Professional Documents
Culture Documents
Alternative Energy - Refers To Energy Sources Which Are No Based On The
Alternative Energy - Refers To Energy Sources Which Are No Based On The
Uploaded by
MORAN DianaCopyright:
Available Formats
You might also like
- Experiment No.2 Ferranti Effect: Objective: ApparatusDocument6 pagesExperiment No.2 Ferranti Effect: Objective: ApparatusMian TauseefNo ratings yet
- Undercurrents 16 June-July 1976Document127 pagesUndercurrents 16 June-July 1976Christopher J Squire50% (2)
- Planetary TalismanDocument30 pagesPlanetary TalismanJones Tagoe100% (18)
- Unit 6Document6 pagesUnit 6Ernie CoachNo ratings yet
- 4.5 - 4.8 Renewable and Non Renewable EnergyDocument55 pages4.5 - 4.8 Renewable and Non Renewable EnergyShereena FaisalNo ratings yet
- Physics 9 - Energy ResourcesDocument36 pagesPhysics 9 - Energy ResourcesHakim Abbas100% (1)
- Physics 9 - Energy ResourcesDocument32 pagesPhysics 9 - Energy Resourcesccbpxprwd2No ratings yet
- Physical Science: Sources of EnergyDocument2 pagesPhysical Science: Sources of EnergyJames Ryan AlzonaNo ratings yet
- ElectricityDocument19 pagesElectricityAdie Alie PumpNo ratings yet
- Energy ResourcesDocument26 pagesEnergy ResourcesAnum ObaidNo ratings yet
- STD-X Sub - Sci-Ii 5. Towards Green EnergyDocument8 pagesSTD-X Sub - Sci-Ii 5. Towards Green EnergySagar WaghmareNo ratings yet
- Renewable Energy ResourcesDocument132 pagesRenewable Energy Resourcesabhinavbansal112211No ratings yet
- Technology and Livelihood EducationDocument63 pagesTechnology and Livelihood EducationNo RiNo ratings yet
- Ib DP Ess Unit 7 NotesDocument8 pagesIb DP Ess Unit 7 NotesAnnaya Rangkuty0% (1)
- M1 SEP-Ktuassist - inDocument26 pagesM1 SEP-Ktuassist - inAir SevakkNo ratings yet
- 2024 EnergyDocument11 pages2024 Energygamerazizlol123No ratings yet
- Sourses of EnergyDocument5 pagesSourses of EnergyArshid WaniNo ratings yet
- ElectricityDocument1 pageElectricitySheryll Almira HilarioNo ratings yet
- Chapter 2 - EnergyDocument17 pagesChapter 2 - Energyamrptl9010No ratings yet
- TLE ModuleDocument73 pagesTLE ModuleDexter Bola100% (1)
- Module 4 SCE NotesDocument35 pagesModule 4 SCE NotesPreethi V AnandNo ratings yet
- Harnessing Energy From Different SourcesDocument3 pagesHarnessing Energy From Different SourcesMARVIN HILARIONo ratings yet
- Harnessing Energy From Different SourcesDocument3 pagesHarnessing Energy From Different SourcesMARVIN HILARIONo ratings yet
- Toyol EnergyDocument1 pageToyol EnergyzinilNo ratings yet
- Energy: Franziska Palme, Vincent Kalnin and Hamza OuerfelliDocument26 pagesEnergy: Franziska Palme, Vincent Kalnin and Hamza OuerfelliHamza OuerfelliNo ratings yet
- Solar EnergyDocument78 pagesSolar EnergySai VikasNo ratings yet
- Engg Chem Finals ReviewerDocument15 pagesEngg Chem Finals ReviewerCarl Jasper RabiNo ratings yet
- Technology and ComputingDocument16 pagesTechnology and ComputingAntwan MeloNo ratings yet
- Module 1 SEP-Ktunotes - inDocument24 pagesModule 1 SEP-Ktunotes - inmujeebNo ratings yet
- Energy ResourcesDocument51 pagesEnergy ResourcesPRATHA PATELNo ratings yet
- How Energy Is Produced and ManagedDocument21 pagesHow Energy Is Produced and ManagedAmiel ObusanNo ratings yet
- Building Utilities 2 ReviewerDocument29 pagesBuilding Utilities 2 ReviewerandreaNo ratings yet
- Topic 9 Alternative and Renewable EnergyDocument41 pagesTopic 9 Alternative and Renewable EnergyChuan Ung SiiNo ratings yet
- Earth Science S-WPS OfficeDocument14 pagesEarth Science S-WPS OfficeBai Johaira BenitoNo ratings yet
- Chapter EightDocument17 pagesChapter EightSeble GetachewNo ratings yet
- Physical Science Quarter 1 Week 7 1Document16 pagesPhysical Science Quarter 1 Week 7 1CrimstonNo ratings yet
- On Non Conventional Energy ResourcesDocument27 pagesOn Non Conventional Energy ResourcesAnonymous HyOfbJ6No ratings yet
- P3 Energy ResourcesDocument1 pageP3 Energy ResourcesArun DonteNo ratings yet
- Energy Efficiency in BuildingDocument30 pagesEnergy Efficiency in BuildingS.K. RecruitingNo ratings yet
- Geothermal Energy: - A Renewable Energy Source For Electricity GenerationDocument22 pagesGeothermal Energy: - A Renewable Energy Source For Electricity GenerationSuraj SunnyNo ratings yet
- Sources of EnergyDocument27 pagesSources of Energym49328968No ratings yet
- Master of Science in Engineering in Energy Systems Planning and ManagementDocument51 pagesMaster of Science in Engineering in Energy Systems Planning and ManagementRabi SarmaNo ratings yet
