Professional Documents
Culture Documents
0 ratings0% found this document useful (0 votes)
7 viewsOxalate 2
Oxalate 2
Uploaded by
Giorgi GhambashidzeCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (350)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Calcium - Oxalic Acid - Technological Importance: F. Jacob, Munich-WeihenstephanDocument2 pagesCalcium - Oxalic Acid - Technological Importance: F. Jacob, Munich-WeihenstephanGiorgi GhambashidzeNo ratings yet
- Beverage Application Guide: Cellulose GumDocument4 pagesBeverage Application Guide: Cellulose GumGiorgi GhambashidzeNo ratings yet
- 023101.101 Hardness CorrectionDocument5 pages023101.101 Hardness CorrectionGiorgi GhambashidzeNo ratings yet
- 10 - Beer Stabilization Technology-Clearly A Matter of ChoiceDocument42 pages10 - Beer Stabilization Technology-Clearly A Matter of ChoiceGiorgi GhambashidzeNo ratings yet
- 02 - Haze FormationDocument22 pages02 - Haze FormationGiorgi GhambashidzeNo ratings yet
- Instruction Manual: Phaseguard C/ T/ HTDocument32 pagesInstruction Manual: Phaseguard C/ T/ HTGiorgi GhambashidzeNo ratings yet
- RFM PhaseGuard 11027E-1Document90 pagesRFM PhaseGuard 11027E-1Giorgi GhambashidzeNo ratings yet
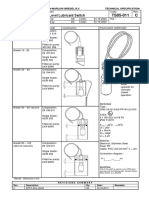
- High Level Lubricant Switch: Pump Type Order Code Construction Float Switch (A230 002)Document1 pageHigh Level Lubricant Switch: Pump Type Order Code Construction Float Switch (A230 002)Giorgi GhambashidzeNo ratings yet
- Datasheet Bredel - 40 50Document2 pagesDatasheet Bredel - 40 50Giorgi GhambashidzeNo ratings yet
Oxalate 2
Oxalate 2
Uploaded by
Giorgi Ghambashidze0 ratings0% found this document useful (0 votes)
7 views1 pageCopyright
© © All Rights Reserved
Available Formats
PDF or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
0 ratings0% found this document useful (0 votes)
7 views1 pageOxalate 2
Oxalate 2
Uploaded by
Giorgi GhambashidzeCopyright:
© All Rights Reserved
Available Formats
Download as PDF or read online from Scribd
Download as pdf
You are on page 1of 1
problems
in brewing
What is the “free calcium
ratio” and what is its relative
importance to beer? How are in-
adequacies treated?
"The “tree calcium ratio,” ale
so expressed as “free Ca as CaSO,
oxalate as CaC0,,"
is a convenient way of expressing
the position of the equilibrium bee
tween calcium and oxalate existing
in a given beet. The ratio is use
ful in predicting beer stability and
gushing tendencies.
‘The natural combination of eal-
cium and oxalic acid and their con-
mash vessel and carry on during
boiling, wort cooling and fermenta-
tion. A change in materials, brewing
water, of method of water treatment
can be responsible for an increase in
calcium oxalate crystals. If insuffi-
cient calcium is present in the wort
to combine with all the available
oxalic acid, the danger of subsequent
turbidity is created. A “crystal
hhaze” may be caused by the for-
mation of deposited crystals and, in
such an instance, the formation of
calcium oxalate has assumed objec
tionable proportions.
Analytically, beer oxalate con
tents are expressed in terms of
calcium oxalate rather than as the
oxalate ion, because some samples
may contain oxalate precipitates at
the time of examination. Also, the
total calcium content of beer is ex-
pressed as calcium sulfate rather
than as the calcium ion, because this
salt is the chief constituent of har-
dening compounds. Furthermore,
relatively litte of the calcium pre
sent in a well stabilized beer can be
attributed to the calcium bicarbo-
nate present in the brewing water or
in the calcium contained in normal
malt.
In predicting beer stability, the
free calcium ratio is used. Extensive
research has shown that free cal-
‘cium ratios of less than 0.25 and
oxalate contents as CaCO, within a
range of 50 ppm produce quite
stable beers, provided that con-
tamination by extraneous calcium
ion is prevented.
‘Beers that have free calcium ra-
tios of 0.25 to 5 and oxalate con-
tents as CaCO, greater than 20
ppm are usually found to be colloi-
dally unstable. Copious sediments of
‘oxalate and other matter, usually of
proteinaceous nature, are gen-
erally observed.
Beers with free calcium ratios of
5 to 13 coupled with oxalate (as
CaC;0,) of 15 to 20 ppm, and cal-
cium ratios greater than 13, with
oxalate (as CaCO) less than 15
ppm, are usually very stable.
