Professional Documents
Culture Documents
Common Polar Aprotic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)
Common Polar Aprotic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)
Uploaded by
sofia quirarteCopyright:
Available Formats
You might also like
- R1 R2 Log 1/H Mass (G) Log P Molar Refractivity Critical (CM /mol) TpsaDocument4 pagesR1 R2 Log 1/H Mass (G) Log P Molar Refractivity Critical (CM /mol) TpsaNoor HanisNo ratings yet
- Acid Mind MapDocument1 pageAcid Mind MapIndianagrofarms100% (2)
- Desitherm BrochureDocument8 pagesDesitherm BrochureBrandon FordNo ratings yet
- Iodine ValueDocument4 pagesIodine ValueRobert Gilmore100% (4)
- Common Polar Protic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)Document1 pageCommon Polar Protic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)sofia quirarteNo ratings yet
- 29 Cyclobutane SynthesisDocument10 pages29 Cyclobutane SynthesisEena BarmanNo ratings yet
- Problem 26 Chemical Structure and Absolute Stereochemistry of ConiineDocument6 pagesProblem 26 Chemical Structure and Absolute Stereochemistry of ConiineLê Hoàng MinhNo ratings yet
- VitanolidiDocument14 pagesVitanolidimarfi1994No ratings yet
- 9-Asymmetric Alkylation of EnolatesDocument10 pages9-Asymmetric Alkylation of EnolatesPARVATHY ANILNo ratings yet
- Dielectric Constants of Common SolventsDocument1 pageDielectric Constants of Common SolventsDrAmit VermaNo ratings yet
- CHEMSKETCHDocument17 pagesCHEMSKETCHUsman GhaniNo ratings yet
- 2444 102611Document147 pages2444 102611anirbanbhowmick88No ratings yet
- Chem SketchDocument17 pagesChem SketchUsman GhaniNo ratings yet
- Process Engineering Flow Diagram Prarancangan Pabrik Kimia Butil Asetat Dari Butanol Dan Asam Asetat Kapasitas 30.000 Ton/TahunDocument2 pagesProcess Engineering Flow Diagram Prarancangan Pabrik Kimia Butil Asetat Dari Butanol Dan Asam Asetat Kapasitas 30.000 Ton/Tahunmuhammad afdalNo ratings yet
- Process Engineering Flow Diagram Prarancangan Pabrik Kimia Butil Asetat Dari Butanol Dan Asam Asetat Kapasitas 30.000 Ton/TahunDocument2 pagesProcess Engineering Flow Diagram Prarancangan Pabrik Kimia Butil Asetat Dari Butanol Dan Asam Asetat Kapasitas 30.000 Ton/Tahunmuhammad afdalNo ratings yet
- Process Engineering Flow Diagram Prarancangan Pabrik Kimia Butil Asetat Dari Butanol Dan Asam Asetat Kapasitas 30.000 Ton/TahunDocument2 pagesProcess Engineering Flow Diagram Prarancangan Pabrik Kimia Butil Asetat Dari Butanol Dan Asam Asetat Kapasitas 30.000 Ton/Tahunmuhammad afdalNo ratings yet
- Sample Name: Thiol Terminated Polystyrene Sample # P18811-SSHDocument1 pageSample Name: Thiol Terminated Polystyrene Sample # P18811-SSHOscar PiñeresNo ratings yet
- ws19302keyDocument7 pagesws19302keyyutika1803No ratings yet
- CERBE Distribution Inc.: Phone: Fax: Internet: EmailDocument14 pagesCERBE Distribution Inc.: Phone: Fax: Internet: EmailMarios AshiotisNo ratings yet
- Syllabus 3006312720200426110931Document18 pagesSyllabus 3006312720200426110931Tushar KanojiyaNo ratings yet
- Organic Name Reactions: Nutshell Review & Preview ofDocument9 pagesOrganic Name Reactions: Nutshell Review & Preview ofSai YashwanthNo ratings yet
- CSL Lab Exam: Shahin Alam CH-004Document6 pagesCSL Lab Exam: Shahin Alam CH-004shahin alamNo ratings yet
- 10 Organic NomenclatureDocument45 pages10 Organic NomenclatureAnubhab100% (2)
- Ester Enolat 3.PptEster Enolat 3Document50 pagesEster Enolat 3.PptEster Enolat 3alief ramdhanNo ratings yet
