Professional Documents
Culture Documents
LC Impaction Hallowell-2015-Equine - Veterinary - Education
LC Impaction Hallowell-2015-Equine - Veterinary - Education
Uploaded by
Turok StaffOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
LC Impaction Hallowell-2015-Equine - Veterinary - Education
LC Impaction Hallowell-2015-Equine - Veterinary - Education
Uploaded by
Turok StaffCopyright:
Available Formats
EQUINE VETERINARY EDUCATION 385
Equine vet. Educ. (2017) 29 (7) 385-390
doi: 10.1111/eve.12530
Review Article
Medical management of large colonic impactions
G. D. Hallowell*
School of Veterinary Medicine and Science, University of Nottingham, Sutton Bonington, Leicestershire, UK.
*Corresponding author email: gayle.d.hallowell@nottingham.ac.uk
Keywords: horse; gastrointestinal; pelvic flexure; colic
Summary masses are primarily composed of struvite (ammonium
Large colonic impactions are a common cause of abdominal magnesium phosphate) (Blue and Wittkopp 1981) and start
pain in the horse. This review aims to discuss normal function around some small nidus (stone, hair, small bits of rope). As
of the large colon, risk factors for development of colonic horses have an abundance of calcium in the gastrointestinal
impactions, diagnosis and optimal strategies for management tract, it is surprising that these masses are not composed of
and prevention based on the evidence available. calcium phosphate (Hassel et al. 2001). However, it is
suggested that there is an excessive solute load of
magnesium in the diet, which may be bound by increased
Introduction amounts of intraluminal protein. Thus diets containing large
Large colonic impactions can be either primary or secondary quantities of alfalfa, which has both a high protein and
to other disease processes that have induced hypovolaemia magnesium content, may predispose animals to developing
such as small intestinal strangulating lesions and subacute these stones. Miniature horses and Arabians have been
equine grass sickness. This article will primarily focus upon shown to be more susceptible in one study (Cohen et al.
primary colonic impactions. Primary large colonic impactions 2000). As these stones take some time to develop and cause
are a common cause of mild to moderate abdominal pain in obstruction, they are rarely identified in animals less than
the horse and generally form gradually leading to the 4 years of age. These obstructions are usually associated with
insidious onset and nature of the clinical signs seen. mild to moderate, intermittent pain, which may lead to these
Impactions of the large colon generally occur at sites of animals presenting with chronic or even recurrent abdominal
luminal narrowing, primarily of the pelvic flexure and right pain (Lloyd et al. 1987). Management of these cases is
dorsal colon, but can affect other intestinal sections (White usually surgical, although dietary manipulation is required
and Dabareiner 1997). post operatively and as such will not be discussed any further.
Faecoliths are a rare cause of colonic impaction,
particularly of the transverse and descending colon. They are
Aetiology and risk factors most commonly seen in ponies and foals (McClure et al.
Many risk factors have been proposed, but not proven. A 1992) and in older horses secondary to poor dentition and
sudden reduction in exercise, often secondary to inappropriate mastication of food material. These animals will
musculoskeletal injuries, is often associated with this condition present as with enteroliths, although may also show signs
(Dabareiner and White 1995). Other factors implicated consistent with tenesmus (Gay et al. 1979).
include parasite migration affecting gastrointestinal motility, Other rarer causes of colonic disease resulting in colonic
poor dentition, coarse roughage, especially horses that eat impactions include adhesions from previous colic surgery or
their straw bed, permanent stabling, oral stereotypies, travel peritonitis, foreign bodies, which are usually seen in younger
in the recent past, administration of pharmacological agents animals and those in the developing world and mural masses
that reduce gastrointestinal motility (Freeman and England such as abscesses, tumours, granulomas and haematomas.
2001; Proudman et al. 2006; Williams et al. 2015) and either The cause of the impaction can be due to direct luminal
dehydration or hypovolaemia (Hillyer et al. 2002). obstruction or indirectly secondary to a change in colonic
The prevalence of colonic impactions with sand is motility.
dramatically affected by geography and soil type as well as
how animals are fed. Some animals also develop a particular
Role of the large colon
predilection for eating sand, which in most cases will not be
due to a mineral deficiency (Blikslager 2010). It is reported In order to fully appreciate the management options for
that fine grain sand generally accumulates in the ventral colonic impactions, it is necessary to understand normal
colonic segments and coarser grains in the dorsal sections colonic function, have a basic understanding of the complex
(Specht and Colahan 1988; Ragle et al. 1989). However, mechanisms involved in normal colonic motility and
changes in gastrointestinal motility may also play a role in appreciate the normal composition of colonic contents.
sand accumulation. Clinical signs are generally similar to The large colon of the horse, compared with other
those with colonic impactions and due to colonic distention monogastric species, is extremely well developed and has
with sand, gaseous distention or inflammation of the mucosa. many functions. It mixes, retains and propulses ingesta. Whilst
Enteroliths leading to ingesta accumulation, usually of the the ingesta is within the colon, it undergoes fermentation and
right dorsal and transverse colonic sections (Lloyd et al. 1987), the process is essentially analogous to that which occurs in
are a relatively uncommon cause of obstruction in the UK, the rumen of farm animals. If the horse did not produce large
but are more prevalent in other parts of the world. These quantities of volatile fatty acids (VFAs) from the fibre it ingests,
© 2016 EVJ Ltd
386 Management of large colonic impactions
it would not survive. These VFAs are absorbed through the The microbiota
caecal and colonic epithelium and are then distributed for
use throughout the body as an energy source. One There is currently a lot of interest in the gastrointestinal
significant difference from ruminants is that the large quantity microbiota, its specific composition and potential
of microbial protein generated in the equine large colon is consequences of change regarding gastrointestinal disease
wasted because there is no opportunity for significant (Schoster et al. 2014). The colon contains bacteria, protozoa
absorption of amino acids. In addition to the generation of and fungi. Changes in composition will affect the efficiency
energy, water absorption in the normal horse primarily occurs of fibre fermentation and locally production of certain
in the large colon. Water in the large intestine acts as a byproducts such as lactic acid and local changes in pH can
reserve in times of need. In an adult horse a volume of up to affect local motility as is well documented in the rumen of
100 l of fluid and associated secretions is absorbed during the farm animals (Enemark 2008). Low faecal pH has been
course of the day (Argenzio et al. 1974, 1975; Argenzio and identified in horses on a high carbohydrate diet (Sykes et al.