- Esci Lesson 6 Energy Geohydrowater ResourcesDocument63 pagesEsci Lesson 6 Energy Geohydrowater ResourcesLearni J. EscoteNo ratings yet
- Geothermal Energy: A Renewable Energy Source For Electricity GenerationDocument23 pagesGeothermal Energy: A Renewable Energy Source For Electricity GenerationJitender Kumar YadavNo ratings yet
- GP5 - Energy, Work & Power 1Document8 pagesGP5 - Energy, Work & Power 1wengiemotshegweNo ratings yet
- Impacts - Considering The Effects On The: Oleum OilDocument4 pagesImpacts - Considering The Effects On The: Oleum OilKrisha DimailigNo ratings yet
- PPE Lect 1Document19 pagesPPE Lect 1A ZNo ratings yet
- EnergíaDocument7 pagesEnergíaJorge MuñozNo ratings yet
- Environmentalsciencemodule2notes 140206195027 Phpapp01Document41 pagesEnvironmentalsciencemodule2notes 140206195027 Phpapp01api-296317938No ratings yet
- Class 10 Science Chapter 14 Revision NotesDocument6 pagesClass 10 Science Chapter 14 Revision NotesMuzafar ahmadNo ratings yet
- Source of EnergyDocument27 pagesSource of EnergyYashKhanijoNo ratings yet
- Energy Sources - PhilippinesDocument1 pageEnergy Sources - PhilippinesMom GieNo ratings yet
- Renewable NonrenewableDocument69 pagesRenewable NonrenewableAbdul HafeezNo ratings yet
- Energy Justification 2018 3 2f4thDocument7 pagesEnergy Justification 2018 3 2f4thapi-375163452No ratings yet
- 3 Energy ResourcesDocument21 pages3 Energy ResourcesJessica May DimeNo ratings yet
- Nonconventgional Energy SourceDocument18 pagesNonconventgional Energy Sourceparamarthasom1974No ratings yet
- Nuclear Energy NotesDocument3 pagesNuclear Energy NotesArianne BatallonesNo ratings yet
- EME Module 1 (Energy Source & Thermodynamics)Document55 pagesEME Module 1 (Energy Source & Thermodynamics)Yuga ChandrashekarNo ratings yet
- Chapter - 14: Sources of EnergyDocument27 pagesChapter - 14: Sources of Energyuma mishraNo ratings yet
- Power CT Syllabus Merged Upto Lec 20Document60 pagesPower CT Syllabus Merged Upto Lec 20avirup SahaNo ratings yet
- 2023TECH L1 3 EnergyDocument39 pages2023TECH L1 3 Energyyannjoseph485No ratings yet
- SCI02-Prelims The Big Bang Theory & NucleosynthesisDocument12 pagesSCI02-Prelims The Big Bang Theory & NucleosynthesisMORAN DianaNo ratings yet
- S W O T: ECO Finals I. Mission and VisionDocument4 pagesS W O T: ECO Finals I. Mission and VisionMORAN DianaNo ratings yet
- Average Speed Distance Traveled Time Taken Cover The DistanceDocument7 pagesAverage Speed Distance Traveled Time Taken Cover The DistanceMORAN DianaNo ratings yet
- Big Bang Nucleosynthesis ExplanationDocument7 pagesBig Bang Nucleosynthesis ExplanationMORAN DianaNo ratings yet
- ComProg Part 2Document5 pagesComProg Part 2MORAN DianaNo ratings yet
- Computer ProgrammingDocument16 pagesComputer ProgrammingMORAN DianaNo ratings yet
- Reg Form (1st Sem)Document1 pageReg Form (1st Sem)MORAN DianaNo ratings yet
- Computer Programming - 1Document9 pagesComputer Programming - 1MORAN DianaNo ratings yet
- Lab Report 3 Heat of CombustionDocument7 pagesLab Report 3 Heat of CombustionLawrence Abram AlcantaraNo ratings yet
- GeM Bidding 5259669Document6 pagesGeM Bidding 5259669Priya RoyNo ratings yet
- rr312404 Design of Machine ElementsDocument8 pagesrr312404 Design of Machine ElementsSRINIVASA RAO GANTANo ratings yet
- Hydroelectric Turbine Generator Detailed Design ReportDocument23 pagesHydroelectric Turbine Generator Detailed Design Reportwvd5028No ratings yet
- API Safety and Fire Protection PublicationDocument6 pagesAPI Safety and Fire Protection PublicationDan PascoNo ratings yet
- 777D Off Highway Truck: Service Training MalagaDocument46 pages777D Off Highway Truck: Service Training Malagajose10001100% (4)
- Measurements and InstrumentationDocument17 pagesMeasurements and Instrumentationdr mbaluNo ratings yet
- Power Designs 5020 Precision Power Source Manual Newer EditionDocument20 pagesPower Designs 5020 Precision Power Source Manual Newer EditionVivi LazuliNo ratings yet
- HS3 - Node 7 - OllieDocument14 pagesHS3 - Node 7 - Ollieollie4hortonNo ratings yet
- Catalogo Generadores - en PDFDocument16 pagesCatalogo Generadores - en PDFAldo David Araujo RojasNo ratings yet
- Teaching of ASME IX Code To Students of GTAW, GMAWFCAW, SMAW and SAW Welding ProcessesDocument4 pagesTeaching of ASME IX Code To Students of GTAW, GMAWFCAW, SMAW and SAW Welding Processesdelta_scopeNo ratings yet
- Rotary Twin Screw CompressorsDocument12 pagesRotary Twin Screw CompressorsOrlando Jose Romero Reyes100% (1)
