Professional Documents
Culture Documents
Vitamin C Write-Up
Vitamin C Write-Up
Uploaded by
annafiiOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Vitamin C Write-Up
Vitamin C Write-Up
Uploaded by
annafiiCopyright:
Available Formats
Aelfgar College
Biology AS
Investigating the vitamin C contents
of different sources
Anna Phizacklea
Introduction
Aim: to use dichlorophenolindolphenol (DCPIP) to determine the vitamin C contents of
different sources.
Hypothesis: The orange will contain the most vitamin C in mg/cm³.
Independent variable: The different sources used: orange juice, lime, lemon, orange.
Dependent variable: The volume of juice (attained from the source) required to
decolourise 2cm³ of DCPIP. This can be used to calcuate, when compared with the control
of 0.1% vitamin C solution, how much vitamin C the source contains in mg/cm³.
Control variable Possible effect Control measure
Age of the source The amount of vitamin C in a Use fruit of the same age; fresh
piece of fruit decreases over is preferable, as vitamin C may
time. This means if they are of be lost at different rates.
different ages, it will not give
accurate results.
Background information: Citrus fruits, such as lemons, contain vitamin C which prevents
the deficiency disease scurvy. Vitamin C is a reducing agent, and decolourises the blue
dye DCPIP. This reaction allows us to estimate the vitamin C content of different sources.
Equipment and materials
Small beakers to collect volumes of juice (100cm³)
Two large beakers (500cm³)
Measuring cylinder (50cm³)
Pipette or syringe to measure 2cm³ volume
Pipette or syringe to accurately measure up to 1cm³
Test tubes
Spatula
Distilled water (50cm³)
0.1% vitamin C solution
DCPIP
The sources used for this example:
A lemon
An orange
A lime
Shop-bought orange juice
Method
Using a pipette or syringe, place exactly 2cm³ of DCPIP solution into a test tube
Using a different graduated pipette or syringe, add the vitamin C solution, drop by
drop, to the DCPIP solution in the test tube. Shake the tube gently after the addition
of each drop and continue adding drops until the DCPIP solution is decolourised.
Record the exact volume of vitamin V solution added.
Repeat this procedure. Average the results to obtain the mean volume of 0.1%
vitamin C which decolourises 2cm³ of DCPIP.
Calculate the mass of vitamin C in this volume of vitamin C solution (it contains
0.001g vitamin C in 1cm³ water). This tells you how much vitamin C is required to
decolourise 2cm³ of DCPIP, which can be used to calculate the volume of vitamin C
in the different sources.
First determine the volume of one of the sources, e.g. the lemon. Slowly submerge
it in a 500cm³ beaker of water, and use another 500cm³ beaker or container to
collect the water displaced. The volume of water displaced is the volume of the
lemon.
Cut your lemon in half. Squeeze the juice of the lemon into a small beaker. Collect
some of the lemon juice using a clean graduated pipette or syringe.
Repeat steps 1 and 2 using the juice attained from your source. Add the juice, drop
by drop, to 2cm³ of DCPIP, until it becomes decolourised.
Repeat steps 5-7 using your different sources. Obviously in the case of orange
juice, you do not need to use displacement to measure the volume – go straight to
step 7.
Results
Raw data
Volume / cm³ required to decolourise 2cm³ DCPIP
Solution 1 2 3 4 Mean
0.1% vitamin C 1.5 2 1.5 1.6 1.65
Orange juice 4.5 4.5 4.3 4.4 4.43
Lime 6.5 7 7.4 7 6.98
Lemon 4 6.4 5.2 5.5 5.28
Orange 6 7.8 6.5 6.3 6.65
Calculated data
From these results, you can calculate the vitamin C content in mg/cm³ of each of the
sources, and using the volume of the source, the mg of vitamin C in each piece of fruit.
1cm³ of 0.1% vitamin C solution is 1mg vitamin C, therefore 1.65mg vitamin C
decolourises 2cm³ DCPIP.