Clarke 1989). 2013) and thus may play a role in motility disorders in the
horse and warrants further evaluation.
Large colonic motility
Diagnosis
Motility of the large colon is essential for mixing, retainment,
propulsion and retropulsion of ingesta. It depends upon a For the majority of colonic obstructions, the mainstay for
complex interaction between neural, hormonal, vascular and diagnosis will be based on history, signs of abdominal
neuromuscular pathways and any disruption to this fine pain, reduced faecal output and presence of a smooth
balance can lead to reduced motility or gastrointestinal distended viscus running laterally in front of, and ventral to
stasis. Regulation of motility requires central, autonomic the pelvis in the case of pelvic flexure impactions and
innervation and the enteric nervous system (myenteric and potentially in other anatomical locations for other areas of
submucosal plexi) (Koenig and Cote 2006). However, control obstruction. Diagnosis of other forms of obstruction,
of intestinal contractility by the enteric nervous system is particularly with sand, will also be aided with history and
independent of the central nervous system (Koenig and Cote geographical location. Other diagnostic aids for sand
2006). Acetylcholine is the main excitatory neurotransmitter impactions include auscultation, where some clinicians
causing smooth muscle contraction through M-2 type advocate being able to ‘hear’ the sand, which sounds
receptors. Sympathetic stimulation inhibits acetylcholine like the sea crashing on the beach, faecal sedimentation
release via activation of alpha-2 receptors (Lester et al. and radiographic assessment (Ragle et al. 1989). There is
1998). There has been a focus on the pelvic flexure regarding controversy regarding the value of ultrasonography; some
large colonic motility and it has long been accepted that an have found that it aids in identification, whereas others
electrical pacemaker exists at the pelvic flexure where the have not and have reported that it does not provide
strong rhythmic contractions of the large intestine begin information regarding the amount of sand present
(Sellers and Lowe 1986). The pacemaker cells of the (Korolainen and Ruohoniemi 2002; Hotwagner and Iben
gastrointestinal tract, the interstitial cells of Cajal (ICC) 2008). In animals that live in areas where sand ingestion is
(Hudson et al. 1999), are responsible for initiating coordinated likely, there appear to be poor ‘cut-offs’ between normal
gastrointestinal motility (Bortoff 1965; Ward et al. 1997; and likely affected animals. Enteroliths and faecoliths may
Sanders et al. 1999). Reduced myenteric neuron densities in be detectable on rectal examination, but abdominal
the large intestine have been identified in horses with radiography may be beneficial to identify these
gastrointestinal obstructive disorders, including impactions, obstructions, especially in smaller cases.
when compared with normal horses (Schusser and White
1997; Schusser et al. 2000) as have ICC density (Fintl et al.
Assessment of hydration status
2004).
One of the key assessments undertaken in the horse with
abdominal pain is of hydration status and this is going to
Large intestinal contents have a significant impact on the most appropriate
The large intestinal contents contain fibrous material management of horses with colonic obstruction. The majority
suspended in water. The size of the fibrous material depends of horses that present with colonic impaction are neither
upon the type of fibre ingested and degree of mastication. hypovolaemic nor dehydrated. Hypovolaemia is defined as a
One experimental study found that high grain diets led to loss of circulating volume, resulting from both water and salt
dehydrated contents of the right dorsal colon when loss. Clinical signs of hypovolaemia include tachycardia,
compared with fibre-based diets (Lopes et al. 2004a), which increased capillary refill time, haemoconcentration
may be due to promotion of water absorption from colonic (increased packed cell volume and total solids), increased
contents secondary to activation of the renin-angiotensin- lactate concentrations, reduced urine output and
aldosterone system post prandially (Clarke et al. 1990), concentrated urine (USG >1.040). Dehydration is defined as a
coupling of absorption of VFA with sodium and water loss of total body water and is much more challenging to
(Argenzio et al. 1977) or fibre holding water within the quantify. Clinical signs of dehydration include decreased
gastrointestinal tract (Warren et al. 1999). The amount of bodyweight (not helpful clinically, but used experimentally),
water that the fibre is contained within is dependent upon sunken eyes, increased skin tent over the upper eyelid and
fluid intake, but also upon how much of that water is tacky mucous membranes. Dehydrated animals are always
absorbed to maintain normal systemic hydration. hypernatraemic.
© 2016 EVJ Ltd
G. D. Hallowell 387
Overall aims of treatment of colonic impactions results in significant haemodynamic changes (Lopes et al.
2002). A more recent study supported previous findings in that
The overall aims of treatment are to provide analgesia, i.v. fluids administered at 3 times maintenance requirements
correct any fluid deficits present, resolve the impaction and were no more efficacious and might be associated with
then identify any potential risk factors to prevent these from adverse physiological findings after withdrawal, whilst boluses
developing again. of water administered nasogastrically can be used to restore
intestinal water with minimal adverse effects (Lester et al.
General management of colonic impactions 2013). Another experimental study proposed that the main
effect of a selection of oral fluids that were compared was
Although withholding food is likely to reduce gastrointestinal activation of the gastrocolic reflex following filling of the
motility (Jones et al. 1991), in the short-term it prevents the stomach (Freeman et al. 1992).
impaction from growing any larger and also provides In clinical cases with colonic impactions, resolution rates
the opportunity for enterally-administered products to reach were fastest in those that received 5 doses of isotonic
the impacted colon. Food should be withheld until there is electrolyte solutions every 30 min or hourly when compared
evidence that the impaction has resolved. As discussed with those that received fluids every 2 h or i.v. Complication
above, these animals are variably painful, but are likely to rates were similar between groups, but severity of abdominal
require some parenteral nonsteroidal anti-inflammatory pain was greatest in those treated every 30 min (Hallowell
agents, which will have direct benefits for managing pain 2008). A similar clinical study demonstrated that 99% of large
and indirect benefits in that a less painful animal will have colon impactions (n = 78) were effectively treated with
reduced activation of the sympathetic nervous system, which enteral fluids with a resolution time of under 24 h (Monreal
itself is likely contributing to gastrointestinal stasis (Koenig and et al. 2010), which is similar to the previous clinical study
Cote 2006). Alpha-2 agonists are known to reduce described above (Hallowell 2008), with no difference in
gastrointestinal motility in normal horses, but will provide resolution time when i.v. fluids were administered concurrently
analgesia for the duration of their sedative actions (Valverde (Monreal et al. 2010).