- Chemistry PracticeDocument13 pagesChemistry PracticeSiddharth KrishnamurthyNo ratings yet
- BIOL1XX7 Final Exam Replacement 2017 For Revision MCQ Answers PDFDocument17 pagesBIOL1XX7 Final Exam Replacement 2017 For Revision MCQ Answers PDFBelinda ZhangNo ratings yet
- Practical Org. Chem. II ReportDocument6 pagesPractical Org. Chem. II Reportسعيد الزهرانيNo ratings yet
- HAHAHAHAHAHAHAHADocument24 pagesHAHAHAHAHAHAHAHAShofiakhoerunnisaNo ratings yet
- Edited - Tugas Komputer Kimia 1 PDFDocument20 pagesEdited - Tugas Komputer Kimia 1 PDFSeptian Eka TruenoNo ratings yet
- Common Solvents For Organic Reactions PDFDocument1 pageCommon Solvents For Organic Reactions PDFRichard OletskyNo ratings yet
- Kuliah Biomedik LipidDocument18 pagesKuliah Biomedik LipidshanksNo ratings yet
- Draw The Following Alkanes.: Hydrocarbon Nomenclature WorksheetDocument3 pagesDraw The Following Alkanes.: Hydrocarbon Nomenclature WorksheetCaseelyn Joy NantizaNo ratings yet
- Accurate Analysis of Glycerol and Glycerides in Biodiesel Oil Using EN 14105 (2011) and Robust Metal MXT ColumnsDocument4 pagesAccurate Analysis of Glycerol and Glycerides in Biodiesel Oil Using EN 14105 (2011) and Robust Metal MXT ColumnsAstiJayatriIINo ratings yet
- 13 Enolate AnionDocument17 pages13 Enolate AnionNova sounds - No copyright musicNo ratings yet
- Basic Organic Chemistry and Mechanisms Revision From M Wills For When You Are Lost and ConfusedDocument128 pagesBasic Organic Chemistry and Mechanisms Revision From M Wills For When You Are Lost and ConfusedJasim Al-rufayeNo ratings yet
- 12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetDocument4 pages12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetZia Rathore100% (1)
- 714X TechnicaldataDocument14 pages714X TechnicaldataQ Duck100% (2)
- CERBE Distribution Inc.: Phone: Fax: Internet: EmailDocument14 pagesCERBE Distribution Inc.: Phone: Fax: Internet: EmailTim SchlankNo ratings yet
- Scientific Tools For Probing The PastDocument52 pagesScientific Tools For Probing The PastAung Htun LinnNo ratings yet
- Pyrolytic Elimination - Syn EliminationsDocument3 pagesPyrolytic Elimination - Syn EliminationssaheedvkNo ratings yet
- 27RS SVT11Document6 pages27RS SVT11Kamal BdNo ratings yet
- Vitamins LipidDocument38 pagesVitamins LipidMohamed HusseiniNo ratings yet
- الامتحان الوطني في الفيزياء والكيمياء 2011 للمسالك العلمية الدورة الاستدراكيةDocument14 pagesالامتحان الوطني في الفيزياء والكيمياء 2011 للمسالك العلمية الدورة الاستدراكية05313893731llllNo ratings yet
- Alcohol, Ether & PhenolDocument8 pagesAlcohol, Ether & Phenolshashwat.gupta.707No ratings yet
- PDFDocument30 pagesPDFAlok RanjanNo ratings yet
- TutorialDocument27 pagesTutorialSiti NuraqidahNo ratings yet
- IUPAC Exercise-3Document6 pagesIUPAC Exercise-3sumit parasharNo ratings yet
- How To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inDocument2 pagesHow To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inmyiitchemistry81% (16)
- IUPAC Nomenclature Exercises in Organic ChemistryDocument11 pagesIUPAC Nomenclature Exercises in Organic ChemistryAdiChemAdi88% (41)
- Amine Unit Materials and Corrosion (Updated)Document36 pagesAmine Unit Materials and Corrosion (Updated)harrinsonfNo ratings yet
- Diagram Alir Kualitatif FixDocument1 pageDiagram Alir Kualitatif FixRifki AzharNo ratings yet
- Taller Quimica 6Document1 pageTaller Quimica 6Paula Andrea Saenz HernandezNo ratings yet