- Direct and InverseDocument2 pagesDirect and InverseRichaBhardwajBhatiaNo ratings yet
- Steven Greer TranscriptDocument44 pagesSteven Greer Transcriptpgeorg100% (1)
- 1701 - Marcelino AngginataDocument4 pages1701 - Marcelino AngginataVando WanesNo ratings yet
- Ideal Solution and Excess functions-Ch11-IVDocument32 pagesIdeal Solution and Excess functions-Ch11-IVmominhadiNo ratings yet
- p11 AnsDocument8 pagesp11 AnsAnonymous ncBe0B9bNo ratings yet
- Failure Mode and Effect Analysis (FMEA) of Redundant SystemsDocument57 pagesFailure Mode and Effect Analysis (FMEA) of Redundant SystemsJuan Manuel SolarNo ratings yet
- Producing Ricc-Gas-Condensate Reservoirs (Case Study)Document6 pagesProducing Ricc-Gas-Condensate Reservoirs (Case Study)Daniel DamboNo ratings yet
- 1927 06 The Electric ArcDocument16 pages1927 06 The Electric ArcdeyvimaycolNo ratings yet
- HD637S1 CenelecDocument7 pagesHD637S1 CenelecMiljenko TomicNo ratings yet
- Science DLPDocument2 pagesScience DLPJesusa Gregory HabigNo ratings yet
- LCM & LEM 320 Control and Expander Modules Data SheetDocument2 pagesLCM & LEM 320 Control and Expander Modules Data SheetGabriel OsorioNo ratings yet
- Petrochemical Level Indicator and Controller With Temperature Monitoring For Spinning or Cotton Process IndustriesDocument3 pagesPetrochemical Level Indicator and Controller With Temperature Monitoring For Spinning or Cotton Process Industriesieee4mybusinessonly100% (1)
- Manual Ingles de Drive Optodriver - Variador 220Document20 pagesManual Ingles de Drive Optodriver - Variador 220Paul GuerreroNo ratings yet
- DC Charger (BTL 10)Document16 pagesDC Charger (BTL 10)chdi100% (1)
- User ManualDocument24 pagesUser Manual123mcrowleyNo ratings yet
Alternative Energy - Refers To Energy Sources Which Are No Based On The
Alternative Energy - Refers To Energy Sources Which Are No Based On The
Uploaded by
MORAN DianaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Alternative Energy - Refers To Energy Sources Which Are No Based On The
Alternative Energy - Refers To Energy Sources Which Are No Based On The
Uploaded by
MORAN DianaCopyright:
Available Formats
SCI02 Midterms
Energy Sources
I. Energy V. Electrochemical: Introduction
- The ability to do work - Batteries give electricity via an electrochemical reaction.
- Takes many forms: Mechanical, Chemical, Thermal, Electrical - Has three parts:
- Alternative Energy – refers to energy sources which are no based on the 1. Anode – Negative End
burning of fossil fuels or the splitting of atoms 2. Cathode – Positive End
3. Electrolyte – Medium between anode and cathode
II. Converting Energy into Electricity - First battery was invented by Alessandro Volta
1. Heat Energy - energy from bond breaking/formation in fuels is converted
to heat energy. (Step is not necessary if power generation does not require VI. Electrochemical: Usage
burning of fuels) - Low intensity (low power output) but are commonly used because of
2. Kinetic energy - heat energy is used to convert water to steam which has portability and efficiency
kinetic energy - Two main types of battery:
3. Mechanical Energy - steam’s kinetic energy becomes mechanical energy 1. Primary or Disposable Battery
which makes turbines and other mechanisms turn (ex: Alkaline, Mercury, Silver-Oxide, and Zinc-Carbon Batteries)
4. Electrical Energy - mechanical energy from the mechanisms create 2. Secondary or Rechargeable Battery
electricity or electrical energy via electromagnetism (ex: Lead-Acid, and Lithium-Ion Batteries)
III. Major Groups of Energy Sources
VII. Electrochemical Energy: Downsides
Methods of electricity generation can be split into two major classifications:
- Environmental concerns: Toxic Metal Pollution
1. Renewable Sources
- Battery manufacture consumes resources and often involves hazardous
Geothermal Energy
chemicals
Hydroelectric Energy
- Used batteries contribute to electronic waste
Wind Energy
- Recycling batteries is an expensive and labor-intensive process
Solar Energy
◦ Prevents dangerous elements (lead, mercury, and cadmium) from
Biomass energy
entering the environment
2. Non-Renewable Sources
Fossil Fuels (coal, petroleum, natural gas)
69% fossil fuels in PH VIII. Nuclear Energy: Introduction
75% fossil fuels in Earth - Bataan
Electrochemical (batteries) - Splitting of atoms (fission) or combination of atoms (fusion) at the nuclear
Nuclear (fusion/fission reactions) level releases large amounts of heat energy as well as radioactive materials.