2010). In the author’s opinion, products containing
N-butylscolopamine are not warranted for the treatment of
colonic impactions as they have a short duration of action, Enteral fluid administration
reduce gastrointestinal motility and on their own have no Enteral fluids can be delivered in the form of water from a
intrinsic analgesic activity. Other analgesic agents have bucket, via an indwelling narrow nasogastric tube to allow for
variable effects on gastrointestinal motility in normal animals, continuous administration of enteral fluids or a conventional
but no studies have been undertaken to address the effects nasogastric tube providing intermittent fluid administration.
of these drugs on motility in experimentally or clinically Fluids provided in the stable can be simply water alone or
affected animals with intestinal obstruction. Turning horses out combined with a second bucket containing either
into small paddocks in order that they gently ambulate may electrolytes or sweetening agents such as fruit juice or
be appropriate and beneficial in some cases (Williams et al. molasses to encourage increased water intake. Some horses
2015), but these animals should not be lunged or ridden. For will select oral fluids containing electrolytes when they have
further information on analgesics for horses with abdominal specific derangements, whereas others will not. Fluids with
pain, see Michou and Leese 2012. electrolyte supplementation should be isotonic (i.e. 9 g NaCl/l
of water or equivalent), or they will not be consumed.
Intermittent fluid administration via nasogastric tube allows
Fluid therapy – intravenous, enteral or both?
the clinician to have complete control over the amount and
If the horse has clinical signs consistent with hypovolaemia, timing of fluids administrated. The stomach tube can be left
which is rare, then the animal needs intravenous (i.v.) fluid in and capped to prevent air entering the stomach or
therapy to restore circulating volume (Corley 2008). In the removed after each fluid administration. This approach can
hypovolaemic horse, in order to protect the vital organs, lead to worsening of signs of abdominal pain due to gastric
blood flow is diverted from the gastrointestinal tract. Once and small intestinal distention and as such should be
blood flow is reduced, so too is gastrointestinal motility and monitored for signs of abdominal pain.
absorption. In addition, hypovolaemia manifests as variable Continuous enteral fluid therapy prevents the abdominal
obtundation, which ultimately results in a reduced thirst drive discomfort seen with intermittent fluid administration. This is
(Corley 2008). It is for this reason that using oral fluid therapy relatively easily undertaken either using commercially
in hypovolaemic animals is unsuccessful at best and available enteral fluid therapy kits or equine nasogastric
detrimental in certain scenarios. If the horse has no fluid feeding tubes. As these tubes are thin, it is prudent to ensure
deficits, or shows signs only of dehydration, then it will be they are positioned in the oesophagus, which may require
more clinically and cost effective to manage the horse with endoscopy or radiography. They need to be appropriately
enteral fluids (Hallowell 2008). Absolute contraindications secured and a muzzle will help to prevent horses rubbing
would include ileus and subsequent excessive nasogastric them out. Clean, but nonsterile, coiled fluid administration
reflux. sets and i.v. fluid bags can be used to hold the water or
Experimental (Lopes et al. 2002; Lester et al. 2013) and electrolyte solutions.
clinical studies (Hallowell 2008; Monreal et al. 2010) have A maximum intermittent rate of enteral fluids would be
shown that correction of large colonic impactions is more 6–8 l for a 500 kg horse (approximately 1.5 l/100 kg) at no less
effective using enteral than parenteral fluids unless significant than 30 min intervals. Checking for nasogastric reflux prior to
haemodilution occurs. The reported parenteral fluid rates the next dose is essential. The maximum rate usually possible
required to hydrate impactions is not only expensive but for continuous administration would be 5 l/500 kg/h (1 l/
© 2016 EVJ Ltd
388 Management of large colonic impactions
100 kg/h) and maximum rates should be 10 l/500 kg/h (2 l/ a)
100 kg/h) (Corley 2008).
Fluids used enterally
A variety of enteral fluids are administered to manage
colonic impactions. These include water, hypotonic or
isotonic fluids supplemented with electrolytes (e.g. sodium
chloride and potassium chloride) and also hypertonic
magnesium sulphate. Hypotonic fluids given enterally are
absorbed from the proximal gastrointestinal tract, whereas
isotonic fluids are more likely to remain within the
gastrointestinal lumen and thus hydrate the colonic contents
(Lopes et al. 2004a,b). b)
Magnesium sulphate is used enterally as a cathartic
agent as an initial treatment for large colonic impactions.
Initial use of 0.5 g/kg bwt is recommended diluted in water.
Doses up to 1 g/kg bwt can be used, but can result in
hypermagnesaemia and repeated administration of this
product is not recommended as it can lead to signs
associated with magnesium toxicity. These include
tachycardia, increased respiratory rate, sweating,
hyperexcitability, muscle tremors, flaccid paralysis and
recumbency. Magnesium sulphate is not as effective
experimentally at increasing colon water content when
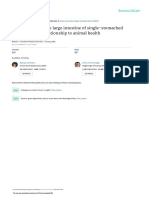
Fig 1: Two photographs of some hard faecal balls immersed in
compared with a balanced electrolyte solution, but does water (a) and liquid paraffin (b) for 24 h. The faeces in the water
increase the water content of faeces in the small colon (a) has dissipated into the solution, whereas the faecal balls in
(Lopes et al. 2004b), so may be of value for primary small liquid paraffin (b) are still hard and solid and remained this way
colon impactions. for a further 6 days (images courtesy of Dr M. Bowen).
Balanced electrolyte solutions can be made from sodium
chloride and combined sodium and potassium chloride
(LoSalt) with approximately 5 g of each per 1 l of water and
the author has found these to be effective, even in Oral glucose in fluids does not provide sufficient nutrition
longstanding, severe large colonic impactions. Other to be valuable in horses and glucose and glycine containing
formulations have been shown to be effective that also add fluids in an experimental diarrhoea model resulted in
sodium bicarbonate, but these are less easy to remember incomplete fluid absorption (Ecke et al. 1998).
and formulate in general equine practice. If only using In summary, enteral fluids can be beneficial for treatment
sodium chloride, add a maximum of 9 g/1 l of water. of large colonic impactions and electrolyte supplementation,
but are not appropriate for hypovolaemic animals or those
with gastrointestinal ileus.
