Professional Documents
Culture Documents
CHEM3218 Revision Questions 2015
CHEM3218 Revision Questions 2015
Uploaded by
keatyCopyright:
Available Formats
You might also like
- Crospovidone EP 10.6 PDFDocument2 pagesCrospovidone EP 10.6 PDFxuan y phanNo ratings yet
- AAS and AES Spectroscopy Exam QuestionsDocument2 pagesAAS and AES Spectroscopy Exam QuestionskeatyNo ratings yet
- SY-8100 Manual OperacionDocument36 pagesSY-8100 Manual OperacionJOse ArmentaNo ratings yet
- TUT 1 Introduction To ChromatographyDocument3 pagesTUT 1 Introduction To Chromatographysikho0ndevuNo ratings yet
- Activity McGuire Chromatographic ResolutionDocument6 pagesActivity McGuire Chromatographic ResolutionArdhi aNo ratings yet
- Answer Any Six Question Including Question No. 1 Which Is CompulsoryDocument2 pagesAnswer Any Six Question Including Question No. 1 Which Is CompulsoryRahul SinghNo ratings yet
- Tutor Chap 4 CHM260Document3 pagesTutor Chap 4 CHM260nathirahjainiNo ratings yet
- CMO 310 Exam 2019Document11 pagesCMO 310 Exam 2019Orlando Wilson Da Sousa MelimNo ratings yet
- Btech 1 Sem Applied Chemistry Pacia101 2018 PDFDocument2 pagesBtech 1 Sem Applied Chemistry Pacia101 2018 PDFNew.T.O.N SethiNo ratings yet
- Problem Set 6 Fall 2018Document3 pagesProblem Set 6 Fall 2018rickNo ratings yet
- NR 320801 Mass Transfer Operations IIDocument6 pagesNR 320801 Mass Transfer Operations IISrinivasa Rao G100% (6)
- Be Winter 2022Document2 pagesBe Winter 2022samip shahNo ratings yet
- 471-526 Exam 3 Fall 2015Document5 pages471-526 Exam 3 Fall 2015ok9275No ratings yet
- A Level Chemistry Paper 2 Exam 28Document3 pagesA Level Chemistry Paper 2 Exam 28Anthony AndyNo ratings yet
- Be Summer 2022Document2 pagesBe Summer 2022samip shahNo ratings yet
- Assignment 2 - Pool of QuestionsDocument7 pagesAssignment 2 - Pool of QuestionsNishant KhandelwalNo ratings yet
- B48BA Exam Questions v1Document18 pagesB48BA Exam Questions v1Manmohan SinghNo ratings yet
- Btech 1 Sem Chemistry Kas 102 2018 19Document2 pagesBtech 1 Sem Chemistry Kas 102 2018 19Viraj RuhelaNo ratings yet
- MT Special Topics (Due Date: Monday, October 16, 2017: 13.00)Document3 pagesMT Special Topics (Due Date: Monday, October 16, 2017: 13.00)Irfan AdityaNo ratings yet
- C) 225 S and 305 S: Chromatographic AnalysesDocument3 pagesC) 225 S and 305 S: Chromatographic AnalysesVikash KushwahaNo ratings yet
- Cy0u10a R Engineering Chemistry Apr 2022Document3 pagesCy0u10a R Engineering Chemistry Apr 2022kangirene9705No ratings yet
- Be Summer 2021Document3 pagesBe Summer 2021samip shahNo ratings yet
- AMI B.Tech NOVEMBER 2010Document5 pagesAMI B.Tech NOVEMBER 2010Thirunavukkarasu ANo ratings yet
- A Level Chemistry Paper 2 Exam 5Document5 pagesA Level Chemistry Paper 2 Exam 5Anthony AndyNo ratings yet
- 2002 ExamsDocument22 pages2002 Examsheshammohamed44148No ratings yet
- 2170501Document3 pages2170501Zoher PainterNo ratings yet
- BT 404 - QUANTITATIVE ANALYSIS IN CHROMATOGRAPHY (Compatibility Mode)Document22 pagesBT 404 - QUANTITATIVE ANALYSIS IN CHROMATOGRAPHY (Compatibility Mode)dsdsdNo ratings yet
- Optional Area Examination Analytical ChemistryDocument4 pagesOptional Area Examination Analytical ChemistryMohamed DahmaneNo ratings yet
- A Level Chemistry Paper 2 Exam 6Document4 pagesA Level Chemistry Paper 2 Exam 6majanga johnNo ratings yet
- Cy0u10a R Engineering Chemistry Oct 2021 1Document2 pagesCy0u10a R Engineering Chemistry Oct 2021 1kangirene9705No ratings yet
- CU-2022 B.Sc. (Honours) Chemistry Semester-6 Paper-DSE-A-4 QPDocument2 pagesCU-2022 B.Sc. (Honours) Chemistry Semester-6 Paper-DSE-A-4 QPsanchita MannaNo ratings yet
- ICH 501-May 2022Document3 pagesICH 501-May 2022Jagadeesh YNo ratings yet
- UCB008Document1 pageUCB008lecev28785No ratings yet
- Gujarat Technological UniversityDocument2 pagesGujarat Technological UniversityTNo ratings yet
- Oc 2023Document8 pagesOc 2023Neha NegiNo ratings yet
- 3rd Sem End Semester - September 2015-FinalDocument6 pages3rd Sem End Semester - September 2015-FinalbgroyNo ratings yet
- CRE-2 Semester PapersDocument12 pagesCRE-2 Semester PapersSarvesh KumarNo ratings yet
- General Chemistry QuestionsDocument3 pagesGeneral Chemistry QuestionsSagar JainNo ratings yet
- Practice Problem Set Mixed Chromatography QuestionsDocument14 pagesPractice Problem Set Mixed Chromatography QuestionsMelinda AndersonNo ratings yet
- Final Theory Exam-307 June2012Document13 pagesFinal Theory Exam-307 June2012Jagadeesh EllilNo ratings yet
- Btech Ice 8 Sem Analytical Instrumentation 2011Document4 pagesBtech Ice 8 Sem Analytical Instrumentation 2011Ahmed MansourNo ratings yet