- Must be done in specialized nuclear power plants and produces radioactive
IV. Fossil Fuels waste products.
- Include hydrocarbon chains (coal, oil, natural gas) - Measures must be taken to prevent mishaps at nuclear power plants.
- Energy comes from the sun through the photosynthesis
◦ plants photosynthesize sunlight IX. Downsides of Non-renewable/Nuclear Energy
◦ animals eat the plants - Limited supply
◦ animals die and become fossils that contain energy - Environmental hazards such as pollution or radioactive waste
- Formation of these fuels is due to a series of geologic processes: - Possibility of meltdown in the case of nuclear power
◦ remains of organic life accumulates at the bottom of the ocean - Fukushima, Japan
◦ remains are buried into the crust and become part of the geosphere - Alternative: Use Renewable Energy Sources
- Remains are buried to depths with high temperatures and pressures where
they are converted to coal, natural gas, or oil. X. Geothermal Energy
- In the Philippines, around 69% of our electricity is derived from fossil fuels - Utilizes heat energy from Earth’s crust
- In the entire world, around 75% of our energy is generated by the ◦ Heat raises the temperature of rocks which in turn increase the
combustion of fossil fuels temperature of nearby groundwater
- Power plants burn fossil fuels and the heat generated turns water to steam ◦ Some groundwater turns into underground steam which is then tapped
which moves turbines to create electricity to turn turbines to create electricity
- General reaction for combustion of hydrocarbon is: 𝐻𝑦𝑑𝑟𝑜𝑐𝑎𝑟𝑏𝑜𝑛 + - Main source of energy in the Visayas Region (about 38% of electricity)
𝑂𝑥𝑦𝑔𝑒𝑛 → 𝐶𝑎𝑟𝑏𝑜𝑛 𝐷𝑖𝑜𝑥𝑖𝑑𝑒 + 𝑊𝑎𝑡𝑒r - Less of an environmental hazard compared to fossil fuels but still emits
- Combustion is an exothermic process (releases heat into the surroundings): carbon dioxide, nitrous oxide, and sulfur dioxide.
𝐶𝐻4 + 2𝑂2 → 𝐶𝑂2 + 2𝐻2𝑂 + 𝐻𝑒𝑎𝑡 𝐸𝑛𝑒𝑟𝑔y
- Combustion creates carbon dioxide that is harmful to the environment in XI. Geothermal Energy: Examples
large amounts - Geothermal Reservoirs: Found near: Geysers, Boiling Mud Pots, Volcanos,
- Emissions from fossil fuel power plants also cause acid rain and global-scale Hot springs
pollution by elements such as mercury - Calauan, Laguna
- Therefore: Fossil fuels are not sustainable energy source - Direct Uses: Hot spring spas, water heating at fish farms, provide buildings
with natural heating, raising plants in greenhouses, drying crops
- Indirect Uses: Electricity Generation
© Diana Moran. ABM-A36
XII. Geothermal Energy: Advantages and Disadvantages XV. Solar Energy
Advantages: - Calatagan, Batangas
◦ Available all year-round - sun as the source of energy
◦ Does not involve combustion of fuel - Solar panel - composed of photovoltaic cells that convert light into electricity
◦ Independent of weather
◦ Clean resource – very little emissions/small overall environmental impact XVI. Solar Energy: Advantages and Disadvantages
◦ Economically sound – fuel is free, rate/KWh likely to be competitive Advantages:
◦ Abundant
Disadvantages:
◦ Low Maintenance
◦ Not widespread (limited to locations near geothermal reservoirs)
◦ Environmentally-friendly
◦ High installation costs
◦ Minimal Emissions
◦ Can run out of steam
◦ May release harmful gases Disadvantages:
◦ Possible high transportation costs ◦ Expensive installation
◦ Susceptibility to earthquakes ◦ Requires rare metals
◦ Requires a large amount of space
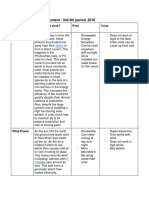
XII. Hydroelectric Energy: Introduction
- Benguet XVII. Biomass
- Utilizes moving water to turn turbines - Refers to organic matter from plants and/or animals.
- Dams installed in strategic locations where there is a substantial drop in ◦ Wastes such as crop remains, manure, and garbage are good sources of
elevation biomass.
- Main source of electricity in Mindanao (38% of energy source in Mindanao) ◦ Wood is also a biomass fuel. (So long as the trees cut are replenished, can
be a renewable energy source)
XIII. Hydroelectric Energy: Advantages and Disadvantages - Biomass is used to create methane and alcohol, fuels that are useful in
Advantages: energy production and powering machinery
◦ Clean and safe - Production of biogas/biofuels involves the action of microorganisms that
◦ Self-sustaining break down organic matter.
