Professional Documents
Culture Documents
Zonisamide in West Syndrome: An Open Label Study: Clinical Commentary
Zonisamide in West Syndrome: An Open Label Study: Clinical Commentary
Uploaded by
ali tidaYou might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (350)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Sel&Ass&Pae&Lis&Car&1 STDocument198 pagesSel&Ass&Pae&Lis&Car&1 STali tida100% (1)
- Treatment: Wilson DiseaseDocument4 pagesTreatment: Wilson Diseaseali tidaNo ratings yet
- Document 1Document1 pageDocument 1ali tidaNo ratings yet
- Marjo S. Van Der Knaap MD, PHD, Jacob Valk MD, PHD Auth. Magnetic Resonance of Myelin, Myelination, and Myelin DisordersDocument570 pagesMarjo S. Van Der Knaap MD, PHD, Jacob Valk MD, PHD Auth. Magnetic Resonance of Myelin, Myelination, and Myelin Disordersali tida100% (1)
- 114 FullDocument8 pages114 Fullali tidaNo ratings yet
- Clinical Sequencing Yield in Epilepsy, Autism Spectrum Disorder, and Intellectual Disability: A Systematic Review and Meta-AnalysisDocument9 pagesClinical Sequencing Yield in Epilepsy, Autism Spectrum Disorder, and Intellectual Disability: A Systematic Review and Meta-Analysisali tidaNo ratings yet
- 1300Document128 pages1300ali tidaNo ratings yet
- 100 Paediatric Picture TestsDocument390 pages100 Paediatric Picture Testsali tidaNo ratings yet
- 130 Hour Advanced TEFLDocument240 pages130 Hour Advanced TEFLali tida100% (1)
- Brain: Part I Plate 3-5: Pileptic YndromesDocument1 pageBrain: Part I Plate 3-5: Pileptic Yndromesali tidaNo ratings yet
- Untitled 1Document1 pageUntitled 1ali tidaNo ratings yet
- Pages From 1416063870 - Illustrat1-3Document1 pagePages From 1416063870 - Illustrat1-3ali tidaNo ratings yet
- Gokul NagarDocument81 pagesGokul NagarNishkarsh MishraNo ratings yet
- Health10 q1 Mod2 Healthcare Providers and Fraudulent Services Ver2 Pages DeletedDocument20 pagesHealth10 q1 Mod2 Healthcare Providers and Fraudulent Services Ver2 Pages DeletedGenesis EspiritNo ratings yet
- Complications of Plaster Cast PATIENT LEAFLETDocument6 pagesComplications of Plaster Cast PATIENT LEAFLETRadiyan MeidhiyantoNo ratings yet
- High Risk PregnancyDocument2 pagesHigh Risk PregnancysamitbisenNo ratings yet
- Words and PrepositionsDocument2 pagesWords and PrepositionsDarek JakubowskiNo ratings yet
- PACS QuestionnaireDocument4 pagesPACS QuestionnaireakashniranjaneNo ratings yet
- MM Kit User GuidlineDocument4 pagesMM Kit User GuidlineRussell JohnNo ratings yet
- Pathologic Fractures: H.T. Temple, MD Walter W. Virkus, MDDocument68 pagesPathologic Fractures: H.T. Temple, MD Walter W. Virkus, MDb BNo ratings yet
- Report PDFDocument6 pagesReport PDFAayushiNo ratings yet
- WPW With Af Case ReportDocument4 pagesWPW With Af Case Report王怡君No ratings yet
- GMP FinalDocument37 pagesGMP FinalekramNo ratings yet
- The Making of Modern Chinese Medicine Authors ProofDocument288 pagesThe Making of Modern Chinese Medicine Authors ProofCorvus CoraxNo ratings yet