- CHM580Document8 pagesCHM580Azreen AnisNo ratings yet
- Analog Communication Systems: 08/09 Sem 2 ExamDocument8 pagesAnalog Communication Systems: 08/09 Sem 2 ExamYong JiaNo ratings yet
- Assignment L01 (Thursday, 11.30 Am) Marking SchemeDocument12 pagesAssignment L01 (Thursday, 11.30 Am) Marking SchemeMawareNo ratings yet
- R5312305-Mass Transfer AndseperationDocument4 pagesR5312305-Mass Transfer AndseperationsivabharathamurthyNo ratings yet
- 6519 2011bme 502 (5115) PDFDocument4 pages6519 2011bme 502 (5115) PDFNoor AhmedNo ratings yet
- 6519 2011bme 502 (5115) PDFDocument4 pages6519 2011bme 502 (5115) PDFNoor AhmedNo ratings yet
- National Institute of Technology, Rourkela - 769 008, OrissaDocument1 pageNational Institute of Technology, Rourkela - 769 008, OrissaSubrat kumar SahooNo ratings yet
- rr422301 Chromatographic SeparationsDocument4 pagesrr422301 Chromatographic SeparationsSRINIVASA RAO GANTANo ratings yet
- 2012 Non-Conventional Energy Sources: CS/B.Tech (ME/PE) /SEM-8/ME-806/2012Document7 pages2012 Non-Conventional Energy Sources: CS/B.Tech (ME/PE) /SEM-8/ME-806/2012Krishna Prakash KPNo ratings yet
- Glance Through The Entire Questions and Solve The Easiest Problems FirstDocument4 pagesGlance Through The Entire Questions and Solve The Easiest Problems FirstRutul JainNo ratings yet
- 1pu Chem Midterm QP Bangalore SouthDocument3 pages1pu Chem Midterm QP Bangalore Southredej66556No ratings yet
- Btech CH 6 Sem Chemical Reaction Engineering 2 NCH 601 2016 17Document2 pagesBtech CH 6 Sem Chemical Reaction Engineering 2 NCH 601 2016 17Anmol YadavNo ratings yet
- From Final ExamDocument9 pagesFrom Final ExamThrishnaa BalasupurManiamNo ratings yet
- XI Mid Term QPDocument3 pagesXI Mid Term QPtechnical SiteNo ratings yet
- Test 2, SMJC 2202 - Sec 02Document1 pageTest 2, SMJC 2202 - Sec 02norsiahNo ratings yet
- S16 CPSDDocument4 pagesS16 CPSDGohit BhatNo ratings yet
- Guía de Estudio EspectrosDocument5 pagesGuía de Estudio EspectrosCésar CidNo ratings yet
- WWW - Manaresults.co - In: Set No. 1Document4 pagesWWW - Manaresults.co - In: Set No. 1Sathya Bhuvaneswari KavalaNo ratings yet
- Gujarat Technological UniversityDocument3 pagesGujarat Technological Universityfeyayel990No ratings yet
- Tutorial 1Document7 pagesTutorial 1Harshi ChandraferiNo ratings yet
- School of Engineering & Physical Sciences Chemical EngineeringDocument10 pagesSchool of Engineering & Physical Sciences Chemical EngineeringIdlan IzharNo ratings yet
- Standard and Super-Resolution Bioimaging Data Analysis: A PrimerFrom EverandStandard and Super-Resolution Bioimaging Data Analysis: A PrimerNo ratings yet
- EetdagboekDocument1 pageEetdagboekkeatyNo ratings yet
- Green Chemistry Exam QuestionsDocument1 pageGreen Chemistry Exam QuestionskeatyNo ratings yet
- Surface Chemistry Short QuestionsDocument1 pageSurface Chemistry Short QuestionskeatyNo ratings yet
- 2015 Rekening ExamDocument10 pages2015 Rekening ExamkeatyNo ratings yet
- Catalysis & Catalysts: Facts and Figures About CatalystsDocument88 pagesCatalysis & Catalysts: Facts and Figures About CatalystskeatyNo ratings yet
- Sadettin Ozturk Aug2009 UFRJDocument31 pagesSadettin Ozturk Aug2009 UFRJkeatyNo ratings yet
- Transport of Dangerous GoodsDocument16 pagesTransport of Dangerous Goodskeaty100% (1)
- Answers To Suggested Problems For Chapter 9Document3 pagesAnswers To Suggested Problems For Chapter 9keatyNo ratings yet
- DT261/3, DT299/3: Dr. Andrew KnoxDocument45 pagesDT261/3, DT299/3: Dr. Andrew KnoxkeatyNo ratings yet
- BSC (Medicinal Chemistry and Pharmaceutical Sciences) : Section A Is Compulsory Answer One Question From Section B andDocument4 pagesBSC (Medicinal Chemistry and Pharmaceutical Sciences) : Section A Is Compulsory Answer One Question From Section B andkeatyNo ratings yet
- CHEM3218 P.Behan-3Document66 pagesCHEM3218 P.Behan-3keatyNo ratings yet
- CHEM 3210 Review Questions 2015Document3 pagesCHEM 3210 Review Questions 2015keatyNo ratings yet
- Separation Processss Lecture NotesDocument17 pagesSeparation Processss Lecture NoteskeatyNo ratings yet
- CHEM 3210 - Sample Numerical Problems and SolutionsDocument2 pagesCHEM 3210 - Sample Numerical Problems and SolutionskeatyNo ratings yet
- BSC (Medicinal Chemistry and Pharmaceutical Sciences) : Section A Is Compulsory Answer One Question From Section B andDocument4 pagesBSC (Medicinal Chemistry and Pharmaceutical Sciences) : Section A Is Compulsory Answer One Question From Section B andkeatyNo ratings yet