Products not recommended for enteral
administration
Management of horses following colonic
Mineral oil can be used as a marker of gastrointestinal transit impactions
(18 h to anus if transit time is normal). For impactions, it works
its way around as well as hindering water penetration (Fig 1). For some horses the cause of a colonic impaction is clear.
Based on no benefit of using this product in the scientific The horse has been stabled following a musculoskeletal injury,
literature, increased cost over water and the likely fatality if has competed on a warm day and/or been travelled, or for
this product is inadvertently administered into the horse’s some other reason diet or water intake has been modified
lungs (Metcalfe et al. 2010) means that it is not (Hillyer et al. 2002), but for others it may be necessary to
recommended for treatment of large colonic impactions. further evaluate management practices in order to prevent
Dioctyl sodium sulphosuccinate is a detergent that recurrence in the future.
should penetrate impacted faecal material by affecting Horses that have had one or multiple colonic impactions
surface tension, thus allowing water to enter the faeces. should have their anthelmintic regime evaluated. This
Care should be taken as a three-fold overdose is fatal includes considering the pharmacological agents
(Moffat et al. 1975) and also increases absorption of mineral administered and at what frequency, methods used to
oil so should not be administered concurrently. There is no reduce pasture contamination and faecal worm egg counts.
benefit of this over water based on an experimental study Faecal worm egg counts have to be interpreted with caution
comparing faecal output and composition (Freeman et al. as many of the clinical signs associated with endoparasitic
1992). disease are seen in the prepatent period and worm egg
Sodium sulphate is an even more potent cathartic than counts do not always correlate with worm burden. In
magnesium sulphate, but consistently causes hypernatraemia addition, the current recommendations (Nielsen et al. 2013)
and hypocalcaemia and is therefore not recommended are to perform modified faecal worm egg counts (Proudman
(Lopes et al. 2004b). and Edwards 1992), which have a high specificity.
© 2016 EVJ Ltd
G. D. Hallowell 389
A dental examination should be performed in horses Argenzio, R.A., Southworth, M., Lowe, J.E. and Stevens, C.E. (1977)
treated for a colonic impaction, particularly if regular dental Interrelationship of Na, HCO3, and volatile fatty acid transport by
equine large intestine. Am. J. Physiol. 233, E469-E478.
work in that case is not undertaken by the clinician
personally. The author has seen several horses that have Blikslager, A.T. (2010) Obstructive disorders of the gastrointestinal tract.
In: Equine Internal Medicine, Eds: S.M. Reed, W.M. Bayly and D.C.
presented with recurrent colonic impactions which on dental Sellon, Saunders, St Louis, Missouri. pp 882-892.
examination have smooth dental arcades from overzealous
Blue, M.G. and Wittkopp, R.W. (1981) Clinical and structural features of
use of power rasps, likely leading to a reduction in forage equine enteroliths. J. Am. Vet. Med. Ass. 179, 79-82.
breakdown.
Bortoff, A. (1965) Electrical transmission of slow waves from longitudinal
The majority of horses are fed a balanced diet, but in the to circular intestinal muscle. Am. J. Physiol. 209, 1254-1260.
author’s opinion, excessive carbohydrates or particularly Clarke, L.L., Roberts, M.C. and Argenzio, R.A. (1990) Feeding and
coarse forage may increase the likelihood of colonic digestive problems in horses. Physiologic responses to a
impactions (Hillyer et al. 2002). concentrated meal. Vet. Clin. N. Am.: Equine Pract. 6, 433-450.
Stereotypies, particularly crib-biting and wind-sucking, Cohen, N.D., Vontur, C.A. and Rakestraw, P.C. (2000) Risk factors for
have been associated with the development of colonic enterolithiasis among horses in Texas. J. Am. Vet. Med. Ass. 216,
impactions. However, it has been shown that there is minimal 1787-1794.
aerophagia associated with these stereotypies (McGreevy Corley, K.T.T. (2008) Fluid therapy. In: The Equine Hospital Manual, Eds:
et al. 1995). Stereotypies in the author’s opinion could be K.T.T. Corley and J. Stephen, Blackwell Publishing Ltd, Chichester,
West Sussex. pp 364-392.
thought of as ‘coping’ mechanisms and thus their association
Dabareiner, R.M. and White, N.A. (1995) Large colon impaction in
with abdominal pain is likely due to increased sympathetic
horses: 147 cases (1985-1991). J. Am. Vet. Med. Ass. 206, 679-685.
nervous system activation and reduced dietary intake
Ecke, P., Hodgson, D.R. and Rose, R.J. (1998) Induced diarrhoea in
leading to changes in gastrointestinal motility. However,
horses. Part 2: Response to administration of an oral rehydration
simply stopping horses performing these stereotypical solution. Vet J. 155, 161–170.
activities is likely to lead to more, rather than less ‘stress’ and Enemark, J.M. (2008) The monitoring, prevention and treatment of
sympathetic activation. As such, it is imperative to try and sub-acute ruminal acidosis (SARA): a review. Vet. J. 176, 32-43.
manage these horses in an optimal environment, taking into Fintl, C., Hudson, N.P., Mayhew, I.G., Edwards, G.B., Proudman, C.J.
consideration group dynamics when at pasture, amount of and Pearson, G.T. (2004) Interstitial cells of Cajal (ICC) in equine
time stabled and at pasture and optimising what is preferred colic: an immunohistochemical study of horses with obstructive
and carefully managing travel to and attendance at disorders of the small and large intestines. Equine Vet. J. 36, 474-
479.
competitions.
Freeman, S.L. and England, G.C. (2001) Effect of romifidine on
gastrointestinal motility, assessed by transrectal ultrasonography.
Conclusions Equine Vet. J. 33, 570-576.
Freeman, D.E., Ferrante, P.L. and Palmer, J.E. (1992) Comparison of the
In conclusion, large colonic impactions are a common cause effects of intragastric infusions of equal volumes of water, dioctyl
of abdominal pain in the horse. Many of the risk factors have sodium sulfosuccinate, and magnesium sulfate on fecal
been identified, but further evaluation of the effects of composition and output in clinically normal horses. Am. J. Vet. Res.
53, 1347-1353.
intestinal pH and changes in the microbiota are warranted.
