Professional Documents
Culture Documents
Mm204E Thermodynamics-Ii Second Midterm Exam: Q1 Q2 Q3 Q4 Q5 Total /20 /20 /20 /20 /20 /100
Mm204E Thermodynamics-Ii Second Midterm Exam: Q1 Q2 Q3 Q4 Q5 Total /20 /20 /20 /20 /20 /100
Uploaded by
Ali DoğruOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mm204E Thermodynamics-Ii Second Midterm Exam: Q1 Q2 Q3 Q4 Q5 Total /20 /20 /20 /20 /20 /100
Mm204E Thermodynamics-Ii Second Midterm Exam: Q1 Q2 Q3 Q4 Q5 Total /20 /20 /20 /20 /20 /100
Uploaded by
Ali DoğruCopyright:
Available Formats
Gazi Üniversitesi/Gazi University
Mühendislik-Mimarlık Fakültesi/Faculty of Engineering and Architecture
Makina Mühendisliği Bölümü/Mechanical Engineering Department
Ankara/Türkiye
Instructor: Dr. Atilla Bıyıkoğlu
Student’s Name :
Q1 Q2 Q3 Q4 Q5 TOTAL Student’s Number :
/20 /20 /20 /20 /20 /100
MM204E THERMODYNAMICS-II
SECOND MIDTERM EXAM
Date: May 28th, 2021 Time Span: 18:30-21:00
(Closed book)
(20%) Q1. Carbon dioxide gas flowing through a heat exchanger operating at steady state is
heated at an essentially constant pressure of 7 MPa from 27oC to 427oC. Neglecting kinetic
and potential energy changes,
(a) Determine the heat transfer, in kJ per kg of CO2 flowing, and compare with the result
obtained using the ideal gas model.
(b) Determine the irreversibility during this constant pressure heating process
(20%) Q2. For a gas obeying the van der Waals equation of state, determine the relations
between T and v and between p and v in isentropic processes for which cv is constant.
(20%) Q3. Methane gas (CH4) at 25oC, 1 atm and a volumetric flow rate of 27 m3/h enters a
furnace operating at steady state. The methane burns completely with 140% of theoretical air,
entering at 127oC, 1 atm. Products of combustion exit at 427 oC, 1 atm. Determine
(a) The volumetric flow rate of the air, in m3/h.
(b) The rate of heat transfer from the furnace, in kJ/h
Hint: Kinetic and potential energy effects are negligible.
(20%) Q4. Coal with a mass analysis of 80% carbon, 3% sulfur, 17% noncombustible ash
burns completely with 120% of theoretical air. Determine the amount of SO2 produced, in kg
SO2 per kg of coal.
(20%) Q5. (a) Propane (g) at 25oC and 100 kPa is burned with 400% theoretical air at 25oC
and 100 kPa. Assume that the reaction occurs reversibly at 25oC, that the oxygen and nitrogen
are separated before the reaction takes place (each at 100 kPa, 25oC), that the constituents in
the products are separated, and that each is at 25oC, 100 kPa. Determine the reversible work
for this process. (b) If the above reaction occurs adiabatically, and each constituent in the
products is at 100 kPa pressure and at the adiabatic flame temperature, compute (a) the
increase in entropy during combustion, (b) the irreversibility of the process, and (c) the
availability of the products of combustion.