- Assignment1. Time Managment ExerciseDocument2 pagesAssignment1. Time Managment ExercisekeatyNo ratings yet
- Chemical Technology LC 2 NotesDocument5 pagesChemical Technology LC 2 NoteskeatyNo ratings yet
- Notes 3 Material Balance CalculationsDocument21 pagesNotes 3 Material Balance CalculationskeatyNo ratings yet
- Chem & Phram Proc Technology LC 1 NotesDocument10 pagesChem & Phram Proc Technology LC 1 NoteskeatyNo ratings yet
- Chem Proc LC 4 NotesDocument11 pagesChem Proc LC 4 NoteskeatyNo ratings yet
- Selected Answers For Exercises: Product KG Waste KG EDocument7 pagesSelected Answers For Exercises: Product KG Waste KG EkeatyNo ratings yet
- Quantification of Piperine in Different Varieties of Piper Nigrum by A Validated High Performance Thin Layer Chromatography Densitometry MethodDocument10 pagesQuantification of Piperine in Different Varieties of Piper Nigrum by A Validated High Performance Thin Layer Chromatography Densitometry MethodArtem KulikovNo ratings yet
- Ep LisinoprilDocument2 pagesEp LisinoprillopebutetNo ratings yet
- Method 515.3 Determination of Chlorinated Acids in DrinkingDocument56 pagesMethod 515.3 Determination of Chlorinated Acids in DrinkingPedro FrancoNo ratings yet
- Method For The Determination of Beta Carotene in Supplements and Raw Materials by Reversed Phase Liquid Chromatography Single Laboratory ValidationDocument13 pagesMethod For The Determination of Beta Carotene in Supplements and Raw Materials by Reversed Phase Liquid Chromatography Single Laboratory ValidationChris JohnsonNo ratings yet
- 1511152635981Document14 pages1511152635981Lolo OmarNo ratings yet
- HPTLC Paper of Berberis PDFDocument5 pagesHPTLC Paper of Berberis PDFAnisahMahardianiNo ratings yet
- 6522-An156 LPN1548Document6 pages6522-An156 LPN1548anushka chadhaNo ratings yet
- Null 2Document30 pagesNull 2Sara EltayiebNo ratings yet
- Homelabmanual204 PDFDocument35 pagesHomelabmanual204 PDFMaii AlaarajNo ratings yet
- D 5739 - 00 Rdu3mzkDocument13 pagesD 5739 - 00 Rdu3mzkAnonymous xMQd4zNo ratings yet
- Radial Chromatography For The Separation of Nitroaniline IsomersDocument3 pagesRadial Chromatography For The Separation of Nitroaniline IsomersAndrianNo ratings yet
- Isoflavone 2Document17 pagesIsoflavone 2Elis ApriyantiNo ratings yet
- Manual CromatografoDocument10 pagesManual CromatografoVerónicaJiménezOlmedoNo ratings yet
- Pharmasist LoksewaDocument5 pagesPharmasist LoksewaAashish BhattaraiNo ratings yet
- Instant Download Ebook PDF Encyclopedia of Analytical Science 3rd Edition PDF ScribdDocument41 pagesInstant Download Ebook PDF Encyclopedia of Analytical Science 3rd Edition PDF Scribdhoward.linkovich475100% (61)
- 00 - Gavilanes (1982) Int J Peptide Protein Res - Pigeon Egg White LysozymeDocument8 pages00 - Gavilanes (1982) Int J Peptide Protein Res - Pigeon Egg White Lysozyme1810mNo ratings yet
- T.Y.B.Sc. Chemistry (6 Units) : Choice Based Credit SystemDocument12 pagesT.Y.B.Sc. Chemistry (6 Units) : Choice Based Credit SystempratikNo ratings yet
- Approval Sheet: Mutmainnah Umar Rahmatia ID. 1513440003 ID. 1613040013Document15 pagesApproval Sheet: Mutmainnah Umar Rahmatia ID. 1513440003 ID. 1613040013rulmadhaniNo ratings yet
- BiotechnologyDocument21 pagesBiotechnologyShivangi Mishra50% (2)
- Separation of Ink Mixture Using Paper Chromatography TechniqueDocument2 pagesSeparation of Ink Mixture Using Paper Chromatography TechniqueSevar AbdullahNo ratings yet
- Name of The Experiment: Fatty Acid Analysis by Using Gas ChromatographyDocument2 pagesName of The Experiment: Fatty Acid Analysis by Using Gas ChromatographyWayMeen PangNo ratings yet
- Diclofenac Injection: TestsDocument2 pagesDiclofenac Injection: TestsMuhammad ImranNo ratings yet
- CH12 - GOC - Shobhit NirwanDocument61 pagesCH12 - GOC - Shobhit NirwanRao GootleyNo ratings yet
- Isolasi Dan Identifikasi Senyawa Flavonoid Dari Daun Jamblang Syzygium Cumini PeDocument11 pagesIsolasi Dan Identifikasi Senyawa Flavonoid Dari Daun Jamblang Syzygium Cumini PeMuhammad Rifqi FauziNo ratings yet
- Ascorbic AcidDocument2 pagesAscorbic AcidMulayam Singh YadavNo ratings yet
- Diethyl PhthalateDocument2 pagesDiethyl PhthalateMulayam Singh YadavNo ratings yet
- Tech 707Document2 pagesTech 707sadaf_5No ratings yet
CHEM3218 Revision Questions 2015
CHEM3218 Revision Questions 2015
Uploaded by
keatyOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CHEM3218 Revision Questions 2015
CHEM3218 Revision Questions 2015
Uploaded by
keatyCopyright:
Available Formats
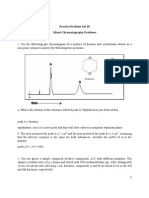
CHEM3218 Pharmaceutical Separation Science Revision Questions Dr P.