As well as correction of impactions with isotonic enteral Gay, C.C., Speirs, V.C., Christie, B.A., Smyth, B. and Parry, B. (1979)
Foreign body obstruction of the small colon in six horses. Equine
electrolyte solutions, consideration of management practices Vet. J. 11, 60-63.
is also warranted.
Hallowell, G.D. (2008) Retrospective study assessing efficacy of
treatment of large colonic impactions. Equine Vet. J. 40, 411-413.
Author’s declaration of interests Hassel, D.M., Schiffman, P.S. and Snyder, J.R. (2001) Petrographic and
geochemic evaluation of equine enteroliths. Am. J. Vet. Res. 62,
No conflicts of interest have been declared. 350-358.
Hillyer, M.H., Taylor, F.G., Proudman, C.J., Edwards, G.B., Smith, J.E. and
French, N.P. (2002) Case control study to identify risk factors for
Ethical animal research simple colonic obstruction and distension colic in horses. Equine
Vet. J. 34, 455-463.
Ethical review not applicable for this review article.
Hotwagner, K. and Iben, C. (2008) Evacuation of sand from the
equine intestine with mineral oil, with and without psyllium. J. Anim.
Physiol. Anim. Nutr. (Berl.) 92, 86-91.
Source of funding
Hudson, N.P., Pearson, G.T., Kitamura, N. and Mayhew, I.G. (1999) An
None. immunohistochemical study of interstitial cells of Cajal (ICC) in the
equine gastrointestinal tract. Res. Vet. Sci. 66, 265-271.
Jones, R.S., Edwards, G.B. and Brearley, J.C. (1991) Commentary on
References prolonged starvation as a factor associated with post-anaesthetic
Argenzio, R.A. and Clarke, L.L. (1989) Electrolyte and water colic. Equine Vet. Educ. 3, 16-18.
absorption in the hind gut of herbivores. Acta Vet. Scand. Suppl. Koenig, J. and Cote, N. (2006) Equine gastrointestinal motility–ileus and
86, 159-167. pharmacological modification. Can. Vet. J. 47, 551-559.
Argenzio, R.A., Lowe, J.E., Pickard, D.W. and Stevens, C.E. (1974) Korolainen, R. and Ruohoniemi, M. (2002) Reliability of ultrasonography
Digesta passage and water exchange in the equine large compared to radiography in revealing intestinal sand
intestine. Am. J. Physiol. 226, 1035-1042. accumulations in horses. Equine Vet. J. 34, 499-504.
Argenzio, R.A., Miller, N. and von Engelhardt, W. (1975) Effect of Lester, G.D., Merritt, A.M., Neuwirth, L., Vetro-Widenhouse, T., Steible,
volatile fatty acids on water and ion absorption from the goat C. and Rice, B. (1998) Effect of alpha 2-adrenergic, cholinergic,
colon. Am. J. Physiol. 229, 997-1002. and nonsteroidal anti-inflammatory drugs on myoelectric activity
© 2016 EVJ Ltd
390 Management of large colonic impactions
of ileum, cecum, and right ventral colon and on cecal emptying Proudman, C.J., Dugdale, A.H., Senior, J.M., Edwards, G.B., Smith, J.E.,
of radiolabeled markers in clinically normal ponies. Am. J. Vet. Res. Leuwer, M.L. and French, N.P. (2006) Pre-operative and
59, 320-327. anaesthesia-related risk factors for mortality in equine colic cases.
Vet. J. 171, 89-97.
Lester, G.D., Merritt, A.M., Kuck, H.V. and Burrow, J.A. (2013) Systemic,
renal, and colonic effects of intravenous and enteral rehydration Ragle, C.A., Meagher, D.M., Lacroix, C.A. and Honnas, C.M. (1989)
in horses. J. Vet. Intern. Med. 27, 554-566. Surgical treatment of sand colic. Results in 40 horses. Vet. Surg. 18,
Lloyd, K., Hintz, H.F., Wheat, J.D. and Schryver, H.F. (1987) Enteroliths in 48-51.
horses. Cornell. Vet. 77, 172-186. Sanders, K.M., Ordog, T., Koh, S.D., Torihashi, S. and Ward, S.M. (1999)
Development and plasticity of interstitial cells of Cajal.
Lopes, M.A., Walker, B.L., White, N.A. 2nd and Ward, D.L. (2002)
Neurogastroenterol. Motil. 11, 311-338.
Treatments to promote colonic hydration: enteral fluid therapy
versus intravenous fluid therapy and magnesium sulphate. Equine Schoster, A., Weese, J.S. and Guardabassi, L. (2014) Probiotic use in
Vet. J. 34, 505-509. horses - what is the evidence for their clinical efficacy? J. Vet.
Intern. Med. 28, 1640-1652.
Lopes, M.A., White, N.A. 2nd, Crisman, M.V. and Ward, D.L. (2004a)
Effects of feeding large amounts of grain on colonic contents and Schusser, G.E. and White, N.A. (1997) Morphologic and quantitative
feces in horses. Am. J. Vet. Res. 65, 687-694. evaluation of the myenteric plexuses and neurons in the large
Lopes, M.A., White, N.A. 2nd, Donaldson, L., Crisman, M.V. and Ward, colon of horses. J. Am. Vet. Med. Ass. 210, 928-934.
D.L. (2004b) Effects of enteral and intravenous fluid therapy, Schusser, G.F., Scheidemann, W. and Huskamp, B. (2000) Muscle
magnesium sulfate, and sodium sulfate on colonic contents and thickness and neuron density in the caecum of horses with chronic
feces in horses. Am. J. Vet. Res. 65, 695-704. recurrent caecal impaction. Equine Vet. J. 32, Suppl. 32, 69-73.
McClure, J.T., Kobluk, C., Voller, K., Geor, R.J., Ames, T.R. and Sivula, N. Sellers, A.F. and Lowe, J.E. (1986) Review of large intestinal motility and
(1992) Fecalith impaction in four miniature foals. J. Am. Vet. Med. mechanisms of impaction in the horse. Equine Vet. J. 18, 261-263.