You might also like
- Hibbeler Dynamics 12th Edition Solutions Chapter 12 Soup IoDocument3 pagesHibbeler Dynamics 12th Edition Solutions Chapter 12 Soup IoAli Doğru33% (3)
- English: Giving Expanded Definitions of WordsDocument24 pagesEnglish: Giving Expanded Definitions of WordsHellen Dea60% (5)
- 13 Partial Pressures of GasesDocument6 pages13 Partial Pressures of GasesTanisha Marie100% (1)
- Thermo II Asst.Document1 pageThermo II Asst.Sunil AdhikariNo ratings yet
- Final 2012 Chemical MetallurgyDocument7 pagesFinal 2012 Chemical MetallurgyRuby AdijayaNo ratings yet
- Universiti Teknologi Mara Final Examination: Confidential EM/APR 2007/KJM451Document10 pagesUniversiti Teknologi Mara Final Examination: Confidential EM/APR 2007/KJM451Diraf AlipNo ratings yet
- Mm204e HW2 2014-15 SpringDocument2 pagesMm204e HW2 2014-15 Springhittaf_05No ratings yet
- Examen Muestra CombustiónDocument1 pageExamen Muestra CombustiónMarco TorresNo ratings yet
- Thermodynamics Final Make UpDocument1 pageThermodynamics Final Make UpTaha MisirliNo ratings yet
- CH126P.B22.M1Exam (M1.Exam.P2)Document2 pagesCH126P.B22.M1Exam (M1.Exam.P2)Luis Alfonso DañezNo ratings yet
- AE8301 Aero Engineering Thermodynamics, QP, Model (2020 - 2021) - SDocument2 pagesAE8301 Aero Engineering Thermodynamics, QP, Model (2020 - 2021) - SGurunath AeroNo ratings yet
- Power Plant Engg Assignment-1Document2 pagesPower Plant Engg Assignment-1keyredin selmanNo ratings yet
- Eng209 PQDocument1 pageEng209 PQChizzy56No ratings yet
- THT ExamDocument7 pagesTHT Examabdilrhman sulimanNo ratings yet
- Assignment 1 - 20194Document1 pageAssignment 1 - 20194NURIEN ZAHIRAH MUHAMMAD ZAFRANNo ratings yet
- Mec551 PDFDocument10 pagesMec551 PDFShah GrungeNo ratings yet
- Thermodynamics I (MEP1203) Model Answer (Document11 pagesThermodynamics I (MEP1203) Model Answer (Kerlos SaeedNo ratings yet
- ME 6301 Engineering Thermodynamics Nov Dec 2014Document3 pagesME 6301 Engineering Thermodynamics Nov Dec 2014BIBIN CHIDAMBARANATHANNo ratings yet
- Final Fall 1432-1433H SolutionDocument9 pagesFinal Fall 1432-1433H SolutionMuhammad HaziqNo ratings yet
- 10.213 Chemical Engineering Thermodynamics Spring 2002 Problem Set FDocument2 pages10.213 Chemical Engineering Thermodynamics Spring 2002 Problem Set FPM SHNo ratings yet
- 07a3ec08 ThermodynamicsDocument8 pages07a3ec08 ThermodynamicsandhracollegesNo ratings yet
- 2.thermal ScienceDocument9 pages2.thermal Scienceram jrpsNo ratings yet
- MET303 - Ktu QbankDocument16 pagesMET303 - Ktu QbankANAND V VNo ratings yet
- AET Model Question PaperDocument4 pagesAET Model Question PaperGurunath AeroNo ratings yet
- EAS Thermo 1 Jan 21 - Gasal - 2020 GWDocument1 pageEAS Thermo 1 Jan 21 - Gasal - 2020 GWWida NingsihNo ratings yet
- Etd. It1 2021-2022 OddDocument1 pageEtd. It1 2021-2022 Oddmmk.mech59No ratings yet
- DocDocument5 pagesDoccessareNo ratings yet
- Parcial Examen Final Quiz Validación Otro XDocument2 pagesParcial Examen Final Quiz Validación Otro XJuan De DiosNo ratings yet
- Me6301 Engineering Thermodynamics May June 2011Document3 pagesMe6301 Engineering Thermodynamics May June 2011BIBIN CHIDAMBARANATHANNo ratings yet
- FinalDocument4 pagesFinalSimge DemirNo ratings yet
- Good Luck: Physical Chemistry 1 (CHE211) Group Mid Exam Time:80 Min 1Document1 pageGood Luck: Physical Chemistry 1 (CHE211) Group Mid Exam Time:80 Min 1binabdo1981No ratings yet
- Assignment 2Document2 pagesAssignment 2Maria SarwatNo ratings yet
- TD QP UPTO Nov 2012 18092012Document31 pagesTD QP UPTO Nov 2012 18092012Narayanan Srinivasan100% (1)