- Naming AlkanesDocument3 pagesNaming Alkanesmercedes.caamalNo ratings yet
- Hidro KarbonDocument43 pagesHidro KarbonElisabet NoviantiNo ratings yet
- đọc tênDocument7 pagesđọc tênthái đức thắngNo ratings yet
- CH CooDocument2 pagesCH CooPawan SharmaNo ratings yet
- Monomers - CatalogDocument36 pagesMonomers - CatalogandersonquimicaNo ratings yet
- AldehidosDocument1 pageAldehidosJuan Sebastian AlarconNo ratings yet
- Struktur Kimia OrganikDocument6 pagesStruktur Kimia OrganikULI PANENo ratings yet
- Organic HWDocument4 pagesOrganic HWLawrence KankamNo ratings yet
- Ejercicio3 Destilación - Quirarte Vizcarra Frida Sofía-3IM63Document4 pagesEjercicio3 Destilación - Quirarte Vizcarra Frida Sofía-3IM63sofia quirarteNo ratings yet
- 2im53 - Lab TC - Corrida 2 - Equipo 3Document23 pages2im53 - Lab TC - Corrida 2 - Equipo 3sofia quirarteNo ratings yet
- UntitledDocument1 pageUntitledsofia quirarteNo ratings yet
- Common Polar Protic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)Document1 pageCommon Polar Protic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)sofia quirarteNo ratings yet
- AromaticDocument173 pagesAromaticParom Waikasikarn100% (1)
- Nitrile rubber-NBRDocument16 pagesNitrile rubber-NBRreilyshawnNo ratings yet
- ChemF5 Ch2 2.1Document14 pagesChemF5 Ch2 2.1SANUSI BIN ABDULLAH MoeNo ratings yet
- Halogen Derivatives SheetDocument6 pagesHalogen Derivatives SheetRajeev GangwarNo ratings yet
- New Values of The Required Hydrophilic-LipophilicBalance For Oil in Water Emulsions of Solid Fatty Acids and AlcoholsDocument4 pagesNew Values of The Required Hydrophilic-LipophilicBalance For Oil in Water Emulsions of Solid Fatty Acids and AlcoholsRicardo100% (3)
- 6 CNE Coupling Reactions 2019-20 PDFDocument39 pages6 CNE Coupling Reactions 2019-20 PDFChisom AdaobiNo ratings yet
- Acids and Bases Chapter - A4 - v1Document27 pagesAcids and Bases Chapter - A4 - v1markmchemNo ratings yet
- CIPW Norm RevisedsurendraDocument20 pagesCIPW Norm RevisedsurendraAmar RachmatNo ratings yet
- Ligand Exchange Chromatography of CarbohydratesDocument10 pagesLigand Exchange Chromatography of CarbohydratesveromendoNo ratings yet
- Chemistry Investigatory ProjectDocument21 pagesChemistry Investigatory ProjectAlfred SumaNo ratings yet
- Laboratory Manual: Prepared byDocument34 pagesLaboratory Manual: Prepared byYuppie RajNo ratings yet
- Porous Organic Polymers: Postgraduate CourseDocument50 pagesPorous Organic Polymers: Postgraduate Coursentnphung.sdh222No ratings yet
- Haemodialysis, Solutions ForDocument4 pagesHaemodialysis, Solutions ForSurafel KebedeNo ratings yet
- Antioxidant Effect of Methanol Extracts From Lotus PlumuleDocument7 pagesAntioxidant Effect of Methanol Extracts From Lotus PlumuleAnne AtesNo ratings yet
- Classnote 548fee8d37792Document46 pagesClassnote 548fee8d37792vinay guttNo ratings yet
- Investigation of Nicotine TobaccoDocument13 pagesInvestigation of Nicotine TobaccoRaman Thakur 5 B ThakurNo ratings yet
- Chem (Ii) 5Document12 pagesChem (Ii) 5Nurul Hasanah100% (1)
- Improved Etherification Procedure For The Preparation of Dibenz (B, F) (1,4) OxazepineDocument3 pagesImproved Etherification Procedure For The Preparation of Dibenz (B, F) (1,4) Oxazepineebi1364No ratings yet
- ChemCom Ch1B pgs023-044Document22 pagesChemCom Ch1B pgs023-044rajayu20002724No ratings yet
- Recent Resin PDFDocument14 pagesRecent Resin PDFblpjNo ratings yet