Behan
Short Questions:
a) Describe the operation of a ‘split’ injection system used in gas chromatography.
b) Outline the operation of a flame ionisation detector.
c) Doubling the length of the column in GC increases the resolution by what factor?
Explain.
d) Write out the Van Deemter equation indicating what each term represents and
describe how it is used in gas chromatography (GC).
e) Briefly explain the difference in polarity between the following:
CH3COCH3 : CH3CH2OH
f) List the following in order of increasing polarity. Explain your reasoning.
CH3CH2OH, CH3CH2Cl, CH3COOH.
g) Calculate the flow rate of carrier gas through a capillary column in cm3/min. given
that the retention time of an unretained species is 1.60 minutes, the column length is
30 m and the internal diameter is 0.25 mm.
h) Define the basic aims of chromatography.
i) The following data was obtained for a two component mixture.
Compound tr (min) Width of peak at base
(min)
A 8.04 0.15
B 8.26 0.15
Calculate the resolution between A and B. Is it suitable for quantitation?
CHEM3218 Revision Question, Dr P. Behan, Page 1 of 8
Long Questions:
Extraction Efficiency & Chromatographic Parameters:
1. Solute A has a partition coefficient of 5 between toluene and water. Suppose that
200ml of 0.02 moldm-3 aqueous solution of A is extracted with toluene What fraction
of A remains in the aqueous phase
a. After 1 extraction with 100ml toluene
b. After 5 extractions with 20ml of toluene
2. The following data were obtained from the chromatogram of a two component
mixture where the retention time of the unretained species was 3.1 minutes.
component Retention time ( min) Width of peak at
base ( min)
A 13.3 1.07
B 14.1 1.16
Calculate
(i) The resolution between A and B.
(ii) The relative retention between A and B.
(iii) The capacity factor for B.
(iv) The number of theoretical plates for B.
3. The following data was obtained from the chromatogram of a three
component mixture. Column length = 10 cm
Compound tR(min) Width of Peak at
base (min)
A 17.8 4.0
B 25.7 5.0
C 29.6 5.5
(i) If the retention time (tR) of an unretained species is 1.7 minutes, determine the
capacity factor for A.
(ii) Calculate the value of N and H (in mm/plate) for peak A, where N is the
number of theoretical plates and H is the height equivalent of a theoretical
plate.
(iii) Calculate the resolution between compounds A and B and between
compounds B and C. Comment on these results in relation to the
quantification of each compound.
4. The following data were obtained from the chromatogram of a two component
mixture separated on a 20 metre capillary column. The retention time of the
unretained species was 1.19 min
component Retention Width of peak
time ( min) at base ( min)
A 8.04 0.15
B 8.26 0.15
CHEM3218 Revision Question, Dr P. Behan, Page 2 of 8
Unretained 1.19
species
Calculate
(i) The capacity factor for A .
(ii) The relative retention between A and B.
(iii) The height equivalent of a theoretical plate, in millimetres , for B.
(iv) The resolution between A and B.
5. The following data were obtained for a two component mixture separated on a 20
metre capillary column.
Compound Retention Time Width of peak at base
(min) (min)
A 8.26 0.15
B 8.46 0.16
Retention time of unretained species = 1.19 min
Calculate (i) the height equivalent of a theoretical plate in
millimetres for A,
(ii) the selectivity factor between A and B,
(iii) the resolution between A and B and
(iv) the length of column required to increase the resolution
between A and B by a factor of two.
Gas Chromatography:
6. Gas chromatography is used to analyse mixtures of organic compounds. Discuss this
technique under each of the following headings:
a. Basic aims.
b. Advantages.
c. Limitations.
7. Write out the Van Deemter equation, discussing what each term represents and
describe how the equation is used in gas chromatography.
8. List the following in order of increasing polarity: ketones, alcohols, halogenated
hydrocarbons, water, hydrocarbons, ethers, esters
9. List the following in order of increasing polarity: propan-2-ol, methanol, butan-1-ol
ethanol, propan-1-ol.
10. Predict the order of elution of the following compounds:
Propanol (b.p. 97oC), heptane (b.p. 98oC), 2-butanone (b.p. 80oC),
3- pentanone (b.p. 102oC)
(i) From a non-polar column.
(ii) From a column of intermediate polarity and
CHEM3218 Revision Question, Dr P. Behan, Page 3 of 8
(iii) from a strongly polar column.
In each case explain your reasoning.
11. Discuss the operation of a “split” injection system used in gas chromatography.
12. Outline the use of temperature programming in gas chromatography.
13. Name three types of detectors (not including a mass spectrometer) used in gas
chromatography and briefly compare their use. Outline the operation principles of any
one of them.