◦ Create habitats for more types of marine life - Technology is currently improving to improve efficiency
◦ Act as flood controllers - Types of Biomass: Wood, Garbage, Crops, Alcohol Fuels, Landfill Gas
◦ Very Efficient (90-95% efficiency)
XVIII. Biomass: Advantages and Disadvantages
Disadvantages
Advantages:
◦ Dam construction may displace marine life and change the ecosystem
◦ Renewable fuel source
around the dam
◦ Minimal environmental impact
◦ Employment generation in rural areas
XIV. Wind Power ◦ Alcohol fuels are efficient and clean-burning
- Bangui, Ilocos ◦ Universal availability
- Has been used for centuries to move ships and pump water
Disadvantages:
- harness mechanical energy from wind thru wind turbines
◦ Combustion contributes directly to global warming
- Wind turbines installed in strategic locations to harness mechanical energy
◦ Expensive to produce and convert
from wind:
◦ On smaller scales, net LOSS of energy. (energy is required to grow plant
◦ Mechanical Energy → Electrical Energy
mass)
Gross Power Generation by Fuel of Philippines (2016)
Chemical Reactions and Heat
© Diana Moran. ABM-A36
Collision – an encounter between particles (such as atoms or molecules) resulting in exchange or transformation of energy (Webster, 2018)
I. Collision Theory
– Explains how reactions occur in a qualitative manner and why different reactions occur at different rates
– When suitable particles of the reactant hit one another, only a small percentage of these collisions create
any significant chemical change.
◦ Successful collisions only occur when they have enough energy for the reaction to take place
Na2S2O3 + 2HCl → 2NaCl + S + SO2 + H2O
– When hydrochloric acid is added to the sodium thiosulphate a reaction occurs which produces a cloudy
suspension of solid sulphur
– The time it takes for the cross to disappear can be used to investigate rate of reaction
II. Energy 2. Cooking: Heat energy is absorbed from the pan in order to cook your food.
– maintains your body temperature close to 37˚C
– enables your heart and lungs to carry on circulation and transpiration
Example on Exothermic Processes
– generates electrical impulses for your nervous system to function
1. Combustion: The burning of Carbon-containing compounds uses Oxygen in
– causes your muscles to contract/expand, allowing you to move
the air and produces Carbon Dioxide, Water, and Heat
– powers machinery and your electronic devices
2. Rain: The condensation of water vapor into rain (changing phases from gas
to liquid) releases Heat
III. Chemical Reactions
– In a chemical reaction:
VI. Enthalpy
1. Existing chemical bonds are broken
– Defined as the heat energy change (∆H) that takes place when reactants
2. Atoms are rearranged
turn into products.
3. New chemical bonds are formed
– ∆H value negative → energy released → exothermic reaction
– Bond Breaking → Requires Energy
– ∆H value positive → energy absorbed → endothermic reaction
– Bond Formation → Releases Energy
– Almost all chemical reactions either absorb or release energy that results in
Example: Enthalphy of Reaction
an energy flow known as Heat
H2(g) + F2(g) = 2HF
◦ Heat → Energy transferred from one object to another due to a
difference in temperature 1. We calculate the ∆H for the chemical equation
1. First we need the Bond Energies of H2, F2, and HF:
IV. Heat – H2 = 436 kJ/mole, F2 = 186 kJ/mole, HF = 568 kJ/mole
– SI unit of energy is Joule (pronounced “jool”), J, in honor of the British – We once again look at the Bond Energies to see how much Heat is
Scientist James Joule who studied work and heat. absorbed/released when that bond is broken/formed.
– 1 J = 1 kgm2/s2 – To break one mole of H2 bonds, the system must absorb 436 kJ
– Kilojoule (kJ) is more commonly used when energies involved in chemical – To break one mole of F2 bonds, the system must absorb 186 kJ
reactions. – To form two moles of HF bonds, the system must release (2x568) kJ
– Energy changes may be expressed in calories (cal) → 1 cal = 4.184 J
– A related unit of energy is used in nutrition, the Calorie (note that the unit 2. We can then use the equation:
is capitalized) ∆H = ∑∆H (bondsbrokeninreactants) – ∑∆H (bondsmadeinproducts)
– To find: ∆ = 436 + 186 − 2 568 = −542
V. Studying Heat – H is negative so Heat is released by the chemical reaction, making it
Thermodynamics – the area of scientific study which examines energy and Exothermic
work transformations in systems.
Thermochemistry – part of thermodynamics, which deals with the study of
the changes in heat in chemical reaction
1. Exothermic Reactions - Involve a release of heat into the surroundings
2. Endothermic Reactions - Involve the absorption of heat into the system
Example: (illustration pg. 17 ppt)
Sam’s salt created an Endothermic Reaction: the reaction absorbed heat
from the flask and its surroundings, lowering their temperature
Julie’s created an Exothermic Reaction: the reaction released heat into the
flask and its surroundings, increasing their temperature
Example on Endothermic Processes
1. Photosynthesis: Plants absorb heat energy from sunlight to convert Carbon
Dioxide and Water into Glucose and Oxygen
VII. The rate of a chemical reaction
© Diana Moran. ABM-A36
Chemical Kinetics – Area of chemistry which deals with the speed at which chemical reactions occur.
– Also deals with all factors that affect the speed of chemical reactions
Example: The formation of diamond and graphite, which occur below the earth’s crust require very high pressure and temperature, are very slow reactions.
VIII. Reaction Rates
– The change in concentration of reactants and products in a certain amount of time.
– One way of expressing is by molarity and the reaction rate is expressed in molarity per second (M/s).