- United States Patent Office: Patented Apr. 7, 1953Document3 pagesUnited States Patent Office: Patented Apr. 7, 1953Syahrul SandreaNo ratings yet
- Chemicals Forecasting: Sr. # Chemical Unit Consumption Forecast Jan-Jun 2022 Jul-Dec 2022 Jan-Jun 2023 Jul-Dec 2023Document9 pagesChemicals Forecasting: Sr. # Chemical Unit Consumption Forecast Jan-Jun 2022 Jul-Dec 2022 Jan-Jun 2023 Jul-Dec 2023Muhammad JunaidNo ratings yet
- Effect of Refining On Quality and Composition of Sunflower OilDocument6 pagesEffect of Refining On Quality and Composition of Sunflower OilGodsend SinagaNo ratings yet
- Coordination Compounds 12thDocument34 pagesCoordination Compounds 12thShahidNo ratings yet
- Worksheet Solution Equilibrium SP 06Document16 pagesWorksheet Solution Equilibrium SP 06rilaNo ratings yet
- Iron & Manganese RemovalDocument47 pagesIron & Manganese RemovalJulio LoredoNo ratings yet
- Atorvastatin Calcium Trihydrate Farmacopea BPDocument3 pagesAtorvastatin Calcium Trihydrate Farmacopea BPGuadalupe EnriquezNo ratings yet
Common Polar Aprotic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)
Common Polar Aprotic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)
Uploaded by
sofia quirarteOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Common Polar Aprotic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)
Common Polar Aprotic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)
Uploaded by
sofia quirarteCopyright:
Available Formats
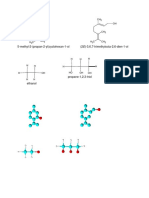
COMMON POLAR APROTIC SOLVENTS
Aprotic solvents with moderately higher dielectric constants than nonpolar solvents
(between 5 and 20) make adequate general-purpose solvents for a wide range of
reactions. Aprotic solvents with dielectric constants greater than 20 and large dipole
moments can dissolve charged species such as various anions used as nucleophiles.
Without hydrogen bonding in the solvent, these nucleophiles are relatively free in
solution, making them more reactive.
Dielectric Dipole Boiling
Solvent Structure
Constant Moment Point (°C)
O
••
••
Dimethylsulfoxide
46.68 3.96 189
(DMSO) H3C S CH3
• •
O
••
••
Dimethylformamide • •
H C N CH3 36.71 3.86 153
(DMF)
CH3
H
Acetonitrile H C C N 38.8 3.92 81.6
• •
(MeCN)
H
CH3
N CH3
• •
Hexamethylphosphoric
30.0 5.54 233
acid (HMPA) H3C
• •
N P N
• •
CH3
CH3 O CH3
••
••
O
••
••
Acetone 20.7 2.88 56
H3C C CH3
H
• • • •
Dichloromethane Cl C Cl 8.93 1.60 39.6
• •
• •
• • • •
O
••
••
Tetrahydrofuran 7.58 1.75 66
(THF)
O
••
••
Methyl acetate 6.7 1.69 57
• •
H3C OCH
• • 3
O
••
••
Ethyl acetate 6.02 1.78 77.1
• •
H3C OCH
• • 2CH3
You might also like
- R1 R2 Log 1/H Mass (G) Log P Molar Refractivity Critical (CM /mol) TpsaDocument4 pagesR1 R2 Log 1/H Mass (G) Log P Molar Refractivity Critical (CM /mol) TpsaNoor HanisNo ratings yet
- Acid Mind MapDocument1 pageAcid Mind MapIndianagrofarms100% (2)
- Desitherm BrochureDocument8 pagesDesitherm BrochureBrandon FordNo ratings yet
- Iodine ValueDocument4 pagesIodine ValueRobert Gilmore100% (4)
- Common Polar Protic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)Document1 pageCommon Polar Protic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)sofia quirarteNo ratings yet
- 29 Cyclobutane SynthesisDocument10 pages29 Cyclobutane SynthesisEena BarmanNo ratings yet
- Problem 26 Chemical Structure and Absolute Stereochemistry of ConiineDocument6 pagesProblem 26 Chemical Structure and Absolute Stereochemistry of ConiineLê Hoàng MinhNo ratings yet