Ass. 200, 205-207. Specht, T.E. and Colahan, P.T. (1988) Surgical treatment of sand colic
McGreevy, P.D., Richardson, J.D., Nicol, C.J. and Lane, J.G. (1995) in equids: 48 cases (1978-1985). J. Am. Vet. Med. Ass. 193, 1560-
Radiographic and endoscopic study of horses performing an oral 1564.
based stereotypy. Equine Vet. J. 27, 92-95. Sykes, B.W., Sykes, K.M. and Hallowell, G.D. (2013) Efficacy of a
Metcalfe, L., Cummins, C., Maischberger, E. and Katz, L. (2010) combination of a unique pectin-lecithin complex (Apolectolâ), live
latrogenic lipoid pneumonia in an adult horse. Ir. Vet. J. 63, 303- yeast and magnesium hydroxide in the prevention of EGUS and
306. faecal acidosis in Thoroughbred racehorses: a randomised, blinded,
Michou, J. and Leese, E. (2012) Sedation and analgesia in the placebo controlled clinical trial. Equine Vet. J. 45, Suppl. 44, 13.
standing horse 1. Drugs used for sedation and systemic analgesia. Valverde, A. (2010) Alpha-2 agonists as pain therapy in horses. Vet.
In Pract. 34, 524-531. Clin. N. Am.: Equine Pract. 26, 515-532.
Moffat, R., Kramer, L., Lerner, D. and Jones, R. (1975) Studies on dioctyl Ward, S.M., Harney, S.C., Bayguinov, J.R., McLaren, G.J. and Sanders,
sodium sulfosuccinate toxicity: clinical, gross and microscopic K.M. (1997) Development of electrical rhythmicity in the murine
pathology in the horse and guinea pig. Can. J. Comp. Med. 39, gastrointestinal tract is specifically encoded in the tunica
434-441. muscularis. J. Physiol. 505(Pt 1), 241-258.
Monreal, L., Navarro, M., Armengou, L., Jose-Cunilleras, E., Cesarini, C. Warren, L.K., Lawrence, L.M., Brewster-Barnes, T. and Powell, D.M.
and Segura, D. (2010) Enteral fluid therapy in 108 horses with large (1999) The effect of dietary fibre on hydration status after
colon impactions and dorsal displacements. Vet. Rec. 166, 259- dehydration with frusemide. Equine Vet. J. 31, Suppl. 30, 508-513.
263. White, N.A. 2nd and Dabareiner, R.M. (1997) Treatment of impaction
Nielsen, M.K., Mittel, L., Grice, A., Erskine, M., Graves, E., Vaala, W., colics. Vet. Clin. N. Am.: Equine Pract. 13, 243-259.
Tully, R.C., French, D.D., Bowman, R. and Kaplan, R.M. (2013). In: Williams, S., Horner, J., Orton, E., Green, M., McMullen, S., Mobasheri,
AAEP Parasite Control Guidelines. pp 1-24.
A. and Freeman, S.L. (2015) Water intake, faecal output and
Proudman, C.J. and Edwards, G.B. (1992) Validation of a intestinal motility in horses moved from pasture to a stabled
centrifugation/flotation technique for the diagnosis of equine management regime with controlled exercise. Equine Vet. J. 47,
cestodiasis. Vet. Rec. 131, 71-72. 96-100.
© 2016 EVJ Ltd
You might also like
- Elite 100 NBME Concepts OutlineDocument158 pagesElite 100 NBME Concepts OutlineDeborah Peters100% (3)
- Microbiology: For The Students of Pharmacy Technicians (Category-B)Document49 pagesMicrobiology: For The Students of Pharmacy Technicians (Category-B)dr amjad100% (3)
- MYP BIOLOGY SyllabusDocument2 pagesMYP BIOLOGY SyllabusManjunath BN100% (3)
- 3 Marc D. Hauser-The Evolution of Communication (1997) PDFDocument762 pages3 Marc D. Hauser-The Evolution of Communication (1997) PDFhorrityNo ratings yet
- Colic AwazDocument23 pagesColic AwazRayan AzizNo ratings yet
- Articulo #2 OropelDocument10 pagesArticulo #2 Oropeldaniela betancurNo ratings yet
- Urolithiasis in Guinea PigsDocument11 pagesUrolithiasis in Guinea PigsCici PutraNo ratings yet
- Gallbladder Mucocele A ReviewDocument6 pagesGallbladder Mucocele A ReviewThaís ChouinNo ratings yet
- 2012 - The Role of Diet in The Prevention and Management of Several Equine Diseases 1Document16 pages2012 - The Role of Diet in The Prevention and Management of Several Equine Diseases 1Jaime Andres HernandezNo ratings yet
- DISCUSSIONDocument16 pagesDISCUSSIONos krishnaNo ratings yet
- Pgy Git AssigmentDocument6 pagesPgy Git Assigmentlawrence mulengaNo ratings yet
- Fodmap 1 PDFDocument23 pagesFodmap 1 PDFSara FaddaNo ratings yet
- Ijcmes201504567 1428047625 PDFDocument5 pagesIjcmes201504567 1428047625 PDFFebelina KuswantiNo ratings yet
- Gastric Dilation and Volvulus Syndrome in DogDocument4 pagesGastric Dilation and Volvulus Syndrome in DogPutu SuandhikaNo ratings yet
- CANINE-Pathophysiology and Management of Canine Colonic DiseasesDocument14 pagesCANINE-Pathophysiology and Management of Canine Colonic Diseasestaner_soysuren100% (1)
- Acidifier As An Alternative Material To Antibiotics in Animal FeedDocument13 pagesAcidifier As An Alternative Material To Antibiotics in Animal FeedGen SoibamNo ratings yet
- Seow-Choen F. Surgery For Haemorrhoids: Ablation or Correction. Asian J Surg 2002 25: 265-266Document2 pagesSeow-Choen F. Surgery For Haemorrhoids: Ablation or Correction. Asian J Surg 2002 25: 265-266l10n_assNo ratings yet
- Chronic Gastric Dilatation in Horses Diagnosis, Treatment and Feeding Management A Survey of 20 Clinical CasesDocument8 pagesChronic Gastric Dilatation in Horses Diagnosis, Treatment and Feeding Management A Survey of 20 Clinical CasesXp Julieth HernandezNo ratings yet