– Can be written out as: Rate = –
[ Reactant at t 2 ]−[Reactant at t 1 ]
t 2−t 1
– Note the negative (-) sign at the beginning, this is because rate is always a positive number.
∆ [ Reactant ] ∆[ Product ]
Rate = – or Rate = –
time time
IX. Factors Affecting Reaction Rate
1. Nature of Reactants – refers to the complexity of the reactants and the 2. Temperature – Higher temperatures means more heat for the system to
bonds that have to be broken and formed in order for the reaction to occur. absorb. More heat means more energetic particles in the system. More
(Ex: Gases tend to react faster than solids or liquids: It takes energy to energetic particles mean more collisions. More collisions means more
separate particles from each other. In order to burn candle wax, the solid chemical changes in a smaller amount of time
wax has to be melted and then vaporized before it reacts with oxygen. (Ex: Plants grow faster in warm or hot environments compared to cold
Methane gas is already in the gas state so it burns faster than wax.) environments.)
FAST: CH4 (g) + 2O2(g) → CO2(g) + 2H2O(g)
Slow: C25H52 (s) + 38 O2(g) → 25 CO2(g) + 26H2O(g)
X. Catalyst Examples
– The use of catalyst is a common way of increasing the rates of chemical 1. Enzymes – Catalyst found in the body include enzymes such as those that
reactions. help breakdown sugars.
– Substances that increase the rate if chemical reactions without itself being 2. Catalytic Converter – Catalytic converter of automobiles, a well known
used in the reaction. catalyst which is used to accelerate the reaction of carbon monoxide and
– A catalyst does not appear in the overall chemical equation as the either a oxygen to form non toxic carbon dioxide.
reactant or a product. – The use of catalytic converters are critical in controlling air pollution due to
– In other words, the mass of a catalyst is the same before and after a the increasing number of automobiles in the world
reaction occurs. – The job of the catalytic converter is to convert harmful pollutants into less
harmful emissions before they ever leave the car's exhaust system
Heterogeneous Catalysis
The most common examples of heterogeneous catalysis in industry involve the reactions of gases being passed over the surface of a solid, often a metal, a metal
oxide or a zeolite
Process Catalyst Equation
Making Amonia Iron
Produces:
Making synthesis gas (carbon monoxide
Nickel a gas (e.g. ethene, propene) a liquid (e.g.petrol)
and hydrogen)
a residue (e.g. fuel oil)
Catalytic cracking of gas oil Zeolite
Reforming of naphtha Platinum and rhenium on alumina
Making epoxyethane Silver on alumina
Making sulfuric acid Vanadium(V) oxide on silica
Making nitric acid Platinum and rhodium
© Diana Moran. ABM-A36
You might also like
- Experiment No.2 Ferranti Effect: Objective: ApparatusDocument6 pagesExperiment No.2 Ferranti Effect: Objective: ApparatusMian TauseefNo ratings yet
- Undercurrents 16 June-July 1976Document127 pagesUndercurrents 16 June-July 1976Christopher J Squire50% (2)
- Planetary TalismanDocument30 pagesPlanetary TalismanJones Tagoe100% (18)
- Unit 6Document6 pagesUnit 6Ernie CoachNo ratings yet
- 4.5 - 4.8 Renewable and Non Renewable EnergyDocument55 pages4.5 - 4.8 Renewable and Non Renewable EnergyShereena FaisalNo ratings yet
- Physics 9 - Energy ResourcesDocument36 pagesPhysics 9 - Energy ResourcesHakim Abbas100% (1)
- Physics 9 - Energy ResourcesDocument32 pagesPhysics 9 - Energy Resourcesccbpxprwd2No ratings yet
- Physical Science: Sources of EnergyDocument2 pagesPhysical Science: Sources of EnergyJames Ryan AlzonaNo ratings yet
- ElectricityDocument19 pagesElectricityAdie Alie PumpNo ratings yet
- Energy ResourcesDocument26 pagesEnergy ResourcesAnum ObaidNo ratings yet
- STD-X Sub - Sci-Ii 5. Towards Green EnergyDocument8 pagesSTD-X Sub - Sci-Ii 5. Towards Green EnergySagar WaghmareNo ratings yet
- Renewable Energy ResourcesDocument132 pagesRenewable Energy Resourcesabhinavbansal112211No ratings yet
- Technology and Livelihood EducationDocument63 pagesTechnology and Livelihood EducationNo RiNo ratings yet
- Ib DP Ess Unit 7 NotesDocument8 pagesIb DP Ess Unit 7 NotesAnnaya Rangkuty0% (1)
- M1 SEP-Ktuassist - inDocument26 pagesM1 SEP-Ktuassist - inAir SevakkNo ratings yet
- 2024 EnergyDocument11 pages2024 Energygamerazizlol123No ratings yet
- Sourses of EnergyDocument5 pagesSourses of EnergyArshid WaniNo ratings yet