- Rauma Epar: Soetamto Wibowo Bagian Bedah FK UNAIR / RSUD DR Soetomo SurabayaDocument37 pagesRauma Epar: Soetamto Wibowo Bagian Bedah FK UNAIR / RSUD DR Soetomo SurabayaMuhammad FirdausNo ratings yet
- Annex4 (CTD)Document178 pagesAnnex4 (CTD)Sajimars SajimarNo ratings yet
- Perioperative Thermoregulation and Heat BalanceDocument10 pagesPerioperative Thermoregulation and Heat BalanceGio VandaNo ratings yet
- Precision in MSDocument62 pagesPrecision in MSSnezana MihajlovicNo ratings yet
- California Edition: DMHC Orders Retroactive PaymentsDocument6 pagesCalifornia Edition: DMHC Orders Retroactive PaymentsPayersandProvidersNo ratings yet
- Test Bank For Egans Fundamentals of Respiratory Care 11th Edition by KacmarekDocument24 pagesTest Bank For Egans Fundamentals of Respiratory Care 11th Edition by KacmarekMichaelHensleyomkra100% (58)
- Essential Facts in Geriatric MedicineDocument26 pagesEssential Facts in Geriatric MedicineDastan HadiNo ratings yet
- FORM RKO BPJS 2019.. EditDocument3 pagesFORM RKO BPJS 2019.. EditMitha yuniatyNo ratings yet
- List of Private Labs Approved by State For Covid Testing As On 29 Aug 2020Document16 pagesList of Private Labs Approved by State For Covid Testing As On 29 Aug 2020FavasNo ratings yet
- Relaxing MassageDocument12 pagesRelaxing MassageFred BelloNo ratings yet
- Medical PodcastDocument14 pagesMedical PodcastAhmed Mohammed omarNo ratings yet
- Tropical Diseases An Unsolved ChallengeDocument9 pagesTropical Diseases An Unsolved ChallengeYet Barreda BasbasNo ratings yet
- Activity 3.3.2 - Measuring Lung Capacity - Human Body SystemsDocument13 pagesActivity 3.3.2 - Measuring Lung Capacity - Human Body SystemsHerman Henson [Northwest CTA]No ratings yet
- Editorial: TP ChaturvediDocument1 pageEditorial: TP ChaturvediAdvanced Research PublicationsNo ratings yet
- Pediatric MnemonicsDocument8 pagesPediatric Mnemonicsadwait marhatta100% (1)
- IVM (In Vitro Maturation) WWWWWWWWWDocument17 pagesIVM (In Vitro Maturation) WWWWWWWWWMirza HassanNo ratings yet
- Transfusion Reaction PDFDocument1 pageTransfusion Reaction PDFKah Man GohNo ratings yet
- Principles of RadiotherapyDocument9 pagesPrinciples of RadiotherapyJuanRuHurtadoNo ratings yet
Zonisamide in West Syndrome: An Open Label Study: Clinical Commentary
Zonisamide in West Syndrome: An Open Label Study: Clinical Commentary
Uploaded by
ali tidaOriginal Title
Copyright
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentZonisamide in West Syndrome: An Open Label Study: Clinical Commentary
Zonisamide in West Syndrome: An Open Label Study: Clinical Commentary
Uploaded by
ali tidaClinical commentary
Epileptic Disord 2009; 11 (4): 339-44
Zonisamide in West syndrome:
an open label study
Mi-Sun Yum, Tae-Sung Ko
Department of Pediatrics, Asan Medical Center, University of Ulsan College of Medicine,
Seoul, Korea
Copyright © 2021 John Libbey Eurotext. Downloaded by MONSIEUR TALIB ABDULQADIR on 19/04/2021.