- Rajiv Gandhi University of Knowledge Technologies, Basar Mechanical EngineeringDocument7 pagesRajiv Gandhi University of Knowledge Technologies, Basar Mechanical EngineeringPunith YadavNo ratings yet
- Power Plant Engineeringmech 4242 - 2022Document6 pagesPower Plant Engineeringmech 4242 - 2022kingsukbarman07No ratings yet
- Me6301 Engineering Thermodynamics May June 2013Document3 pagesMe6301 Engineering Thermodynamics May June 2013BIBIN CHIDAMBARANATHANNo ratings yet
- ME132 Thermodynamics Nov Dec 2007Document4 pagesME132 Thermodynamics Nov Dec 2007ARUNGREESMANo ratings yet
- 2023-Dec CH-213 116Document2 pages2023-Dec CH-213 116Jeison Estiven Lopez OrtegaNo ratings yet
- Politecnico Di Milano: Department of Energy School of Industrial and Information EngineeringDocument2 pagesPolitecnico Di Milano: Department of Energy School of Industrial and Information EngineeringEliot KhNo ratings yet
- Thermal Unit 2 14 MarksDocument3 pagesThermal Unit 2 14 MarksKumar SubramanianNo ratings yet
- ME FinalDocument81 pagesME FinalAnkit DahiyaNo ratings yet
- THD3602 Major Test 1 2023Document3 pagesTHD3602 Major Test 1 2023LeighNo ratings yet
- Heat and Mass TransferDocument2 pagesHeat and Mass TransferRuby SmithNo ratings yet
- Thermodynamics Question Solve 2011Document3 pagesThermodynamics Question Solve 2011MD SR ShantoNo ratings yet
- 8.assignment Tutorial QPDocument13 pages8.assignment Tutorial QPvsureshkannanmsecNo ratings yet
- Refrigeration and Air-Conditioning (Professional Elective-II) (Autonomy 2020 Pattern)Document11 pagesRefrigeration and Air-Conditioning (Professional Elective-II) (Autonomy 2020 Pattern)do it for funNo ratings yet
- Assignment SolutionDocument3 pagesAssignment SolutionSuraj ChaudharyNo ratings yet
- Power Plant EngineeringDocument4 pagesPower Plant EngineeringKishore KrishNo ratings yet
- COMSATS University Islamabad: Sahiwal Campus (Department of Mechanical Engineering)Document15 pagesCOMSATS University Islamabad: Sahiwal Campus (Department of Mechanical Engineering)baba sugarNo ratings yet
- MECHANICAL ENGINEERING 2019 Scheme S4 Syllabus Ktustudents - inDocument91 pagesMECHANICAL ENGINEERING 2019 Scheme S4 Syllabus Ktustudents - inashnbNo ratings yet
- Modernization of Unit For Elimination of Vocs by Catalytic OxidationDocument6 pagesModernization of Unit For Elimination of Vocs by Catalytic Oxidationoverlord5555No ratings yet
- Assignment 1, Autumn 2023Document2 pagesAssignment 1, Autumn 2023cocodarshi2022No ratings yet
- Thermo Final SummerDocument2 pagesThermo Final SummerMAk StudiosNo ratings yet
- Me8391 - EtdDocument3 pagesMe8391 - Etdsyed1188No ratings yet
- 08r059210304 ThermodynamicsDocument8 pages08r059210304 ThermodynamicsandhracollegesNo ratings yet
- ETD Final Exam 4Document4 pagesETD Final Exam 4Bhargav Srinivas PadamataNo ratings yet
- 7157902Document62 pages7157902Red RedNo ratings yet
- 15CHE111 - Spring 2019 ProjectDocument5 pages15CHE111 - Spring 2019 ProjectPunith KumarNo ratings yet
- Me 6301 - Engineering Thermodynamics Unit Test 1 - Set 1Document2 pagesMe 6301 - Engineering Thermodynamics Unit Test 1 - Set 1BIBIN CHIDAMBARANATHANNo ratings yet
- ME Con-3Document8 pagesME Con-3vidya chakitwarNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Review On Thermal Energy Storage With Phase Change: Materials, Heat Transfer Analysis and ApplicationsDocument33 pagesReview On Thermal Energy Storage With Phase Change: Materials, Heat Transfer Analysis and ApplicationsAli DoğruNo ratings yet
- Optimization of A Phase Change MaterialDocument30 pagesOptimization of A Phase Change MaterialAli DoğruNo ratings yet