14. Hexane and 1-methylethylmethanoate both have boiling points of 68oC. They are
unresolved on an intermediate polarity column. State what the order of elution
would be on a non polar column and a polar column and give reasons for your
answer. Explain why they are unresolved on an intermediate polarity column.
15. Differentiate between packed and capillary columns in gas chromatography.
Liquid Chromatography:
16. “HPLC is a more versatile analytical technique than GC” Discuss this statement.
17. Draw a labelled block diagram of a HPLC. Write a brief note on each component.
18. Describe the differences between normal and reverse phase HPLC.
19. Describe the use of gradient elution in HPLC.
20. Use a diagram explain the fixed loop injection process of HPLC.
21. Describe how you would develop a method for the separation of a mixture of methyl,
ethyl and propylparaben using high performance liquid chromatography (HPLC).
Paraben (R = Methyl, Ethyl or Propyl)
CHEM3218 Revision Question, Dr P. Behan, Page 4 of 8
Mass Spectrometry:
22. Distinguish between electron impact ionisation and chemical ionisation as used in gas
chromatography mass spectrometry (GC/MS).
23. In relation to gas chromatography/mass spectroscopy (GC/MS), write a detailed note
on each of the following:
a. The operation of an ion trap mass spectrometer.
b. Electron impact ionisation (EI) versus chemical ionisation (CI).
c. Total Ion Chromatogram (TIC) versus Selected Ion monitoring (SIM).
d. Continuous dynode electron multiplier detector.
24. Outline the operation of an ion-trap mass spectrometer as used in a hyphenated system
such as GC/MS. Include in your answer a brief description of the advantages of using
such a detector in the hyphenated system compared to a general purpose detector such
as a flame ionisation detector.
25. Below is the GC-MS mass spectrum of benzoic acid (C₇H₆O₂).
(i) From the mass spectrum, identify the m/z for the base peak, the molecular ion
peak and the major fragmentation peaks.
(ii) Suggest and describe ionisation method explaining how the peaks at m/z 122,
105 and 77 in the benzoic acid GC-MS spectrum are formed.
20. The Ethanol electron ionisation mass spectrum shows peaks at m/z 46, 45, 31, 29 &
27
(i) Write the reaction which represents the electron ionisation of ethanol
(ii) What causes the major peaks at m/z = 46, 45, 31 & 29 in the ethanol mass
spectrum?
CHEM3218 Revision Question, Dr P. Behan, Page 5 of 8
21. Below is the mass spectrum of ethyl benzene (C6H5CH2CH3) showing major peaks at
m/z = 106, 911 & 77.
(i) Suggest an ionisation source used in this method and justify your answer.
(ii) Write the reaction for EI for ethylbenzene
(iii) What causes the major peaks at m/z = 106, 91, & 77 in the ethylbenzene mass
spectrum?
26. Discuss the advantages of using a mass spectrometer as a detector with gas
chromatography ( GC/MS).
27. Using the mass spectrum of Neon (Ne) shown below, calculate the average atomic
mass of Ne.
CHEM3218 Revision Question, Dr P. Behan, Page 6 of 8
Quantification:
28. A 0.520 g sample of handcream was shaken vigorously in 20 cm3 of methanol until
well dispersed. 5 cm3 of 100 ppm butyl paraben (internal standard) was added and the
solution made up to 100 cm3 with methanol. Standard solutions of methyl paraben
were made up each containing the same amount of internal standard as the sample. 20
l of each solution were injected into a liquid chromatograph and the following results
obtained:
Standards (ppm) Peak Area (cm2)
Methyl Internal
Paraben Standard
2 0.52 1.04
4 0.95 0.95
6 1.64 1.10
8 2.00 1.01
10 2.80 1.09
Handcream 1.07 0.85
Using this data plot an appropriate graph and use it to determine the % w/w of methyl
paraben in the handcream.
29. The concentration of ethanol in a wine sample was determined by GC using the
internal standard method. A 25 cm3 sample of the wine together with 10 cm3 of
the internal standard, propanol, were made up to 50 cm3 with water.
Standard solutions of ethanol were made up, each containing the same amount
of internal standard as the sample. 1.0 µL samples of each solution were
chromatographed and the following results were obtained.
Ethanol standards Peak height Peak height
( cm) (cm)
%vol/vol Ethanol Propanol
2.0 1.04 2.08
4.0 1.90 1.90
6.0 3.28 2.20
8.0 4.00 2.02
10.0 5.50 2.18
wine 2.11 1.70
Plot an appropriate graph and use it to determine the %vol/vol of ethanol in the
wine. Discuss your result in relation to the label value of 11.5% vol/vol.
30. The concentration of ethanol in a wine sample was determined by GC using the
internal standard method. A 2 cm3 sample of wine together with 5 cm3 of the internal
standard, propanol, were made up to 50 cm3 with water.
CHEM3218 Revision Question, Dr P. Behan, Page 7 of 8
Standard solutions of ethanol were made up each containing the same amount of
internal standard as the sample. 1.0 µL samples of each solution were
chromatographed and the following results obtained:
Ethanol standards Peak area Peak area
(integrator units) (integrator units)
%wt/wt ethanol propanol
0.24 1685 2407
0.48 3483 2488
0.72 4887 2327
0.96 6821 2436
wine 2749 2390
Plot an appropriate graph and use it to determine the % wt/wt of ethanol in the wine.
The density of ethanol is 0.79g cm-3. Compare your result to the label value of 12.5%
vol/vol.