Received April 3, 2009; Accepted November 18, 2009-11-19
ABSTRACT – Aims. Infantile spasms are usually resistant to conventional anti-
epileptic drugs. Although adrenocorticotropic hormones or vigabatrin are
regarded as standard agents for the treatment of infantile spasms, there are still
limitations of their use. We determined the efficacy and tolerability of high-dose
zonisamide in patients with recent-onset infantile spasms. Methods. Seventeen
patients with infantile spasms, who were admitted to our hospital between
October 2005 and November 2007, were eligible for enrolment within two
months of diagnosis. Zonisamide was administered at a starting dose of
2-8 mg/kg/day, increasing by 2-5 mg/kg/day every three to four days until the
seizures disappeared or the dose reached 30 mg/kg/day. Complete response
was defined as clinical cessation of infantile spasms over 28 consecutive days
and disappearance of hypsarrhythmia by EEG analysis. Results. Of the 17 trea-
ted patients (nine who received initial monotherapy and eight add-on therapy),
five of 12 (42.0%) cryptogenic patients and two of five (40.0%) symptomatic
patients showed complete disappearance of spasms. The maximum daily dose
was 10–28 mg/kg, and the effective daily dose was 10-22 mg/kg. Mean time
period before disappearance of spasms and hypsarrhythmia was eight days.
Seizure recurrence was observed in three of the seven patients who showed
complete disappearance of spasms. Adverse effects included irritability in four
patients and poor oral intake in two patients. Conclusion. Although further study
of zonisamide is warranted, high-dose zonisamide can be effective and safe in
some infants with newly diagnosed West syndrome.
Key words: West syndrome, zonisamide, hypsarrhythmia, high-dose, safety
West syndrome is an age-related used to treat this disorder are not
epilepsy syndrome occurring in infancy fully understood. Because infantile
that is characterized by infantile spasms in many patients are resistant
spasms, hypsarrhythmia and develop- to conventional antiepileptic drugs,
mental regression (West, 1841; Lux the treatments of choice have included
and Osborne, 2004). In view of the pres- adrenocorticotropic hormones and
ence of profound neurodevelopmental steroids, but these agents have been
sequelae, treatment of infantile spasms associated with severe adverse effects
Correspondence: is usually initiated quickly and aggres- (Dulac and Tuxhorn, 2005).
doi: 10.1684/epd.2009.0283
T.-S. Ko, M.D.
Department of Pediatrics,
sively after diagnosis, with the aim of Zonisamide (3-sulfamoylmethyl-1,2-
Asan Medical Center, changing the natural history of the benzisoxazole; ZNS) is a new anti-
University of Ulsan College of Medicine, disease. Due to the vague underlying epileptic drug with an efficacy profile
388-1, Poongnap-dong, pathophysiology, treatment of infantile similar to those of phenytoin and
Songpa-ku,
Seoul, 138-736, Korea spasms has remained empirical, and carbamazepine (Peters and Sorkin,
<tsko@amc.seoul.kr> the mechanisms of action of drugs 1993). Although the exact mechanism
Epileptic Disord Vol. 11, No. 4, December 2009 339
M.-S. Yum, T.-S. Ko
A
Copyright © 2021 John Libbey Eurotext. Downloaded by MONSIEUR TALIB ABDULQADIR on 19/04/2021.
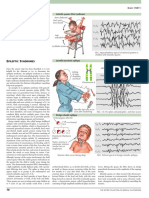
Figure 1. A) Interictal EEG of Patient 4 at six months of age showing hypsarrhythmia while asleep. B) EEG of Patient 4 at seven months of age
after treatment with zonisamide revealing normal background activity with symmetric sleep spindles.
of action of ZNS is not known, the drug most likely acts Although several recent reports have described the efficacy
by blocking T-type calcium channels (Suzuki et al., of ZNS in infantile spasms, these reports were restricted
1992), inhibiting slow sodium channels (Schauf, 1987) mainly to Japanese populations (Suzuki, 2001; Yanagaki
and/or inhibiting glutamate release (Okada et al., 1998). et al., 2005; Yanai et al., 1999). We have assessed the
In addition, ZNS may block the propagation of seizure efficacy, safety, and tolerability of high-dose ZNS in the
discharge and suppress the epileptogenic focus (Takano treatment of infantile spasms in patients eligible for
et al., 1995). treatment within two months of diagnosis.
340 Epileptic Disord Vol. 11, No. 4, December 2009
Zonisamide in West syndrome
A
Copyright © 2021 John Libbey Eurotext. Downloaded by MONSIEUR TALIB ABDULQADIR on 19/04/2021.
