For A Free ElectronDocument5 pagesElectron Spin Resonance Spectroscopy: Calculating The Land e G-Factor For A Free ElectronRahul DevarakondaNo ratings yet
- Tudent Worksheet Ubble Chamber Pictures: Main Body of The 2 M Bubble Chamber © CERNDocument15 pagesTudent Worksheet Ubble Chamber Pictures: Main Body of The 2 M Bubble Chamber © CERNChai Min HiungNo ratings yet
- IITBHU Physics Assignmnet2Document2 pagesIITBHU Physics Assignmnet2Yash BhosaleNo ratings yet
- Chapter 4Document47 pagesChapter 4biniyam kefyalewNo ratings yet
- Lec 4 Equation of PlaneDocument6 pagesLec 4 Equation of PlaneMahad ElahiNo ratings yet
- Matti Pitkanen - Magnetospheric ConsciousnessDocument388 pagesMatti Pitkanen - Magnetospheric ConsciousnessJoaokaa100% (1)
- AE01 M1 SolutionsDocument154 pagesAE01 M1 Solutionshemanthindia19No ratings yet
- Chemistry Presentation Report - MT2Document7 pagesChemistry Presentation Report - MT2Nisarg BediyaNo ratings yet
- Comparison Between Geometric Quantisation and Covariant Quantum MechanicsDocument21 pagesComparison Between Geometric Quantisation and Covariant Quantum MechanicsresbickrayNo ratings yet
- Regarding The Formalism of Quantum MechanicsDocument2 pagesRegarding The Formalism of Quantum MechanicsΚουταντου ΕφηNo ratings yet
- TRM 2 15Document143 pagesTRM 2 15tabilinNo ratings yet
- CSEC Physics - The AtomDocument5 pagesCSEC Physics - The AtomCornflakes ToastedNo ratings yet