CHEM3218 Revision Question, Dr P. Behan, Page 8 of 8
You might also like
- Crospovidone EP 10.6 PDFDocument2 pagesCrospovidone EP 10.6 PDFxuan y phanNo ratings yet
- AAS and AES Spectroscopy Exam QuestionsDocument2 pagesAAS and AES Spectroscopy Exam QuestionskeatyNo ratings yet
- SY-8100 Manual OperacionDocument36 pagesSY-8100 Manual OperacionJOse ArmentaNo ratings yet
- TUT 1 Introduction To ChromatographyDocument3 pagesTUT 1 Introduction To Chromatographysikho0ndevuNo ratings yet
- Activity McGuire Chromatographic ResolutionDocument6 pagesActivity McGuire Chromatographic ResolutionArdhi aNo ratings yet
- Answer Any Six Question Including Question No. 1 Which Is CompulsoryDocument2 pagesAnswer Any Six Question Including Question No. 1 Which Is CompulsoryRahul SinghNo ratings yet
- Tutor Chap 4 CHM260Document3 pagesTutor Chap 4 CHM260nathirahjainiNo ratings yet
- CMO 310 Exam 2019Document11 pagesCMO 310 Exam 2019Orlando Wilson Da Sousa MelimNo ratings yet
- Btech 1 Sem Applied Chemistry Pacia101 2018 PDFDocument2 pagesBtech 1 Sem Applied Chemistry Pacia101 2018 PDFNew.T.O.N SethiNo ratings yet
- Problem Set 6 Fall 2018Document3 pagesProblem Set 6 Fall 2018rickNo ratings yet
- NR 320801 Mass Transfer Operations IIDocument6 pagesNR 320801 Mass Transfer Operations IISrinivasa Rao G100% (6)
- Be Winter 2022Document2 pagesBe Winter 2022samip shahNo ratings yet
- 471-526 Exam 3 Fall 2015Document5 pages471-526 Exam 3 Fall 2015ok9275No ratings yet
- A Level Chemistry Paper 2 Exam 28Document3 pagesA Level Chemistry Paper 2 Exam 28Anthony AndyNo ratings yet
- Be Summer 2022Document2 pagesBe Summer 2022samip shahNo ratings yet
- Assignment 2 - Pool of QuestionsDocument7 pagesAssignment 2 - Pool of QuestionsNishant KhandelwalNo ratings yet
- B48BA Exam Questions v1Document18 pagesB48BA Exam Questions v1Manmohan SinghNo ratings yet
- Btech 1 Sem Chemistry Kas 102 2018 19Document2 pagesBtech 1 Sem Chemistry Kas 102 2018 19Viraj RuhelaNo ratings yet
- MT Special Topics (Due Date: Monday, October 16, 2017: 13.00)Document3 pagesMT Special Topics (Due Date: Monday, October 16, 2017: 13.00)Irfan AdityaNo ratings yet
- C) 225 S and 305 S: Chromatographic AnalysesDocument3 pagesC) 225 S and 305 S: Chromatographic AnalysesVikash KushwahaNo ratings yet
- Cy0u10a R Engineering Chemistry Apr 2022Document3 pagesCy0u10a R Engineering Chemistry Apr 2022kangirene9705No ratings yet
- Be Summer 2021Document3 pagesBe Summer 2021samip shahNo ratings yet
- AMI B.Tech NOVEMBER 2010Document5 pagesAMI B.Tech NOVEMBER 2010Thirunavukkarasu ANo ratings yet
- A Level Chemistry Paper 2 Exam 5Document5 pagesA Level Chemistry Paper 2 Exam 5Anthony AndyNo ratings yet
- 2002 ExamsDocument22 pages2002 Examsheshammohamed44148No ratings yet
- 2170501Document3 pages2170501Zoher PainterNo ratings yet
- BT 404 - QUANTITATIVE ANALYSIS IN CHROMATOGRAPHY (Compatibility Mode)Document22 pagesBT 404 - QUANTITATIVE ANALYSIS IN CHROMATOGRAPHY (Compatibility Mode)dsdsdNo ratings yet
- Optional Area Examination Analytical ChemistryDocument4 pagesOptional Area Examination Analytical ChemistryMohamed DahmaneNo ratings yet
- A Level Chemistry Paper 2 Exam 6Document4 pagesA Level Chemistry Paper 2 Exam 6majanga johnNo ratings yet
- Cy0u10a R Engineering Chemistry Oct 2021 1Document2 pagesCy0u10a R Engineering Chemistry Oct 2021 1kangirene9705No ratings yet
- CU-2022 B.Sc. (Honours) Chemistry Semester-6 Paper-DSE-A-4 QPDocument2 pagesCU-2022 B.Sc. (Honours) Chemistry Semester-6 Paper-DSE-A-4 QPsanchita MannaNo ratings yet
- ICH 501-May 2022Document3 pagesICH 501-May 2022Jagadeesh YNo ratings yet
- UCB008Document1 pageUCB008lecev28785No ratings yet
- Gujarat Technological UniversityDocument2 pagesGujarat Technological UniversityTNo ratings yet
- Oc 2023Document8 pagesOc 2023Neha NegiNo ratings yet
- 3rd Sem End Semester - September 2015-FinalDocument6 pages3rd Sem End Semester - September 2015-FinalbgroyNo ratings yet
- CRE-2 Semester PapersDocument12 pagesCRE-2 Semester PapersSarvesh KumarNo ratings yet
- General Chemistry QuestionsDocument3 pagesGeneral Chemistry QuestionsSagar JainNo ratings yet
- Practice Problem Set Mixed Chromatography QuestionsDocument14 pagesPractice Problem Set Mixed Chromatography QuestionsMelinda AndersonNo ratings yet
- Final Theory Exam-307 June2012Document13 pagesFinal Theory Exam-307 June2012Jagadeesh EllilNo ratings yet