Figure 2. A) Interictal EEG of Patient 7 at seven months of age showing right side dominant asymmetric modified hypsarrhythmia and left pos-
terior focal spike discharges during sleeping. B) EEG of Patient 7 at eight months of age after treatment with zonisamide revealing remaining
asymmetric background activity and disappearance of modified hypsarrhythmia.
Materials and methods at the Asan Medical Center were eligible for this
study. Prior to study entry, we obtained Ethical
From October 2005 to November 2007, patients with Committee and Institutional Review Board approval,
infantile spasms beginning at ≤ 12 months of age and and the risks and benefits of ZNS and alternative
hypsarrhythmia identified by electroencephalogram treatment regimens were discussed with each patient’s
(EEG) analysis at the Pediatric Neurology department caregivers.
Epileptic Disord Vol. 11, No. 4, December 2009 341
M.-S. Yum, T.-S. Ko
Patients were treated with ZNS within two months of diag- Results
nosis as initial monotherapy or as add-on therapy. Patients
older than 12 months at diagnosis were excluded, as were During the inclusion period, 17 patients newly diagnosed
patients in whom spasms were successfully treated with with infantile spasms were admitted to our hospital and
other drugs before the introduction of ZNS. treated with ZNS. The group comprised 13 males and
The initial daily dose of ZNS was 2-8 mg/kg body weight, four females, and in all but one patient, developmental
administered orally in one or two doses. Daily dosage was milestones at the time of diagnosis were markedly inferior
increased by 2-5 mg/kg every three to four days until the to age-matched normal profiles. The maximum daily ZNS
spasms disappeared or to a maximum daily dosage of dosage was 9.9-27.8 mg/kg.
30 mg/kg. Routine EEGs were repeated at the time care- Of these 17 patients, seven (41.2%) showed complete
givers reported clinical spasm cessation. Outcome mea- resolution of spasms and disappearance of spasms
surements included clinical response, EEG evaluation, (figures 1, 2), including five of 12 (42.0%) cryptogenic
dosing parameters and tolerability. Complete response patients and two of five (40.0%) symptomatic patients.
Copyright © 2021 John Libbey Eurotext. Downloaded by MONSIEUR TALIB ABDULQADIR on 19/04/2021.
was defined as clinical cessation of infantile spasms The effective daily dose ranged from 10-22.5 mg/kg
reported by their caregivers for ≥ 28 consecutive days and the mean time interval before spasm disappear-
from the time of the last witnessed spasm, according to ance was eight days. Spasm recurrence within six
the West Delphi consensus (Lux and Osborne, 2004), months was observed in three of the seven patients
and disappearance of hypsarrhythmia on EEG between (table 1).
study days 14 and 28 (figure 1). The exit criteria were ZNS treatment was not effective in 10 patients;
defined as no cessation of spasms and extant hypsarrhy- these 10 patients received a maximum daily dose of
thmia of EEG 21 days from start of ZNS therapy. For those 9.9-27.8 mg/kg (mean 19.8 mg/kg). There was no significant
who met the exit criteria, therapy was started without any difference in age at clinical spasm onset, lead time from
delay. onset of spasms or dose of ZNS between responders and
Table 1. Summary of the patient characteristics (n = 17).
Patient Onset age Etiology Concomitant Max. ZNS At spasm control Spasm Add-on
number/ (mo.) drug dose (mg/kg) recurrence therapy after
Time EEG (day) ZNS*
sex (day)
1/M 7 Crypto. VPA 17.9 20 Normal 48** CLB
2/M 8 Crypto. VPA 22.2 15 Normal - -
3/M 6 Crypto. - 22.5 5 Normal 36** VGB†
4/F 5 Crypto. - 10.0 8 Both P-O 115§ VGB
spikes
5/M 8 Crypto. VPA, VGB 11.0 3 Normal - -
6/M 11 PVL - 12.4 6 Normal - -
7/M 7 Infarct CBZ, CLB, VGB 16.5 4 Normal - -
8/M 4 Crypto. VGB, TPM 9.9 - - - KD
9/F 12 Crypto. - 14.3 - - - VGB
10/M 12 Crypto. - 20.4 - - - VGB†
11/F 6 Crypto. - 24.1 - - - VGB†
12/M 2 Crypto. - 27.3 - - - VGB†
13/F 8 Crypto. - 19.6 - - - VGB
14/M 6 Crypto. - 22.2 - - - VGB†
15/M 12 TS VGB 17.4 - - - TPM, KD†
16/M 2 TS PHB 15.3 - - - VGB†
17/M 12 Ventriculomegaly VPA 27.8 - - - F/U loss
* For the patients who did not respond to zonisamide or the patients who experienced recurrence, the add-on therapy was started
without any delay.