- ElectricityDocument1 pageElectricitySheryll Almira HilarioNo ratings yet
- Chapter 2 - EnergyDocument17 pagesChapter 2 - Energyamrptl9010No ratings yet
- TLE ModuleDocument73 pagesTLE ModuleDexter Bola100% (1)
- Module 4 SCE NotesDocument35 pagesModule 4 SCE NotesPreethi V AnandNo ratings yet
- Harnessing Energy From Different SourcesDocument3 pagesHarnessing Energy From Different SourcesMARVIN HILARIONo ratings yet
- Harnessing Energy From Different SourcesDocument3 pagesHarnessing Energy From Different SourcesMARVIN HILARIONo ratings yet
- Toyol EnergyDocument1 pageToyol EnergyzinilNo ratings yet
- Energy: Franziska Palme, Vincent Kalnin and Hamza OuerfelliDocument26 pagesEnergy: Franziska Palme, Vincent Kalnin and Hamza OuerfelliHamza OuerfelliNo ratings yet
- Solar EnergyDocument78 pagesSolar EnergySai VikasNo ratings yet
- Engg Chem Finals ReviewerDocument15 pagesEngg Chem Finals ReviewerCarl Jasper RabiNo ratings yet
- Technology and ComputingDocument16 pagesTechnology and ComputingAntwan MeloNo ratings yet
- Module 1 SEP-Ktunotes - inDocument24 pagesModule 1 SEP-Ktunotes - inmujeebNo ratings yet
- Energy ResourcesDocument51 pagesEnergy ResourcesPRATHA PATELNo ratings yet
- How Energy Is Produced and ManagedDocument21 pagesHow Energy Is Produced and ManagedAmiel ObusanNo ratings yet
- Building Utilities 2 ReviewerDocument29 pagesBuilding Utilities 2 ReviewerandreaNo ratings yet
- Topic 9 Alternative and Renewable EnergyDocument41 pagesTopic 9 Alternative and Renewable EnergyChuan Ung SiiNo ratings yet
- Earth Science S-WPS OfficeDocument14 pagesEarth Science S-WPS OfficeBai Johaira BenitoNo ratings yet
- Chapter EightDocument17 pagesChapter EightSeble GetachewNo ratings yet
- Physical Science Quarter 1 Week 7 1Document16 pagesPhysical Science Quarter 1 Week 7 1CrimstonNo ratings yet
- On Non Conventional Energy ResourcesDocument27 pagesOn Non Conventional Energy ResourcesAnonymous HyOfbJ6No ratings yet
- P3 Energy ResourcesDocument1 pageP3 Energy ResourcesArun DonteNo ratings yet
- Energy Efficiency in BuildingDocument30 pagesEnergy Efficiency in BuildingS.K. RecruitingNo ratings yet
- Geothermal Energy: - A Renewable Energy Source For Electricity GenerationDocument22 pagesGeothermal Energy: - A Renewable Energy Source For Electricity GenerationSuraj SunnyNo ratings yet
- Sources of EnergyDocument27 pagesSources of Energym49328968No ratings yet
- Master of Science in Engineering in Energy Systems Planning and ManagementDocument51 pagesMaster of Science in Engineering in Energy Systems Planning and ManagementRabi SarmaNo ratings yet
- Esci Lesson 6 Energy Geohydrowater ResourcesDocument63 pagesEsci Lesson 6 Energy Geohydrowater ResourcesLearni J. EscoteNo ratings yet
- Geothermal Energy: A Renewable Energy Source For Electricity GenerationDocument23 pagesGeothermal Energy: A Renewable Energy Source For Electricity GenerationJitender Kumar YadavNo ratings yet
- GP5 - Energy, Work & Power 1Document8 pagesGP5 - Energy, Work & Power 1wengiemotshegweNo ratings yet
- Impacts - Considering The Effects On The: Oleum OilDocument4 pagesImpacts - Considering The Effects On The: Oleum OilKrisha DimailigNo ratings yet
- PPE Lect 1Document19 pagesPPE Lect 1A ZNo ratings yet
- EnergíaDocument7 pagesEnergíaJorge MuñozNo ratings yet
- Environmentalsciencemodule2notes 140206195027 Phpapp01Document41 pagesEnvironmentalsciencemodule2notes 140206195027 Phpapp01api-296317938No ratings yet
- Class 10 Science Chapter 14 Revision NotesDocument6 pagesClass 10 Science Chapter 14 Revision NotesMuzafar ahmadNo ratings yet
- Source of EnergyDocument27 pagesSource of EnergyYashKhanijoNo ratings yet
- Energy Sources - PhilippinesDocument1 pageEnergy Sources - PhilippinesMom GieNo ratings yet
- Renewable NonrenewableDocument69 pagesRenewable NonrenewableAbdul HafeezNo ratings yet
- Energy Justification 2018 3 2f4thDocument7 pagesEnergy Justification 2018 3 2f4thapi-375163452No ratings yet
- 3 Energy ResourcesDocument21 pages3 Energy ResourcesJessica May DimeNo ratings yet
- Nonconventgional Energy SourceDocument18 pagesNonconventgional Energy Sourceparamarthasom1974No ratings yet
- Nuclear Energy NotesDocument3 pagesNuclear Energy NotesArianne BatallonesNo ratings yet
- EME Module 1 (Energy Source & Thermodynamics)Document55 pagesEME Module 1 (Energy Source & Thermodynamics)Yuga ChandrashekarNo ratings yet