- 9-3 Laminar Flat-Plate Boundary Layer: Exact Solution: U X y VDocument5 pages9-3 Laminar Flat-Plate Boundary Layer: Exact Solution: U X y VAli DoğruNo ratings yet
- Heat Transfer Efficiency of Metal Honeycombs - KopyaDocument10 pagesHeat Transfer Efficiency of Metal Honeycombs - KopyaAli DoğruNo ratings yet
- Me302 Fluid Mechanics Ii: Rectangular Coordinate SystemDocument8 pagesMe302 Fluid Mechanics Ii: Rectangular Coordinate SystemAli DoğruNo ratings yet
- Chapter 2 Earth and Earth Systems SummaryDocument3 pagesChapter 2 Earth and Earth Systems SummaryLord LlenoNo ratings yet
- How Do You Convert BTU To CFM?Document6 pagesHow Do You Convert BTU To CFM?sanjay975No ratings yet
- Gas Exchange in Humans CIE IGCSE Biology 610 Classified Paster Paper 2Document21 pagesGas Exchange in Humans CIE IGCSE Biology 610 Classified Paster Paper 2IGCSE Physics & ChemistryNo ratings yet
- Q1Air Pollution QuestionsDocument3 pagesQ1Air Pollution QuestionsSREEKUMARA GANAPATHY V S stellamaryscoe.edu.inNo ratings yet
- 3 - Weather PowerPoint NotesDocument135 pages3 - Weather PowerPoint NotesErmias AbelnehNo ratings yet
- Climate Change by Humanity's Hand 1111Document4 pagesClimate Change by Humanity's Hand 1111Kyle ErosidoNo ratings yet
- Lecture 11 The AnthroposphereDocument14 pagesLecture 11 The AnthroposphereKiara TongNo ratings yet
- Breeze CapstoneDocument52 pagesBreeze CapstoneKurt JimenezNo ratings yet
- PressureDocument35 pagesPressureHarsh GuptaNo ratings yet
- Chapter 14 Review Q AnswersDocument2 pagesChapter 14 Review Q Answersapi-269764684No ratings yet
- Ee8703 Re Unit1Document43 pagesEe8703 Re Unit1manimaranNo ratings yet
- 1.4 Mine Gases - Methane IDocument9 pages1.4 Mine Gases - Methane IDeepakKattimani100% (1)
- Nso Class-7Document3 pagesNso Class-7Swati AshtakeNo ratings yet
- Sở Giáo Dục Và Đào Tạo Thanh Hóa Đề Thi Thử Số 1 Kỳ Thi Vào Lớp 10 Thpt Chuyên Lam Sơn Năm học 2022-2023 Môn thi: Tiếng AnhDocument8 pagesSở Giáo Dục Và Đào Tạo Thanh Hóa Đề Thi Thử Số 1 Kỳ Thi Vào Lớp 10 Thpt Chuyên Lam Sơn Năm học 2022-2023 Môn thi: Tiếng AnhLê thị YếnNo ratings yet
- Overview Glass Furnace1Document34 pagesOverview Glass Furnace1Yogesh BadheNo ratings yet
- bài tập bổ íchDocument83 pagesbài tập bổ íchtrung100% (1)
- Ficha Técnica Activated Carbon Air Filter PDFDocument2 pagesFicha Técnica Activated Carbon Air Filter PDFMeliza SuarezNo ratings yet
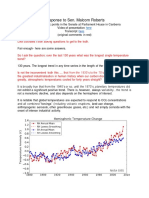
- Response To Senator - Climate ChangeDocument5 pagesResponse To Senator - Climate ChangeChris ColoseNo ratings yet
- Earth Astronomical Object Experiment, Ariny Amos (Astronomer)Document1,900 pagesEarth Astronomical Object Experiment, Ariny Amos (Astronomer)Dr. Amos ArinyNo ratings yet
- How Airplanes Fly: A Physical Description of Lift Level 3Document14 pagesHow Airplanes Fly: A Physical Description of Lift Level 3delaneylukeNo ratings yet
- Topic 1 Pyschrometry NotesDocument50 pagesTopic 1 Pyschrometry NotesCia Lev Lim100% (1)
- The Composition and Structure of The AtmosphereDocument87 pagesThe Composition and Structure of The AtmosphereMarch Sadaran DequiñaNo ratings yet
- Gaia and The Earth SystemDocument8 pagesGaia and The Earth Systemken455No ratings yet
- Climate ZonesDocument26 pagesClimate ZonesKhiZra ShahZadNo ratings yet
- Cooling Tower LabDocument24 pagesCooling Tower LabMuhammad Tayyib Abdul Manan0% (1)
- Concept R3 AnswerDocument44 pagesConcept R3 Answershsh.shetty4456No ratings yet
- EnergyDocument10 pagesEnergyDennis Paul Paz LopezNo ratings yet
- Reviewer No AnswerDocument27 pagesReviewer No AnswerReydale CachoNo ratings yet