** Spasm recurrence without EEG at the time of recurrence.
§
Spasm recurrence with hypsarrhythmia.
†
Patients who showed complete response after add-on therapy.
ZNS: zonisamide; Crypto: cryptogenic; VPA: valproate; Both P-O spikes: both parieto-occipital spikes; VGB: vigabatrin; PVL: periven-
tricular leukomalacia; CBZ: carbamazepine; CLB: clobazam; TPM: topiramate; KD: ketogenic diet; TS: tuberous sclerosis;
PHB: phenobarbital; F/U loss: follow-up loss.
342 Epileptic Disord Vol. 11, No. 4, December 2009
Zonisamide in West syndrome
Table 2. Summary of studies examining zonisamide efficacy in patients with infantile spasms.
References N Response rate* (%) Dosage (mean), S/E
(responder/total numbers of patients) mg/kg/day
Overall Initial Cryptogenic Symptomatic
monotherapy**
Yanagihara et al., 1995 9 33.3 - 0 33.3 (3/9) - None
Yanai et al., 1999 27 33.3 0 100 (2/2) 28.0 (7/25) 5-12.5 (7.8) None
Kawawaki et al., 1999 16 25.0 25.0 (4/16) 66.7 (2/3) 15.4 (2/13) 4-8 (5.8) 1/16
Suzuki, 2001; Suzuki et al., 54 20.4 20.4† (11/54). 28.6 (4/14) 17.5 (7/40) 10-13 (-) None
1997
Lotze and Wilfong, 2004 23 26.1 30.0 (3/10) 0 26.1 (6/26) 8-32 (18) 5/23
Copyright © 2021 John Libbey Eurotext. Downloaded by MONSIEUR TALIB ABDULQADIR on 19/04/2021.
Santos and Brotherton, 2005 7 0 0 - - 3.3-35 (15.9) -
Lee et al., 2009 26 0 0 - - 7.2-14 (8.5) -
Our study 17 41.2 33.3 (3/9) 42.0 (5/12) 40 (2/5) 9.9-27.8 (18.3) 4/17
* Response indicates complete spasm cessations and some patients did not show clearing of the hypsarrhythmia.
** Response rate of the patients who were treatment-naive before zonisamide administration.
†
All patients failed to respond to high-dose vitamin B6 therapy and vitamin B6 was discontinued.
-: data are not available.
non-responders. Adverse effects included irritability in four States (Lotze and Wilfong, 2004), all previous studies
patients (patient 5, 13, 14, and 15) and poor oral intake in reported lower response rates to the present study; how-
two (patient 5 and 14). ever, these previous studies used a lower dose, suggesting
that the better outcome reported in our study may be due
to the higher dose of ZNS (Yanagaki et al., 2005; Yanai
Discussion et al., 1999; Suzuki et al., 1997). Analysis of response
rates in these previous studies according to etiology indi-
A review of the current treatment for West syndrome in cated that the patients with cryptogenic etiology (8/19,
Japan (Tsuji et al., 2007) noted that ZNS use has signifi- 42.1%) showed better responses than those with
cantly increased in recent years and that ZNS is now the symptomatic etiology (26/113, 23.0%).
second or third choice among the non-hormonal, antiepi-
In contrast, our study showed similar outcomes in both
leptic drugs for treating West syndrome in Japan. Here,
groups, suggesting the need for additional, larger studies.
we report a relatively high response rate, with complete
clinical efficacy in 41.2% of patients with newly diag- The present observation that there were no serious side
nosed infantile spasms. We administered ZNS as mono- effects associated with high dose and rapid titration of
therapy in nine patients and as add-on therapy in eight ZNS in infants is in agreement with previous reports
patients. Three of the nine patients (33.3%) who received (Yanagaki et al., 2005; Mandelbaum et al., 2005).