- VitanolidiDocument14 pagesVitanolidimarfi1994No ratings yet
- 9-Asymmetric Alkylation of EnolatesDocument10 pages9-Asymmetric Alkylation of EnolatesPARVATHY ANILNo ratings yet
- Dielectric Constants of Common SolventsDocument1 pageDielectric Constants of Common SolventsDrAmit VermaNo ratings yet
- CHEMSKETCHDocument17 pagesCHEMSKETCHUsman GhaniNo ratings yet
- 2444 102611Document147 pages2444 102611anirbanbhowmick88No ratings yet
- Chem SketchDocument17 pagesChem SketchUsman GhaniNo ratings yet
- Process Engineering Flow Diagram Prarancangan Pabrik Kimia Butil Asetat Dari Butanol Dan Asam Asetat Kapasitas 30.000 Ton/TahunDocument2 pagesProcess Engineering Flow Diagram Prarancangan Pabrik Kimia Butil Asetat Dari Butanol Dan Asam Asetat Kapasitas 30.000 Ton/Tahunmuhammad afdalNo ratings yet
- Process Engineering Flow Diagram Prarancangan Pabrik Kimia Butil Asetat Dari Butanol Dan Asam Asetat Kapasitas 30.000 Ton/TahunDocument2 pagesProcess Engineering Flow Diagram Prarancangan Pabrik Kimia Butil Asetat Dari Butanol Dan Asam Asetat Kapasitas 30.000 Ton/Tahunmuhammad afdalNo ratings yet
- Process Engineering Flow Diagram Prarancangan Pabrik Kimia Butil Asetat Dari Butanol Dan Asam Asetat Kapasitas 30.000 Ton/TahunDocument2 pagesProcess Engineering Flow Diagram Prarancangan Pabrik Kimia Butil Asetat Dari Butanol Dan Asam Asetat Kapasitas 30.000 Ton/Tahunmuhammad afdalNo ratings yet
- Sample Name: Thiol Terminated Polystyrene Sample # P18811-SSHDocument1 pageSample Name: Thiol Terminated Polystyrene Sample # P18811-SSHOscar PiñeresNo ratings yet
- ws19302keyDocument7 pagesws19302keyyutika1803No ratings yet
- CERBE Distribution Inc.: Phone: Fax: Internet: EmailDocument14 pagesCERBE Distribution Inc.: Phone: Fax: Internet: EmailMarios AshiotisNo ratings yet
- Syllabus 3006312720200426110931Document18 pagesSyllabus 3006312720200426110931Tushar KanojiyaNo ratings yet
- Organic Name Reactions: Nutshell Review & Preview ofDocument9 pagesOrganic Name Reactions: Nutshell Review & Preview ofSai YashwanthNo ratings yet
- CSL Lab Exam: Shahin Alam CH-004Document6 pagesCSL Lab Exam: Shahin Alam CH-004shahin alamNo ratings yet
- 10 Organic NomenclatureDocument45 pages10 Organic NomenclatureAnubhab100% (2)
- Ester Enolat 3.PptEster Enolat 3Document50 pagesEster Enolat 3.PptEster Enolat 3alief ramdhanNo ratings yet
- Chemistry PracticeDocument13 pagesChemistry PracticeSiddharth KrishnamurthyNo ratings yet
- BIOL1XX7 Final Exam Replacement 2017 For Revision MCQ Answers PDFDocument17 pagesBIOL1XX7 Final Exam Replacement 2017 For Revision MCQ Answers PDFBelinda ZhangNo ratings yet
- Practical Org. Chem. II ReportDocument6 pagesPractical Org. Chem. II Reportسعيد الزهرانيNo ratings yet
- HAHAHAHAHAHAHAHADocument24 pagesHAHAHAHAHAHAHAHAShofiakhoerunnisaNo ratings yet
- Edited - Tugas Komputer Kimia 1 PDFDocument20 pagesEdited - Tugas Komputer Kimia 1 PDFSeptian Eka TruenoNo ratings yet
- Common Solvents For Organic Reactions PDFDocument1 pageCommon Solvents For Organic Reactions PDFRichard OletskyNo ratings yet
- Kuliah Biomedik LipidDocument18 pagesKuliah Biomedik LipidshanksNo ratings yet
- Draw The Following Alkanes.: Hydrocarbon Nomenclature WorksheetDocument3 pagesDraw The Following Alkanes.: Hydrocarbon Nomenclature WorksheetCaseelyn Joy NantizaNo ratings yet