Solution Mean volume mg required ÷ mg/cm³ mg/100cm³ Volume mg of
to decolourise volume of of fruit vitamin C
2cm³ DCPIP solution required piece/cm³ per piece
of fruit
0.1% 1.65 1.65 ÷1.65 1 100 – –
vitamin C
Orange 4.43 1.65 ÷ 4.43 0.37 37.3 – –
juice
Lime 6.98 1.65 ÷ 6.98 0.24 23.7 94 22.24
Lemon 5.28 1.65 ÷ 5.28 0.31 31.3 100 31.27
Orange 6.65 1.65 ÷ 6.65 0.25 24.8 180 44.66
Conclusion
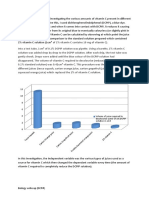
As can be seen on the graph, orange juice contains the most vitamin C, at 0.37mg/cm³,
making it the best source of vitamin C. The second best source was the lemon, at
0.31mg/cm³. Contrasting with the hypothesis, the third best was the orange, at
0.25mg/cm³. The least vitamin C containing source was the lime, at 0.24mg/cm³. However,
if related to size, the orange contained 44.66mg of vitamin C in one piece of fruit,
compared to 31.27mg in a lemon, and 22.24mg in a lime, meaning the orange would be
the best source overall if a person were to choose one specific fruit to eat, as the amount
of vitamin C in orange juice would vary depending on the volume of juice.
Evaluation
There were numerous sources of error throughout the experiment. One error was the
argument of when the DCPIP had gone clear, as many people had different views of when
it was. Also, as the juices obtained and used were coloured, it was difficult to decide when
it had gone clear as it was tinted with the colour of the juice. Within each group, this was
avoided by having the same person decide when it was clear. However, there were
discrepancies between groups. This could be avoided by a pre-arranged definition of clear
from the whole class.
Another source of error was the pipettes used. Some of them were graduated with smaller
measures, and were therefore more accurate than others, so in some cases it was
uncertain exactly how much juice from a source had been added. This could be avoided
through everyone using the same kind of pipette for each purpose, preferably one with
small graduations for optimum accuracy. Another problem with the pipettes was that some
of the ones used did not hold the liquid in well, and a small amount escaped before
reaching the test tube. This could have had an effect in the case of measuring DCPIP, as
there would be less to be decolourised, tainting the results. This could be avoided by
ensuring the pipettes used were fully functional beforehand, and making sure the pipettes
still contained the correct amount before placing in the test tube.
An additional source of error could be the amount the test tube was shaken after each
drop of a vitamin C containing solution was added. Some people may have shaken it for
longer than others, which could have meant the vitamin C dispersed more or acted faster
than in other cases. This could be avoided by having a pre-set amount of time to shake for,
e.g. 20 seconds, and having the same person in each group do so each time.
A problem with the results obtained is that they are based on solely the juice taken from a
fruit. This ignores the other parts of the fruit, i.e. the rind and fleshy parts. This makes the
assumption about how much vitamin C is contained in a piece of fruit unreliable. To
improve this, the experiment could be tried by cutting up and processing a whole piece of
fruit, and adding the obtained liquid to the DCPIP. This would take into account the whole
of the fruit, although there may be further issues with viscosity and clearness.