ZNS monotherapy and four of the eight patients (50%) Despite the limitations of our study, including the open-
who received ZNS add-on therapy achieved a seizure- label design and the lack of a control group, our results
free outcome. In the natural course of infantile spasms, provide evidence that ZNS may be effective and safe in
though, there is a known spontaneous remission rate of patients newly diagnosed with infantile spasms. □
approximately 20% during the first year of diagnosis
(Hrachovy and Frost, 2003). Considering some of our Disclosure.
patients relapsed, the response rate was 2/12 (16.7%) for None of the authors has any conflict of interest to disclose.
cryptogenic cases and 4/17 (23.5%) for all patients, which
does not seem too different from the potential spontane-
ous remission rate. References
A review of the literature revealed six reports of the clini-
cal response of ZNS in West syndrome (table 2). In sum- Dulac O, Tuxhorn I. Infantile spasms and west syndrome. In:
mary, for patients with infantile spasms, 3.3-35 mg/kg of Roger J, Bureau M, Dravet C, Genton P, Tassinari CA, Wolf P,
ZNS was associated with an overall clinical response rate eds. Epileptic Syndromes in Infancy, Childhood and Adolescence.
of 0-33.3%. Including our cases, 40 patients of a total of Paris: John Libbey Eurotext, 2005: 53-72.
163 (24.5%) responded completely to ZNS. When admin- Hrachovy RA, Frost JD Jr. Infantile epileptic encephalopathy
istered as initial monotherapy, 21 of 89 patients (23.6%) with hypsarrhythmia (infantile spasms/West syndrome). J Clin
completely responded. Except for one study in the United Neurophysiol 2003; 20: 408-25.
Epileptic Disord Vol. 11, No. 4, December 2009 343
M.-S. Yum, T.-S. Ko
Kawawaki H, Tomiwa K, Shiraishi K, Murata R. Efficacy of zoni- Schauf CL. Zonisamide enhances slow sodium inactivation in
samide in West syndrome. No To Hattatsu 1999; 31: 263-7. Myxicola. Brain Res 1987; 413: 185-8.
Lee YJ, Kang HC, Seo JH, Lee JS, Kim HD. Efficacy and tolerability Suzuki S, Kawakami K, Nishimura S, et al. Zonisamide blocks
of adjunctive therapy with zonisamide in childhood intractable T-type calcium channel in cultured neurons of rat cerebral cortex.
epilepsy. Brain Dev 2009 (in press). Epilepsy Res 1992; 12: 21-7.
Lotze TE, Wilfong AA. Zonisamide treatment for symptomatic Suzuki Y, Nagai T, Ono J, et al. Zonisamide monotherapy in
infantile spasms. Neurology 2004; 62: 296-8. newly diagnosed infantile spasms. Epilepsia 1997; 38: 1035-8.
Lux AL, Osborne JP. A proposal for case definitions and outcome Suzuki Y. Zonisamide in West syndrome. Brain Dev 2001; 23:
measures in studies of infantile spasms and West syndrome: con- 658-61.
sensus statement of the West Delphi group. Epilepsia 2004; 45:
Takano K, Tanaka T, Fujita T, Nakai H, Yonemasu Y. Zonisamide:
1416-28.
electrophysiological and metabolic changes in kainic acid-
Mandelbaum DE, Bunch M, Kugler SL, Venkatasubramanian A, induced limbic seizures in rats. Epilepsia 1995; 36: 644-8.