Such beers have highly favorable
free calcium ratios and they are un-
likely to develop an oxalate insta-
Dility, even if small amounts of ex-
traneous calcium ions accidentally
‘happen to contaminate them during
cellar operations.
To summarize, the relative levels
shown in Table 1 will prove useful.
In order to estimate a beer’s sta-
Dility, it is necessary first to ascer-
tain the oxalate content and the free
calcium ratio of the beer in ques-
Oxalate can be eliminated by
hardening the water at different
points in the brewing process. The
common practice is to do so right
at the start, either by mixing cor-
rective salts with the brewing water
beforehand or by adding them di-
rectly to the main mash, the cooker
mash, and to the mash during
sparging.
JE hardening treatment is applied
in the kettle or at a latter stage, the
beneficial action which the correc-
tive compounds exert during the
mashing process is lost. This loss
‘CaCs0s (opm)
ee than 0:25 test thon 30 Feil table
More than 023, |_—More than 20 Unnabie
Ser leas thon 5 a ms
3 More than 5, toss than 20, Stable
bot more than 15
4 Vary sable
‘August 1973—The BREWERS DIGEST 67
can be minimized by adjusting the
pH of the brewing water with min-
eral acid.
‘Addition of the hardening com-
pound in the cellar is least desirable
because of the difficulty in dissolving
the material in the beer without
thorough agitation.
‘The hardening salts used should
be of a good soluble grade. Harden
ing material of poor solubility is of
little practical value.
Can you inform me as to
1% Chea dabytnces exert acto
ing and inhibitory effects on pro-
teolytic enzyme (papain func-
tion)?
Papain is sensitive to a great
‘many reagents. In general, it is acti-
vated by reducing agents and inhib-
ited by oxidizing agents, the two ac-
tions usually being reversible.
Some common activators are hy-
drogen sulfide, bisulfite, hydrosul-
fite, cysteine, and urea. Examples of
inhibitors are atmospheric oxygen,
iodine, bromine, hydrogen peroxide,
heavy metal ions (¢.¢., Fe, Cu, Ni),
fresh _cuprous oxide, and methyl
bromide.
In addition to these reagents,
many natural products are reported
to contain gs yet unidentified acti-
vators and/or inhibitors. Papai
self is believed to contain materials
that affect its protease components.
Moreover, some agents alternate
in their role as activator and inhibi-
tor depending on their concentra-
tion. Ascorbic acid is a reducing
agent which is readily converted
into dehydroascorbie acid which
‘acts as an oxidizing agent. This ac-
counts for the fact that it is vari-
ously reported as exerting both an
activating and an inhibitory effect.
To further complicate this matter,
many agents affect papain in one
way when it is reduced (thiol state)
and in another when it is oxidized
(disulfide state).
How is malt vinegar pro-
duced? What materials are
used?
Malt forms the basic material in
the production of malt vinegar. The
procedure involved is quite similar
to beer production through the mill-
ing and mashing processes. How-
ever, the sweet wort is not boiled
but is fermented with yeast and the
resultant unhopped beer is acctified
by acetic acid bacteria,
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (350)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Calcium - Oxalic Acid - Technological Importance: F. Jacob, Munich-WeihenstephanDocument2 pagesCalcium - Oxalic Acid - Technological Importance: F. Jacob, Munich-WeihenstephanGiorgi GhambashidzeNo ratings yet
- Beverage Application Guide: Cellulose GumDocument4 pagesBeverage Application Guide: Cellulose GumGiorgi GhambashidzeNo ratings yet
- 023101.101 Hardness CorrectionDocument5 pages023101.101 Hardness CorrectionGiorgi GhambashidzeNo ratings yet
- 10 - Beer Stabilization Technology-Clearly A Matter of ChoiceDocument42 pages10 - Beer Stabilization Technology-Clearly A Matter of ChoiceGiorgi GhambashidzeNo ratings yet
- 02 - Haze FormationDocument22 pages02 - Haze FormationGiorgi GhambashidzeNo ratings yet
- Instruction Manual: Phaseguard C/ T/ HTDocument32 pagesInstruction Manual: Phaseguard C/ T/ HTGiorgi GhambashidzeNo ratings yet
- RFM PhaseGuard 11027E-1Document90 pagesRFM PhaseGuard 11027E-1Giorgi GhambashidzeNo ratings yet
- High Level Lubricant Switch: Pump Type Order Code Construction Float Switch (A230 002)Document1 pageHigh Level Lubricant Switch: Pump Type Order Code Construction Float Switch (A230 002)Giorgi GhambashidzeNo ratings yet
- Datasheet Bredel - 40 50Document2 pagesDatasheet Bredel - 40 50Giorgi GhambashidzeNo ratings yet