- A Review On Urolithiasis in Dogs and Cats: Bulgarian Journal of Veterinary MedicineDocument18 pagesA Review On Urolithiasis in Dogs and Cats: Bulgarian Journal of Veterinary MedicinetattaNo ratings yet
- Gastrointestinal Stasis in Rabbits and Rodents - WSAVA2010 - VINDocument4 pagesGastrointestinal Stasis in Rabbits and Rodents - WSAVA2010 - VINAdrianaNo ratings yet
- Historical Review On Cholelithiasis: January 2015Document6 pagesHistorical Review On Cholelithiasis: January 2015joitNo ratings yet
- Laprak Unit Mencit SuzyyDocument13 pagesLaprak Unit Mencit Suzyynanda syafiraNo ratings yet
- Nutrition and Pathology: F. Lebas, T. Gidenne, J.M. Perez and D. LicoisDocument18 pagesNutrition and Pathology: F. Lebas, T. Gidenne, J.M. Perez and D. LicoisAlejandro BoteroNo ratings yet
- VetPractice 04 2014 Equine AprilDocument1 pageVetPractice 04 2014 Equine AprilsatriaarceusNo ratings yet
- Pancreatic Diseases (Excluding Cystic Fibrosis) : Jacques SarlesDocument5 pagesPancreatic Diseases (Excluding Cystic Fibrosis) : Jacques SarlesAbdelkader HociniNo ratings yet
- Beneficial Bacteria in Pet Rabbits: DigestionDocument8 pagesBeneficial Bacteria in Pet Rabbits: DigestionHouse HuntingNo ratings yet
- Common Emergencies in Rabbits, Guinea Pigs, and ChinchillasDocument19 pagesCommon Emergencies in Rabbits, Guinea Pigs, and ChinchillasErnesto VersailleNo ratings yet
- LD B1a018150 Addin Hayu Prasetya R3 K2 Jurnal2Document2 pagesLD B1a018150 Addin Hayu Prasetya R3 K2 Jurnal2Addin Hayu PNo ratings yet
- Metabolic Health, The Metabolome and Reproduction in Female Cattle: A ReviewDocument11 pagesMetabolic Health, The Metabolome and Reproduction in Female Cattle: A ReviewFatima 999No ratings yet
- The Morphology of The Post-Gastric Alimentary CanalDocument18 pagesThe Morphology of The Post-Gastric Alimentary CanalAnca MihalcescuNo ratings yet
- 10.2478 - Aopf 2020 0010Document4 pages10.2478 - Aopf 2020 0010naciraNo ratings yet
- The Physicochemical Environment of The Ne - 1999 - The American Journal of CliniDocument7 pagesThe Physicochemical Environment of The Ne - 1999 - The American Journal of CliniJoão PedroNo ratings yet
- Physiology, Large Intestine - StatPearls - NCBI Bookshelf PDFDocument3 pagesPhysiology, Large Intestine - StatPearls - NCBI Bookshelf PDFMichael MengNo ratings yet
- Recent Pathophysiology and Therapy For Paralytic IleusDocument5 pagesRecent Pathophysiology and Therapy For Paralytic IleusPrihanant BayuNo ratings yet
- Genetic Control of Obesity and Gut Microbiota Composition in Response To High-Fat, High-Sucrose Diet in MiceDocument12 pagesGenetic Control of Obesity and Gut Microbiota Composition in Response To High-Fat, High-Sucrose Diet in MiceJerônimo CoelhoNo ratings yet
- Current Advances in Surgical Management of Ruminal Disorders of BovineDocument7 pagesCurrent Advances in Surgical Management of Ruminal Disorders of BovineKazi Zihadul AlamNo ratings yet
- Metzler and R. Mosenthin, 2008Document13 pagesMetzler and R. Mosenthin, 2008mirapoNo ratings yet
- Dietary Lectins As Disease Causing ToxicDocument11 pagesDietary Lectins As Disease Causing ToxicHughNo ratings yet
- 2008 Dietary Treatment of NephrolithiasisDocument7 pages2008 Dietary Treatment of NephrolithiasisNayaNo ratings yet
- Diagnosisand Managementofrumen Acidosisandbloatinfeedlots: Nathan F. Meyer,, Tony C. BryantDocument18 pagesDiagnosisand Managementofrumen Acidosisandbloatinfeedlots: Nathan F. Meyer,, Tony C. BryantDaniellu CcNo ratings yet
- Souza2017 Article RefluxEsophagitisAndItsRoleInTDocument10 pagesSouza2017 Article RefluxEsophagitisAndItsRoleInTInne SidaurukNo ratings yet
- DX Anaphysio PathophysioDocument6 pagesDX Anaphysio PathophysioJeofy PamaNo ratings yet
- Nutritional Approaches To GallstonesDocument10 pagesNutritional Approaches To GallstonesPepe1949No ratings yet
- Nutritional Approaches To PreventionDocument11 pagesNutritional Approaches To PreventionBrian Floyd Andrewmer AlbitosNo ratings yet
- Nutritional Stretegies To Prevent Urolithiasis in Animals: Veterinary World March 2011Document4 pagesNutritional Stretegies To Prevent Urolithiasis in Animals: Veterinary World March 2011Bianca FărcaşNo ratings yet
- Lactose Digestion and The Evolutionary Genetics of Lactase Persistance - Ingram Et Al 2009 Human GeneticsDocument13 pagesLactose Digestion and The Evolutionary Genetics of Lactase Persistance - Ingram Et Al 2009 Human GeneticsEmanuele SabettaNo ratings yet
- Asturgeon MetabolismDocument2 pagesAsturgeon MetabolismMirela CrețuNo ratings yet
- Nutritional Support in Gastrointestinal Diseases: Compromised GutDocument18 pagesNutritional Support in Gastrointestinal Diseases: Compromised GutRoxana CristeaNo ratings yet
- Williams, 2001 - Fermentation - in - The - Large - Intestine - MonogastricosDocument23 pagesWilliams, 2001 - Fermentation - in - The - Large - Intestine - MonogastricosÓscar David Ramírez MosqueraNo ratings yet
- Management Pancreas and GallbladderDocument21 pagesManagement Pancreas and Gallbladderapi-19790126No ratings yet
- Omasoabomasal Impaction in A Gersy CowDocument6 pagesOmasoabomasal Impaction in A Gersy CowAli H. Sadiek أ.د. علي حسن صديق100% (1)