You might also like
- Vitamin C Core Practical Write Up PDFDocument2 pagesVitamin C Core Practical Write Up PDFMichael Collin0% (1)
- Vitamin C Content in Fruit JuicesDocument2 pagesVitamin C Content in Fruit JuicesSharifah Nurain100% (3)
- 1 - Aanalysis of Food Dyes in Beverages - S PDFDocument6 pages1 - Aanalysis of Food Dyes in Beverages - S PDFJenny Zhang0% (1)
- HubSpot: Inbound Marketing and Web 2.0Document3 pagesHubSpot: Inbound Marketing and Web 2.0test100% (1)
- RESULT & Discussion Exp 6 AnalyDocument3 pagesRESULT & Discussion Exp 6 AnalyAlimah AzeliNo ratings yet
- Analysis Vitamin C Fruit JuicesDocument9 pagesAnalysis Vitamin C Fruit Juiceskhalifa1122100% (1)
- Biology November ReportDocument3 pagesBiology November ReportIndrani GoswamiNo ratings yet
- The Vitamin C Content of Fruit JuiceDocument16 pagesThe Vitamin C Content of Fruit JuiceTootsie88% (40)
- Chem Project PresentationDocument23 pagesChem Project PresentationSarah LeeNo ratings yet
- Lab 4 LipidDocument8 pagesLab 4 Lipidapi-384770852No ratings yet
- Iodometric Analysis For Vitamin C Lab ReportDocument9 pagesIodometric Analysis For Vitamin C Lab ReportAlleia Mae Urbano Mazo80% (10)
- How Does Exercise Affect Heart Rate Alvin PDFDocument13 pagesHow Does Exercise Affect Heart Rate Alvin PDFapi-134712575No ratings yet
- 11 Fruit JuicesDocument8 pages11 Fruit Juicesjules blancoNo ratings yet
- How To Make TE BufferDocument2 pagesHow To Make TE BufferJoanne HodgsonNo ratings yet
- Chemistry Project: Water Chemistry in Daily LifeDocument29 pagesChemistry Project: Water Chemistry in Daily LifeAnna ThomasNo ratings yet
- Lab Report Water Potential FinalDocument6 pagesLab Report Water Potential Finalade2001fr3113No ratings yet
- Experiment 6.2Document3 pagesExperiment 6.2cindy_lee_11No ratings yet
- If Ye Know These Things Ross DrysdaleDocument334 pagesIf Ye Know These Things Ross DrysdaleBernardo Rasimo100% (1)
- Part 11 Metal Detector Manual PDFDocument24 pagesPart 11 Metal Detector Manual PDFOrlando Melipillan100% (1)
- Vit C Experiment Write UpDocument9 pagesVit C Experiment Write UpSanngeeta100% (2)
- Practical Vitiman CDocument14 pagesPractical Vitiman CLee da DonNo ratings yet
- Bio ReportDocument8 pagesBio ReportTharshini_Indr_6713No ratings yet
- Investigating Vitamin C Concentration in Homemade and Store Bought SmoothiesDocument7 pagesInvestigating Vitamin C Concentration in Homemade and Store Bought SmoothiesisabelleNo ratings yet
- Vitamin C in Fruit JuicesDocument5 pagesVitamin C in Fruit JuicesANMOL journeyNo ratings yet
- Biology Core Practical 2Document6 pagesBiology Core Practical 2Roman Crame50% (2)
- Analysis of Concentration of Vitamin C IDocument21 pagesAnalysis of Concentration of Vitamin C IMahamud Hasan Prince100% (2)
- Vitamin CDocument15 pagesVitamin Czaiy67% (3)
- Which Type of Fruit Juice Provides The Most Vitamin C?Document5 pagesWhich Type of Fruit Juice Provides The Most Vitamin C?Aswathy BijuNo ratings yet
- Measuring The Vitamin C Content of Foods and Fruit JuicesDocument3 pagesMeasuring The Vitamin C Content of Foods and Fruit JuicesJohnNo ratings yet
- Experiment On Vitamins - CONGSONDocument3 pagesExperiment On Vitamins - CONGSONShayne Angelique CongsonNo ratings yet
- DCPIP Write UpDocument2 pagesDCPIP Write UpHashim Chishty0% (1)