- Accurate Analysis of Glycerol and Glycerides in Biodiesel Oil Using EN 14105 (2011) and Robust Metal MXT ColumnsDocument4 pagesAccurate Analysis of Glycerol and Glycerides in Biodiesel Oil Using EN 14105 (2011) and Robust Metal MXT ColumnsAstiJayatriIINo ratings yet
- 13 Enolate AnionDocument17 pages13 Enolate AnionNova sounds - No copyright musicNo ratings yet
- Basic Organic Chemistry and Mechanisms Revision From M Wills For When You Are Lost and ConfusedDocument128 pagesBasic Organic Chemistry and Mechanisms Revision From M Wills For When You Are Lost and ConfusedJasim Al-rufayeNo ratings yet
- 12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetDocument4 pages12 U Orgo - 1 - Hydrocarbon Nomenclature WorksheetZia Rathore100% (1)
- 714X TechnicaldataDocument14 pages714X TechnicaldataQ Duck100% (2)
- CERBE Distribution Inc.: Phone: Fax: Internet: EmailDocument14 pagesCERBE Distribution Inc.: Phone: Fax: Internet: EmailTim SchlankNo ratings yet
- Scientific Tools For Probing The PastDocument52 pagesScientific Tools For Probing The PastAung Htun LinnNo ratings yet
- Pyrolytic Elimination - Syn EliminationsDocument3 pagesPyrolytic Elimination - Syn EliminationssaheedvkNo ratings yet
- 27RS SVT11Document6 pages27RS SVT11Kamal BdNo ratings yet
- Vitamins LipidDocument38 pagesVitamins LipidMohamed HusseiniNo ratings yet
- الامتحان الوطني في الفيزياء والكيمياء 2011 للمسالك العلمية الدورة الاستدراكيةDocument14 pagesالامتحان الوطني في الفيزياء والكيمياء 2011 للمسالك العلمية الدورة الاستدراكية05313893731llllNo ratings yet
- Alcohol, Ether & PhenolDocument8 pagesAlcohol, Ether & Phenolshashwat.gupta.707No ratings yet
- PDFDocument30 pagesPDFAlok RanjanNo ratings yet
- TutorialDocument27 pagesTutorialSiti NuraqidahNo ratings yet
- IUPAC Exercise-3Document6 pagesIUPAC Exercise-3sumit parasharNo ratings yet
- How To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inDocument2 pagesHow To Find No of Structural Isomers by S.K.sinha See Chemistry Animations at HTTP://WWW - Openchemistry.inmyiitchemistry81% (16)
- IUPAC Nomenclature Exercises in Organic ChemistryDocument11 pagesIUPAC Nomenclature Exercises in Organic ChemistryAdiChemAdi88% (41)
- Amine Unit Materials and Corrosion (Updated)Document36 pagesAmine Unit Materials and Corrosion (Updated)harrinsonfNo ratings yet
- Diagram Alir Kualitatif FixDocument1 pageDiagram Alir Kualitatif FixRifki AzharNo ratings yet
- Taller Quimica 6Document1 pageTaller Quimica 6Paula Andrea Saenz HernandezNo ratings yet
- Naming AlkanesDocument3 pagesNaming Alkanesmercedes.caamalNo ratings yet
- Hidro KarbonDocument43 pagesHidro KarbonElisabet NoviantiNo ratings yet
- đọc tênDocument7 pagesđọc tênthái đức thắngNo ratings yet
- CH CooDocument2 pagesCH CooPawan SharmaNo ratings yet
- Monomers - CatalogDocument36 pagesMonomers - CatalogandersonquimicaNo ratings yet
- AldehidosDocument1 pageAldehidosJuan Sebastian AlarconNo ratings yet
- Struktur Kimia OrganikDocument6 pagesStruktur Kimia OrganikULI PANENo ratings yet
- Organic HWDocument4 pagesOrganic HWLawrence KankamNo ratings yet
- Ejercicio3 Destilación - Quirarte Vizcarra Frida Sofía-3IM63Document4 pagesEjercicio3 Destilación - Quirarte Vizcarra Frida Sofía-3IM63sofia quirarteNo ratings yet
- 2im53 - Lab TC - Corrida 2 - Equipo 3Document23 pages2im53 - Lab TC - Corrida 2 - Equipo 3sofia quirarteNo ratings yet
- UntitledDocument1 pageUntitledsofia quirarteNo ratings yet
- Common Polar Protic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)Document1 pageCommon Polar Protic Solvents: Solvent Structure Dielectric Constant Dipole Moment Boiling Point (°C)sofia quirarteNo ratings yet