- Differential Diagnosis and Treatment of Acute Diarrhoea in The Dog and Cat PDFDocument9 pagesDifferential Diagnosis and Treatment of Acute Diarrhoea in The Dog and Cat PDFDani OrtizNo ratings yet
- Getting Crap Out of A CatDocument5 pagesGetting Crap Out of A CatLarasati FadhlenNo ratings yet
- Genetic Influences On Carbohydrate DigestionDocument8 pagesGenetic Influences On Carbohydrate DigestionNurma100% (1)
- 3 - Fasciolosis - Case ReportDocument3 pages3 - Fasciolosis - Case ReportMuhammad KamranNo ratings yet
- The Gastrointestinal SystemDocument11 pagesThe Gastrointestinal SystembrettdwsmithNo ratings yet
- Valeric Acid Glyceride Esters in Feed Promote Broiler Performa - 2018 - PoultryDocument9 pagesValeric Acid Glyceride Esters in Feed Promote Broiler Performa - 2018 - Poultrylady mae rufinoNo ratings yet
- GallstoneDocument6 pagesGallstoneMushfique HussainNo ratings yet
- Fecal Impaction A Cause For ConcernDocument7 pagesFecal Impaction A Cause For Concernadkhiatul muslihatinNo ratings yet
- Hypercalcemia and Calcium Oxalate Urolithiasis in Cats: A Report of Five CasesDocument5 pagesHypercalcemia and Calcium Oxalate Urolithiasis in Cats: A Report of Five CasesJean AmorinNo ratings yet
- GallstonesDocument6 pagesGallstonesricky aditNo ratings yet
- Recent Technological Innovations in AquacultureDocument16 pagesRecent Technological Innovations in AquaculturemosiomadNo ratings yet
- 2.biochem - Aspa-Funtanilla - Protein and Amino Acids StructureDocument94 pages2.biochem - Aspa-Funtanilla - Protein and Amino Acids StructureFuntanilla Diangkinay Jay-arNo ratings yet
- DNA PackagingDocument25 pagesDNA PackagingHoor Ul Ain Rounaq100% (1)
- 29075423: Myeloid Sarcoma of The Oral Cavity: A Case Report and Review of 89 Cases From The Literature 3Document5 pages29075423: Myeloid Sarcoma of The Oral Cavity: A Case Report and Review of 89 Cases From The Literature 3Zahrotul UlaNo ratings yet
- Chapter 1 - History of Zebrafish Resea - 2020 - The Zebrafish in Biomedical Rese PDFDocument12 pagesChapter 1 - History of Zebrafish Resea - 2020 - The Zebrafish in Biomedical Rese PDFNicolas BaronNo ratings yet
- Typhonium Flagelliforme Decreases Telomerase Expression: in Hela Cervical Cancer CellsDocument7 pagesTyphonium Flagelliforme Decreases Telomerase Expression: in Hela Cervical Cancer CellsNije AsriNo ratings yet
- Multiple Choice Questions (MCQ) Topic Quiz Biological MembranesDocument22 pagesMultiple Choice Questions (MCQ) Topic Quiz Biological MembranesVeluz Kirt Peter GuiribaNo ratings yet
- Protein StructureDocument66 pagesProtein StructureAjit Suryawanshi100% (2)
- Etiology of Breast CarcinomaDocument11 pagesEtiology of Breast Carcinomashrutik91No ratings yet
- The Protein: Presented by Dr. Shazzad Hosain Asst. Prof. EECS, NSUDocument157 pagesThe Protein: Presented by Dr. Shazzad Hosain Asst. Prof. EECS, NSUAlimushwan AdnanNo ratings yet
- Gene BiotecDocument6 pagesGene BioteclemtechNo ratings yet
- WORKSHEET 15.2 Causes of VariationDocument2 pagesWORKSHEET 15.2 Causes of VariationLim Wai WaiNo ratings yet
- KooBits - Engaging The Digital KidsDocument18 pagesKooBits - Engaging The Digital KidsNiladri ChatterjeeNo ratings yet
- Tatalovic Natcroat 2012 2Document4 pagesTatalovic Natcroat 2012 2Ir MaNo ratings yet
- Tissue Culture Media Composition and TypesDocument18 pagesTissue Culture Media Composition and TypesFemina kaipallyNo ratings yet
- Each Name Must Refer To Only One Species Everyone Must Use The Same NameDocument9 pagesEach Name Must Refer To Only One Species Everyone Must Use The Same NameWe the people Lesson 9No ratings yet
- Biology Project: Chromosomal DisorderDocument44 pagesBiology Project: Chromosomal DisorderArun Kumar ANo ratings yet
- Modern Flow CytometryDocument67 pagesModern Flow CytometryKleber FelipeNo ratings yet
- E) Two: Shah Kamal Institute Thatta (Skit)Document2 pagesE) Two: Shah Kamal Institute Thatta (Skit)Wahaj KhanNo ratings yet
- General Biology 2 DLL October 01 and October 02 2018Document4 pagesGeneral Biology 2 DLL October 01 and October 02 2018Ivy AguasNo ratings yet
- General Biology 1 First Periodical Test TosDocument4 pagesGeneral Biology 1 First Periodical Test TosMA. HAZEL TEOLOGONo ratings yet
- Preliminary Evaluation of Lake Lanao Fish Hypseleotris Agilis Herre For Antimicrobial ActivityDocument8 pagesPreliminary Evaluation of Lake Lanao Fish Hypseleotris Agilis Herre For Antimicrobial Activitynourshamsia barosaNo ratings yet
- Evolution of EukaryotesDocument55 pagesEvolution of EukaryotesMolinaArzabeCarlos100% (1)
- Goljan RR - ImmunopathologyDocument13 pagesGoljan RR - ImmunopathologyShaz ChindhyNo ratings yet
- Mikper 3 Nutrisi Dan Kultur MikroorganismeDocument31 pagesMikper 3 Nutrisi Dan Kultur MikroorganismePa ChannelNo ratings yet
- 22 CH106 Metabolic Paths For Carbohydrates Timberlake 2ndDocument70 pages22 CH106 Metabolic Paths For Carbohydrates Timberlake 2ndEnrique LiKeNo ratings yet
- Full Chapter Neuroprotection Method and Protocols Methods in Molecular Biology 2761 1St Edition Swapan Ray PDFDocument53 pagesFull Chapter Neuroprotection Method and Protocols Methods in Molecular Biology 2761 1St Edition Swapan Ray PDFmahalia.riera944100% (7)