- Vitmin C ReportDocument14 pagesVitmin C ReportOdongo TonnyNo ratings yet
- Vitamin C Lab PDFDocument7 pagesVitamin C Lab PDFJohn Baptist John Bosco100% (1)
- Determination of Vitamin C Concentration by TitrationDocument5 pagesDetermination of Vitamin C Concentration by TitrationMaryam JabiyevaNo ratings yet
- Chemistry Project 3.0Document15 pagesChemistry Project 3.0Lubna Khalid67% (3)
- Exp. 8 (Iodimetric Analysis For Vitamin C)Document4 pagesExp. 8 (Iodimetric Analysis For Vitamin C)Nikko Gabriel AquinoNo ratings yet
- Chem Project - Class 12Document12 pagesChem Project - Class 12M AdithyaNo ratings yet
- Ascorbic Acid PDFDocument18 pagesAscorbic Acid PDFHarshNo ratings yet
- EXP 5 Determination For Ascorbic Acid AnalysisDocument2 pagesEXP 5 Determination For Ascorbic Acid AnalysisthirafauziNo ratings yet
- Biology ExperimentDocument2 pagesBiology Experimentain_azhar96No ratings yet
- Lab Report Bio 330Document4 pagesLab Report Bio 330hyunjeans booNo ratings yet
- Determination of The Concentration of Vitamin C by Using The DCPIP TestDocument2 pagesDetermination of The Concentration of Vitamin C by Using The DCPIP TestBlaireNo ratings yet
- Titration Lab Report MYP ChemistryDocument14 pagesTitration Lab Report MYP ChemistryHardik SinghiNo ratings yet
- Quantitative Analysis of Vitamin C Contained in FoodsDocument8 pagesQuantitative Analysis of Vitamin C Contained in FoodsCleve Hines100% (1)
- Determination of Vitamin CDocument7 pagesDetermination of Vitamin Capi-487208181No ratings yet
- Tesis vITAMIN C CONTENT IN FRUITSDocument24 pagesTesis vITAMIN C CONTENT IN FRUITSHema JothyNo ratings yet
- Effect of PH On Enzyme Activity Lab 3Document8 pagesEffect of PH On Enzyme Activity Lab 3api-340907023No ratings yet
- Vitaminc IodineDocument3 pagesVitaminc IodineMuh. Ma'arifNo ratings yet
- An Alternative Method of Milk TreatmentDocument9 pagesAn Alternative Method of Milk TreatmentA.M.ANo ratings yet
- CHEMISTRY ProjectDocument17 pagesCHEMISTRY ProjectMerin MariamNo ratings yet
- Lab Report 4 sbl1023Document7 pagesLab Report 4 sbl1023api-3850387010% (1)
- AS Biology Unit 3: DurationDocument30 pagesAS Biology Unit 3: DurationShamaNo ratings yet
- The Extraction of Benzoic AcidDocument7 pagesThe Extraction of Benzoic AcidChenling NiNo ratings yet
- P&D Soft Drink and JuiceDocument3 pagesP&D Soft Drink and Juiceanastatia23No ratings yet
- Lab - Rate of TranspirationDocument7 pagesLab - Rate of TranspirationBrianna Mayer100% (1)
- Yeast Cellular Respiration Labfin-2Document7 pagesYeast Cellular Respiration Labfin-2Nabeel UddinNo ratings yet
- Homeostasis Activity - MARY SHESHIRADocument3 pagesHomeostasis Activity - MARY SHESHIRASheshira Reddy YeruvaNo ratings yet
- Lab Report 4Document6 pagesLab Report 4api-392375614No ratings yet
- Experiment 9 SaponificationDocument6 pagesExperiment 9 Saponificationpatrice green - SteadmanNo ratings yet
- Activity 1.21 Vit C Report of Core Practical Edexcel AsDocument3 pagesActivity 1.21 Vit C Report of Core Practical Edexcel AsJesse EnglandNo ratings yet
- Chapter 6 - Nutrition (Vitamin C)Document2 pagesChapter 6 - Nutrition (Vitamin C)Raja Marina IraniNo ratings yet
- Vitamin C: in Fruit JuicesDocument17 pagesVitamin C: in Fruit Juicessss100% (1)
- AbstractDocument17 pagesAbstractRukmani 1011No ratings yet