- AromaticDocument173 pagesAromaticParom Waikasikarn100% (1)
- Nitrile rubber-NBRDocument16 pagesNitrile rubber-NBRreilyshawnNo ratings yet
- ChemF5 Ch2 2.1Document14 pagesChemF5 Ch2 2.1SANUSI BIN ABDULLAH MoeNo ratings yet
- Halogen Derivatives SheetDocument6 pagesHalogen Derivatives SheetRajeev GangwarNo ratings yet
- New Values of The Required Hydrophilic-LipophilicBalance For Oil in Water Emulsions of Solid Fatty Acids and AlcoholsDocument4 pagesNew Values of The Required Hydrophilic-LipophilicBalance For Oil in Water Emulsions of Solid Fatty Acids and AlcoholsRicardo100% (3)
- 6 CNE Coupling Reactions 2019-20 PDFDocument39 pages6 CNE Coupling Reactions 2019-20 PDFChisom AdaobiNo ratings yet
- Acids and Bases Chapter - A4 - v1Document27 pagesAcids and Bases Chapter - A4 - v1markmchemNo ratings yet
- CIPW Norm RevisedsurendraDocument20 pagesCIPW Norm RevisedsurendraAmar RachmatNo ratings yet
- Ligand Exchange Chromatography of CarbohydratesDocument10 pagesLigand Exchange Chromatography of CarbohydratesveromendoNo ratings yet
- Chemistry Investigatory ProjectDocument21 pagesChemistry Investigatory ProjectAlfred SumaNo ratings yet
- Laboratory Manual: Prepared byDocument34 pagesLaboratory Manual: Prepared byYuppie RajNo ratings yet
- Porous Organic Polymers: Postgraduate CourseDocument50 pagesPorous Organic Polymers: Postgraduate Coursentnphung.sdh222No ratings yet
- Haemodialysis, Solutions ForDocument4 pagesHaemodialysis, Solutions ForSurafel KebedeNo ratings yet
- Antioxidant Effect of Methanol Extracts From Lotus PlumuleDocument7 pagesAntioxidant Effect of Methanol Extracts From Lotus PlumuleAnne AtesNo ratings yet
- Classnote 548fee8d37792Document46 pagesClassnote 548fee8d37792vinay guttNo ratings yet
- Investigation of Nicotine TobaccoDocument13 pagesInvestigation of Nicotine TobaccoRaman Thakur 5 B ThakurNo ratings yet
- Chem (Ii) 5Document12 pagesChem (Ii) 5Nurul Hasanah100% (1)
- Improved Etherification Procedure For The Preparation of Dibenz (B, F) (1,4) OxazepineDocument3 pagesImproved Etherification Procedure For The Preparation of Dibenz (B, F) (1,4) Oxazepineebi1364No ratings yet
- ChemCom Ch1B pgs023-044Document22 pagesChemCom Ch1B pgs023-044rajayu20002724No ratings yet
- Recent Resin PDFDocument14 pagesRecent Resin PDFblpjNo ratings yet
- United States Patent Office: Patented Apr. 7, 1953Document3 pagesUnited States Patent Office: Patented Apr. 7, 1953Syahrul SandreaNo ratings yet
- Chemicals Forecasting: Sr. # Chemical Unit Consumption Forecast Jan-Jun 2022 Jul-Dec 2022 Jan-Jun 2023 Jul-Dec 2023Document9 pagesChemicals Forecasting: Sr. # Chemical Unit Consumption Forecast Jan-Jun 2022 Jul-Dec 2022 Jan-Jun 2023 Jul-Dec 2023Muhammad JunaidNo ratings yet
- Effect of Refining On Quality and Composition of Sunflower OilDocument6 pagesEffect of Refining On Quality and Composition of Sunflower OilGodsend SinagaNo ratings yet
- Coordination Compounds 12thDocument34 pagesCoordination Compounds 12thShahidNo ratings yet
- Worksheet Solution Equilibrium SP 06Document16 pagesWorksheet Solution Equilibrium SP 06rilaNo ratings yet
- Iron & Manganese RemovalDocument47 pagesIron & Manganese RemovalJulio LoredoNo ratings yet
- Atorvastatin Calcium Trihydrate Farmacopea BPDocument3 pagesAtorvastatin Calcium Trihydrate Farmacopea BPGuadalupe EnriquezNo ratings yet