- Chapter - 14: Sources of EnergyDocument27 pagesChapter - 14: Sources of Energyuma mishraNo ratings yet
- Power CT Syllabus Merged Upto Lec 20Document60 pagesPower CT Syllabus Merged Upto Lec 20avirup SahaNo ratings yet
- 2023TECH L1 3 EnergyDocument39 pages2023TECH L1 3 Energyyannjoseph485No ratings yet
- SCI02-Prelims The Big Bang Theory & NucleosynthesisDocument12 pagesSCI02-Prelims The Big Bang Theory & NucleosynthesisMORAN DianaNo ratings yet
- S W O T: ECO Finals I. Mission and VisionDocument4 pagesS W O T: ECO Finals I. Mission and VisionMORAN DianaNo ratings yet
- Average Speed Distance Traveled Time Taken Cover The DistanceDocument7 pagesAverage Speed Distance Traveled Time Taken Cover The DistanceMORAN DianaNo ratings yet
- Big Bang Nucleosynthesis ExplanationDocument7 pagesBig Bang Nucleosynthesis ExplanationMORAN DianaNo ratings yet
- ComProg Part 2Document5 pagesComProg Part 2MORAN DianaNo ratings yet
- Computer ProgrammingDocument16 pagesComputer ProgrammingMORAN DianaNo ratings yet
- Reg Form (1st Sem)Document1 pageReg Form (1st Sem)MORAN DianaNo ratings yet
- Computer Programming - 1Document9 pagesComputer Programming - 1MORAN DianaNo ratings yet
- Lab Report 3 Heat of CombustionDocument7 pagesLab Report 3 Heat of CombustionLawrence Abram AlcantaraNo ratings yet
- GeM Bidding 5259669Document6 pagesGeM Bidding 5259669Priya RoyNo ratings yet
- rr312404 Design of Machine ElementsDocument8 pagesrr312404 Design of Machine ElementsSRINIVASA RAO GANTANo ratings yet
- Hydroelectric Turbine Generator Detailed Design ReportDocument23 pagesHydroelectric Turbine Generator Detailed Design Reportwvd5028No ratings yet
- API Safety and Fire Protection PublicationDocument6 pagesAPI Safety and Fire Protection PublicationDan PascoNo ratings yet
- 777D Off Highway Truck: Service Training MalagaDocument46 pages777D Off Highway Truck: Service Training Malagajose10001100% (4)
- Measurements and InstrumentationDocument17 pagesMeasurements and Instrumentationdr mbaluNo ratings yet
- Power Designs 5020 Precision Power Source Manual Newer EditionDocument20 pagesPower Designs 5020 Precision Power Source Manual Newer EditionVivi LazuliNo ratings yet
- HS3 - Node 7 - OllieDocument14 pagesHS3 - Node 7 - Ollieollie4hortonNo ratings yet
- Catalogo Generadores - en PDFDocument16 pagesCatalogo Generadores - en PDFAldo David Araujo RojasNo ratings yet
- Teaching of ASME IX Code To Students of GTAW, GMAWFCAW, SMAW and SAW Welding ProcessesDocument4 pagesTeaching of ASME IX Code To Students of GTAW, GMAWFCAW, SMAW and SAW Welding Processesdelta_scopeNo ratings yet
- Rotary Twin Screw CompressorsDocument12 pagesRotary Twin Screw CompressorsOrlando Jose Romero Reyes100% (1)
- Direct and InverseDocument2 pagesDirect and InverseRichaBhardwajBhatiaNo ratings yet
- Steven Greer TranscriptDocument44 pagesSteven Greer Transcriptpgeorg100% (1)
- 1701 - Marcelino AngginataDocument4 pages1701 - Marcelino AngginataVando WanesNo ratings yet
- Ideal Solution and Excess functions-Ch11-IVDocument32 pagesIdeal Solution and Excess functions-Ch11-IVmominhadiNo ratings yet
- p11 AnsDocument8 pagesp11 AnsAnonymous ncBe0B9bNo ratings yet
- Failure Mode and Effect Analysis (FMEA) of Redundant SystemsDocument57 pagesFailure Mode and Effect Analysis (FMEA) of Redundant SystemsJuan Manuel SolarNo ratings yet
- Producing Ricc-Gas-Condensate Reservoirs (Case Study)Document6 pagesProducing Ricc-Gas-Condensate Reservoirs (Case Study)Daniel DamboNo ratings yet
- 1927 06 The Electric ArcDocument16 pages1927 06 The Electric ArcdeyvimaycolNo ratings yet
- HD637S1 CenelecDocument7 pagesHD637S1 CenelecMiljenko TomicNo ratings yet
- Science DLPDocument2 pagesScience DLPJesusa Gregory HabigNo ratings yet
- LCM & LEM 320 Control and Expander Modules Data SheetDocument2 pagesLCM & LEM 320 Control and Expander Modules Data SheetGabriel OsorioNo ratings yet
- Petrochemical Level Indicator and Controller With Temperature Monitoring For Spinning or Cotton Process IndustriesDocument3 pagesPetrochemical Level Indicator and Controller With Temperature Monitoring For Spinning or Cotton Process Industriesieee4mybusinessonly100% (1)
- Manual Ingles de Drive Optodriver - Variador 220Document20 pagesManual Ingles de Drive Optodriver - Variador 220Paul GuerreroNo ratings yet
- DC Charger (BTL 10)Document16 pagesDC Charger (BTL 10)chdi100% (1)
- User ManualDocument24 pagesUser Manual123mcrowleyNo ratings yet