- Revision Sheet - WaterDocument1 pageRevision Sheet - WaterannafiiNo ratings yet
- Abortion Statistics 00s SummaryDocument3 pagesAbortion Statistics 00s SummaryannafiiNo ratings yet
- Daphnia - Caffeine ExperimentDocument4 pagesDaphnia - Caffeine ExperimentannafiiNo ratings yet
- Biology - Succession PresentationDocument7 pagesBiology - Succession PresentationannafiiNo ratings yet
- Patrick Hughes ResearchDocument1 pagePatrick Hughes ResearchannafiiNo ratings yet
- Exam TechniqueDocument1 pageExam TechniqueannafiiNo ratings yet
- Coming of Age EssayDocument5 pagesComing of Age EssayannafiiNo ratings yet
- Yamabe Flow On Nilpotent Lie GroupsDocument20 pagesYamabe Flow On Nilpotent Lie GroupsEnzo RicNo ratings yet
- N. Mixture, CombinationDocument2 pagesN. Mixture, CombinationYareniNo ratings yet
- Shahetal.2022 TecGeomorpJhelumDocument21 pagesShahetal.2022 TecGeomorpJhelumAyesha EjazNo ratings yet
- StatementOfAccount 6316692309 21072023 222045Document17 pagesStatementOfAccount 6316692309 21072023 222045Asekar AlagarsamyNo ratings yet
- HSC 11 Scalars and Vectors Ch2Document5 pagesHSC 11 Scalars and Vectors Ch2Snehal PanchalNo ratings yet
- Quality Supervisor Job DescriptionDocument8 pagesQuality Supervisor Job Descriptionqualitymanagement246No ratings yet
- Fundamentals of HydraulicsDocument101 pagesFundamentals of HydraulicswissamhijaziNo ratings yet
- TakeawayDocument6 pagesTakeawayWilman VasquezNo ratings yet
- Safety Data Sheet Carbon Cathode SolutionDocument11 pagesSafety Data Sheet Carbon Cathode SolutionJeff BanasekNo ratings yet
- In Re Plagiarism Case Against Justice Del CastilloDocument112 pagesIn Re Plagiarism Case Against Justice Del CastilloRaffyLaguesmaNo ratings yet
- DSF Course Curriculum 1305231045Document8 pagesDSF Course Curriculum 1305231045Gaurav BhadaneNo ratings yet
- How To Download A Windows 10 ISO File - PCWorldDocument3 pagesHow To Download A Windows 10 ISO File - PCWorldRajeev BatraNo ratings yet
- Sword of DestinyDocument435 pagesSword of DestinyJailouise Perez100% (1)
- UNIT HistoryDocument2 pagesUNIT HistorySanders StephenNo ratings yet
- Stable Fixed Points of Card Trick FunctionsDocument10 pagesStable Fixed Points of Card Trick FunctionsDerekNo ratings yet
- Comfort ZoneDocument4 pagesComfort Zonesigal ardanNo ratings yet
- CaneToadsKakadu 2Document3 pagesCaneToadsKakadu 2Matheesha RajapakseNo ratings yet
- Huffman Coding - Base of JPEG Image CompressionDocument13 pagesHuffman Coding - Base of JPEG Image CompressionFikaduNo ratings yet
- SBT Sekolah Berprestasi Tinggi (HPS) High Performing SchoolsDocument14 pagesSBT Sekolah Berprestasi Tinggi (HPS) High Performing SchoolsAminNo ratings yet
- Types of ParentingDocument13 pagesTypes of ParentingViseshNo ratings yet
- Comm 130 PortfolioDocument23 pagesComm 130 PortfolioSami MossNo ratings yet
- Dance As A CompetitionDocument3 pagesDance As A CompetitionJaymie NeriNo ratings yet
- 4411 Studio MonitorDocument4 pages4411 Studio MonitorabraxastributetosantanaNo ratings yet
- Assignment 5 - Profile and Cross Section LevelingDocument3 pagesAssignment 5 - Profile and Cross Section LevelingKeanna Marie TorresNo ratings yet
- Informe Sobre El Manejo de CostasDocument88 pagesInforme Sobre El Manejo de CostasMetro Puerto RicoNo ratings yet
- Adam Weeks ResumeDocument3 pagesAdam Weeks ResumeAdam WeeksNo ratings yet