- Btech Ice 8 Sem Analytical Instrumentation 2011Document4 pagesBtech Ice 8 Sem Analytical Instrumentation 2011Ahmed MansourNo ratings yet
- CHM580Document8 pagesCHM580Azreen AnisNo ratings yet
- Analog Communication Systems: 08/09 Sem 2 ExamDocument8 pagesAnalog Communication Systems: 08/09 Sem 2 ExamYong JiaNo ratings yet
- Assignment L01 (Thursday, 11.30 Am) Marking SchemeDocument12 pagesAssignment L01 (Thursday, 11.30 Am) Marking SchemeMawareNo ratings yet
- R5312305-Mass Transfer AndseperationDocument4 pagesR5312305-Mass Transfer AndseperationsivabharathamurthyNo ratings yet
- 6519 2011bme 502 (5115) PDFDocument4 pages6519 2011bme 502 (5115) PDFNoor AhmedNo ratings yet
- 6519 2011bme 502 (5115) PDFDocument4 pages6519 2011bme 502 (5115) PDFNoor AhmedNo ratings yet
- National Institute of Technology, Rourkela - 769 008, OrissaDocument1 pageNational Institute of Technology, Rourkela - 769 008, OrissaSubrat kumar SahooNo ratings yet
- rr422301 Chromatographic SeparationsDocument4 pagesrr422301 Chromatographic SeparationsSRINIVASA RAO GANTANo ratings yet
- 2012 Non-Conventional Energy Sources: CS/B.Tech (ME/PE) /SEM-8/ME-806/2012Document7 pages2012 Non-Conventional Energy Sources: CS/B.Tech (ME/PE) /SEM-8/ME-806/2012Krishna Prakash KPNo ratings yet
- Glance Through The Entire Questions and Solve The Easiest Problems FirstDocument4 pagesGlance Through The Entire Questions and Solve The Easiest Problems FirstRutul JainNo ratings yet
- 1pu Chem Midterm QP Bangalore SouthDocument3 pages1pu Chem Midterm QP Bangalore Southredej66556No ratings yet
- Btech CH 6 Sem Chemical Reaction Engineering 2 NCH 601 2016 17Document2 pagesBtech CH 6 Sem Chemical Reaction Engineering 2 NCH 601 2016 17Anmol YadavNo ratings yet
- From Final ExamDocument9 pagesFrom Final ExamThrishnaa BalasupurManiamNo ratings yet
- XI Mid Term QPDocument3 pagesXI Mid Term QPtechnical SiteNo ratings yet
- Test 2, SMJC 2202 - Sec 02Document1 pageTest 2, SMJC 2202 - Sec 02norsiahNo ratings yet
- S16 CPSDDocument4 pagesS16 CPSDGohit BhatNo ratings yet
- Guía de Estudio EspectrosDocument5 pagesGuía de Estudio EspectrosCésar CidNo ratings yet
- WWW - Manaresults.co - In: Set No. 1Document4 pagesWWW - Manaresults.co - In: Set No. 1Sathya Bhuvaneswari KavalaNo ratings yet
- Gujarat Technological UniversityDocument3 pagesGujarat Technological Universityfeyayel990No ratings yet
- Tutorial 1Document7 pagesTutorial 1Harshi ChandraferiNo ratings yet
- School of Engineering & Physical Sciences Chemical EngineeringDocument10 pagesSchool of Engineering & Physical Sciences Chemical EngineeringIdlan IzharNo ratings yet
- Standard and Super-Resolution Bioimaging Data Analysis: A PrimerFrom EverandStandard and Super-Resolution Bioimaging Data Analysis: A PrimerNo ratings yet
- EetdagboekDocument1 pageEetdagboekkeatyNo ratings yet
- Green Chemistry Exam QuestionsDocument1 pageGreen Chemistry Exam QuestionskeatyNo ratings yet
- Surface Chemistry Short QuestionsDocument1 pageSurface Chemistry Short QuestionskeatyNo ratings yet
- 2015 Rekening ExamDocument10 pages2015 Rekening ExamkeatyNo ratings yet
- Catalysis & Catalysts: Facts and Figures About CatalystsDocument88 pagesCatalysis & Catalysts: Facts and Figures About CatalystskeatyNo ratings yet
- Sadettin Ozturk Aug2009 UFRJDocument31 pagesSadettin Ozturk Aug2009 UFRJkeatyNo ratings yet
- Transport of Dangerous GoodsDocument16 pagesTransport of Dangerous Goodskeaty100% (1)
- Answers To Suggested Problems For Chapter 9Document3 pagesAnswers To Suggested Problems For Chapter 9keatyNo ratings yet
- DT261/3, DT299/3: Dr. Andrew KnoxDocument45 pagesDT261/3, DT299/3: Dr. Andrew KnoxkeatyNo ratings yet
- BSC (Medicinal Chemistry and Pharmaceutical Sciences) : Section A Is Compulsory Answer One Question From Section B andDocument4 pagesBSC (Medicinal Chemistry and Pharmaceutical Sciences) : Section A Is Compulsory Answer One Question From Section B andkeatyNo ratings yet
- CHEM3218 P.Behan-3Document66 pagesCHEM3218 P.Behan-3keatyNo ratings yet
- CHEM 3210 Review Questions 2015Document3 pagesCHEM 3210 Review Questions 2015keatyNo ratings yet
- Separation Processss Lecture NotesDocument17 pagesSeparation Processss Lecture NoteskeatyNo ratings yet
- CHEM 3210 - Sample Numerical Problems and SolutionsDocument2 pagesCHEM 3210 - Sample Numerical Problems and SolutionskeatyNo ratings yet