Wollack JB. Broad-spectrum efficacy of zonisamide at 12 months
Tsuji T, Okumura A, Ozawa H, Ito M, Watanabe K. Current treat-
Copyright © 2021 John Libbey Eurotext. Downloaded by MONSIEUR TALIB ABDULQADIR on 19/04/2021.
in children with intractable epilepsy. J Child Neurol 2005; 20:
ment of West syndrome in Japan. J Child Neurol 2007; 22: 560-4.
594-7.
Yanagaki S, Oguni H, Yoshii K, et al. Zonisamide for West
Okada M, Kawata Y, Mizuno K, Wada K, Kondo T, Kaneko S.
syndrome: a comparison of clinical responses among different
Interaction between Ca2+, K+, carbamazepine and zonisamide
titration rate. Brain Dev 2005; 27: 286-90.
on hippocampal extracellular glutamate monitored with a micro-
dialysis electrode. Br J Pharmacol 1998; 124: 1277-85. Yanagihara K, Imai K, Otani K, Futagi Y. The effect of zonisamide
Peters DH, Sorkin EM. Zonisamide. A review of its pharmacody- in West syndrome. No To Hattatsu 1995; 27: 500-2.
namic and pharmacokinetic properties, and therapeutic potential Yanai S, Hanai T, Narazaki O. Treatment of infantile spasms with
in epilepsy. Drugs 1993; 45: 760-87. zonisamide. Brain Dev 1999; 21: 157-61.
Santos CC, Brotherton T. Use of zonisamide in pediatric patients. West WJ. On a peculiar form of infantile convulsions. Lancet
Pediatr Neurol 2005; 33: 12-4. 1841; 35: 724-5.
344 Epileptic Disord Vol. 11, No. 4, December 2009
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5834)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1093)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (852)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (590)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (903)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (541)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (350)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (824)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (122)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (405)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Sel&Ass&Pae&Lis&Car&1 STDocument198 pagesSel&Ass&Pae&Lis&Car&1 STali tida100% (1)
- Treatment: Wilson DiseaseDocument4 pagesTreatment: Wilson Diseaseali tidaNo ratings yet
- Document 1Document1 pageDocument 1ali tidaNo ratings yet
- Marjo S. Van Der Knaap MD, PHD, Jacob Valk MD, PHD Auth. Magnetic Resonance of Myelin, Myelination, and Myelin DisordersDocument570 pagesMarjo S. Van Der Knaap MD, PHD, Jacob Valk MD, PHD Auth. Magnetic Resonance of Myelin, Myelination, and Myelin Disordersali tida100% (1)
- 114 FullDocument8 pages114 Fullali tidaNo ratings yet
- Clinical Sequencing Yield in Epilepsy, Autism Spectrum Disorder, and Intellectual Disability: A Systematic Review and Meta-AnalysisDocument9 pagesClinical Sequencing Yield in Epilepsy, Autism Spectrum Disorder, and Intellectual Disability: A Systematic Review and Meta-Analysisali tidaNo ratings yet
- 1300Document128 pages1300ali tidaNo ratings yet
- 100 Paediatric Picture TestsDocument390 pages100 Paediatric Picture Testsali tidaNo ratings yet
- 130 Hour Advanced TEFLDocument240 pages130 Hour Advanced TEFLali tida100% (1)
- Brain: Part I Plate 3-5: Pileptic YndromesDocument1 pageBrain: Part I Plate 3-5: Pileptic Yndromesali tidaNo ratings yet
- Untitled 1Document1 pageUntitled 1ali tidaNo ratings yet
- Pages From 1416063870 - Illustrat1-3Document1 pagePages From 1416063870 - Illustrat1-3ali tidaNo ratings yet
- Gokul NagarDocument81 pagesGokul NagarNishkarsh MishraNo ratings yet
- Health10 q1 Mod2 Healthcare Providers and Fraudulent Services Ver2 Pages DeletedDocument20 pagesHealth10 q1 Mod2 Healthcare Providers and Fraudulent Services Ver2 Pages DeletedGenesis EspiritNo ratings yet
- Complications of Plaster Cast PATIENT LEAFLETDocument6 pagesComplications of Plaster Cast PATIENT LEAFLETRadiyan MeidhiyantoNo ratings yet
- High Risk PregnancyDocument2 pagesHigh Risk PregnancysamitbisenNo ratings yet
- Words and PrepositionsDocument2 pagesWords and PrepositionsDarek JakubowskiNo ratings yet
- PACS QuestionnaireDocument4 pagesPACS QuestionnaireakashniranjaneNo ratings yet
- MM Kit User GuidlineDocument4 pagesMM Kit User GuidlineRussell JohnNo ratings yet
- Pathologic Fractures: H.T. Temple, MD Walter W. Virkus, MDDocument68 pagesPathologic Fractures: H.T. Temple, MD Walter W. Virkus, MDb BNo ratings yet
- Report PDFDocument6 pagesReport PDFAayushiNo ratings yet
- WPW With Af Case ReportDocument4 pagesWPW With Af Case Report王怡君No ratings yet
- GMP FinalDocument37 pagesGMP FinalekramNo ratings yet
- The Making of Modern Chinese Medicine Authors ProofDocument288 pagesThe Making of Modern Chinese Medicine Authors ProofCorvus CoraxNo ratings yet
- Rauma Epar: Soetamto Wibowo Bagian Bedah FK UNAIR / RSUD DR Soetomo SurabayaDocument37 pagesRauma Epar: Soetamto Wibowo Bagian Bedah FK UNAIR / RSUD DR Soetomo SurabayaMuhammad FirdausNo ratings yet
- Annex4 (CTD)Document178 pagesAnnex4 (CTD)Sajimars SajimarNo ratings yet
- Perioperative Thermoregulation and Heat BalanceDocument10 pagesPerioperative Thermoregulation and Heat BalanceGio VandaNo ratings yet
- Precision in MSDocument62 pagesPrecision in MSSnezana MihajlovicNo ratings yet
- California Edition: DMHC Orders Retroactive PaymentsDocument6 pagesCalifornia Edition: DMHC Orders Retroactive PaymentsPayersandProvidersNo ratings yet
- Test Bank For Egans Fundamentals of Respiratory Care 11th Edition by KacmarekDocument24 pagesTest Bank For Egans Fundamentals of Respiratory Care 11th Edition by KacmarekMichaelHensleyomkra100% (58)
- Essential Facts in Geriatric MedicineDocument26 pagesEssential Facts in Geriatric MedicineDastan HadiNo ratings yet
- FORM RKO BPJS 2019.. EditDocument3 pagesFORM RKO BPJS 2019.. EditMitha yuniatyNo ratings yet
- List of Private Labs Approved by State For Covid Testing As On 29 Aug 2020Document16 pagesList of Private Labs Approved by State For Covid Testing As On 29 Aug 2020FavasNo ratings yet
- Relaxing MassageDocument12 pagesRelaxing MassageFred BelloNo ratings yet
- Medical PodcastDocument14 pagesMedical PodcastAhmed Mohammed omarNo ratings yet
- Tropical Diseases An Unsolved ChallengeDocument9 pagesTropical Diseases An Unsolved ChallengeYet Barreda BasbasNo ratings yet
- Activity 3.3.2 - Measuring Lung Capacity - Human Body SystemsDocument13 pagesActivity 3.3.2 - Measuring Lung Capacity - Human Body SystemsHerman Henson [Northwest CTA]No ratings yet
- Editorial: TP ChaturvediDocument1 pageEditorial: TP ChaturvediAdvanced Research PublicationsNo ratings yet
- Pediatric MnemonicsDocument8 pagesPediatric Mnemonicsadwait marhatta100% (1)
- IVM (In Vitro Maturation) WWWWWWWWWDocument17 pagesIVM (In Vitro Maturation) WWWWWWWWWMirza HassanNo ratings yet
- Transfusion Reaction PDFDocument1 pageTransfusion Reaction PDFKah Man GohNo ratings yet
- Principles of RadiotherapyDocument9 pagesPrinciples of RadiotherapyJuanRuHurtadoNo ratings yet