- BSC (Medicinal Chemistry and Pharmaceutical Sciences) : Section A Is Compulsory Answer One Question From Section B andDocument4 pagesBSC (Medicinal Chemistry and Pharmaceutical Sciences) : Section A Is Compulsory Answer One Question From Section B andkeatyNo ratings yet
- Assignment1. Time Managment ExerciseDocument2 pagesAssignment1. Time Managment ExercisekeatyNo ratings yet
- Chemical Technology LC 2 NotesDocument5 pagesChemical Technology LC 2 NoteskeatyNo ratings yet
- Notes 3 Material Balance CalculationsDocument21 pagesNotes 3 Material Balance CalculationskeatyNo ratings yet
- Chem & Phram Proc Technology LC 1 NotesDocument10 pagesChem & Phram Proc Technology LC 1 NoteskeatyNo ratings yet
- Chem Proc LC 4 NotesDocument11 pagesChem Proc LC 4 NoteskeatyNo ratings yet
- Selected Answers For Exercises: Product KG Waste KG EDocument7 pagesSelected Answers For Exercises: Product KG Waste KG EkeatyNo ratings yet
- Quantification of Piperine in Different Varieties of Piper Nigrum by A Validated High Performance Thin Layer Chromatography Densitometry MethodDocument10 pagesQuantification of Piperine in Different Varieties of Piper Nigrum by A Validated High Performance Thin Layer Chromatography Densitometry MethodArtem KulikovNo ratings yet
- Ep LisinoprilDocument2 pagesEp LisinoprillopebutetNo ratings yet
- Method 515.3 Determination of Chlorinated Acids in DrinkingDocument56 pagesMethod 515.3 Determination of Chlorinated Acids in DrinkingPedro FrancoNo ratings yet
- Method For The Determination of Beta Carotene in Supplements and Raw Materials by Reversed Phase Liquid Chromatography Single Laboratory ValidationDocument13 pagesMethod For The Determination of Beta Carotene in Supplements and Raw Materials by Reversed Phase Liquid Chromatography Single Laboratory ValidationChris JohnsonNo ratings yet
- 1511152635981Document14 pages1511152635981Lolo OmarNo ratings yet
- HPTLC Paper of Berberis PDFDocument5 pagesHPTLC Paper of Berberis PDFAnisahMahardianiNo ratings yet
- 6522-An156 LPN1548Document6 pages6522-An156 LPN1548anushka chadhaNo ratings yet
- Null 2Document30 pagesNull 2Sara EltayiebNo ratings yet
- Homelabmanual204 PDFDocument35 pagesHomelabmanual204 PDFMaii AlaarajNo ratings yet
- D 5739 - 00 Rdu3mzkDocument13 pagesD 5739 - 00 Rdu3mzkAnonymous xMQd4zNo ratings yet
- Radial Chromatography For The Separation of Nitroaniline IsomersDocument3 pagesRadial Chromatography For The Separation of Nitroaniline IsomersAndrianNo ratings yet
- Isoflavone 2Document17 pagesIsoflavone 2Elis ApriyantiNo ratings yet
- Manual CromatografoDocument10 pagesManual CromatografoVerónicaJiménezOlmedoNo ratings yet
- Pharmasist LoksewaDocument5 pagesPharmasist LoksewaAashish BhattaraiNo ratings yet
- Instant Download Ebook PDF Encyclopedia of Analytical Science 3rd Edition PDF ScribdDocument41 pagesInstant Download Ebook PDF Encyclopedia of Analytical Science 3rd Edition PDF Scribdhoward.linkovich475100% (61)
- 00 - Gavilanes (1982) Int J Peptide Protein Res - Pigeon Egg White LysozymeDocument8 pages00 - Gavilanes (1982) Int J Peptide Protein Res - Pigeon Egg White Lysozyme1810mNo ratings yet
- T.Y.B.Sc. Chemistry (6 Units) : Choice Based Credit SystemDocument12 pagesT.Y.B.Sc. Chemistry (6 Units) : Choice Based Credit SystempratikNo ratings yet
- Approval Sheet: Mutmainnah Umar Rahmatia ID. 1513440003 ID. 1613040013Document15 pagesApproval Sheet: Mutmainnah Umar Rahmatia ID. 1513440003 ID. 1613040013rulmadhaniNo ratings yet
- BiotechnologyDocument21 pagesBiotechnologyShivangi Mishra50% (2)
- Separation of Ink Mixture Using Paper Chromatography TechniqueDocument2 pagesSeparation of Ink Mixture Using Paper Chromatography TechniqueSevar AbdullahNo ratings yet
- Name of The Experiment: Fatty Acid Analysis by Using Gas ChromatographyDocument2 pagesName of The Experiment: Fatty Acid Analysis by Using Gas ChromatographyWayMeen PangNo ratings yet
- Diclofenac Injection: TestsDocument2 pagesDiclofenac Injection: TestsMuhammad ImranNo ratings yet
- CH12 - GOC - Shobhit NirwanDocument61 pagesCH12 - GOC - Shobhit NirwanRao GootleyNo ratings yet
- Isolasi Dan Identifikasi Senyawa Flavonoid Dari Daun Jamblang Syzygium Cumini PeDocument11 pagesIsolasi Dan Identifikasi Senyawa Flavonoid Dari Daun Jamblang Syzygium Cumini PeMuhammad Rifqi FauziNo ratings yet
- Ascorbic AcidDocument2 pagesAscorbic AcidMulayam Singh YadavNo ratings yet
- Diethyl PhthalateDocument2 pagesDiethyl PhthalateMulayam Singh YadavNo ratings yet
- Tech 707Document2 pagesTech 707sadaf_5No ratings yet