Professional Documents
Culture Documents
Chapter Solutions 14
Chapter Solutions 14
Uploaded by
luizdrOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chapter Solutions 14
Chapter Solutions 14
Uploaded by
luizdrCopyright:
Available Formats
Patrick, An Introduction to Medicinal Chemistry 5e
Chapter 14 – Drug design: optimizing access to the target
Answers to end-of-chapter questions
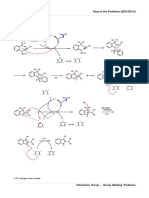
1) The mechanism below shows the release of one molecule of formaldehyde from
methenamine. The mechanism can then be repeated to release a further five molecules
of formaldehyde. Four molecules of ammonia will eventually be formed as well.
Since acid is present, the ammonia molecules would be protonated and form an
ammonium salt.
H
H H H
H H H
O H
N N N O N O
H
N N N
N -H
N N N N
N H
N N N
H H H
N O N
O
N H N H
+ C
N N H H
N N
2)
Ph O
Me
H
H2N HN O N Me
S Me S Me S
-H +H H OH
O N C
N Me N Me N
O Ph N O
H O H
C OH C OH Ph N O
O O H
3) The transport proteins that carry levodopa across the blood brain barrier are present
in order to transport essential amino acids. Levodopa has a normal head group for an
amino acid and is accepted as such. Carbidopa does not have the normal head group.
It has a hydrazine group in place of am amino group. There is also a methyl group at
the -carbon instead of hydrogen. As a result, it is not accepted by the transport
proteins.
© Oxford University Press, 2013. All rights reserved.
Patrick, An Introduction to Medicinal Chemistry 5e
Chapter 14 – Drug design: optimizing access to the target
Amino acid
head group
HO CO2H HO CO2H
H NHNH2
NH2 Me
HO HO
Levodopa Carbidopa
4) Electronic modifications could be carried out to stabilise the ester group. For
example, the ester could be replaced with an amide or a urethane group.
O CH3 O CH3
CH3 O CH3
CH3 CH3
N N
H3C O CH3 N
H3C N CH3 H2N O CH3
H
Acetylcholine
Steric shields could be added to hinder the access of nucleophiles or water to the
carbonyl group.
O CH3 O CH3 CH3 O CH3
CH3 CH3 CH3
N N H3C N
H3C O CH3 H3C O CH3 O CH3
Acetylcholine CH3
A combination of both tactics could be used e.g.
O CH3
O CH3 CH3 CH3
CH3 H3C N
N N CH3
H2N O CH3 H
CH3
Structures such as these should be more stable, but it is important to consider whether
the changes made will affect the binding interactions of the compound. There is little
point in designing a stable analogue of acetylcholine if it cannot fit the receptor
binding site (see also section 22.7).
5) A metabolic reaction is required which would either neutralise the positive charges
on the nitrogen or split the molecule such that both charges are no longer present on
the same structure. One way of splitting the molecule in two would be to introduce an
ester into the alkyl chain. It is well known that esters are easily hydrolysed by
esterases. The following structures are examples of molecules which would be worth
investigating (see also section 22.10.2.2).
© Oxford University Press, 2013. All rights reserved.
Patrick, An Introduction to Medicinal Chemistry 5e
Chapter 14 – Drug design: optimizing access to the target
Me Me O
Me Me
N O Me N O Me
Me N Me O N
Me Me
O Me O Me
6) Miotine, as shown in the diagram, has a positive charge and should not be able to
cross the blood brain barrier. However, it is possible for the molecule to lose a proton
to form the free base and this structure can cross the blood brain barrier to produce the
CNS effects observed.
Me Me
MeHN O -H MeHN O
NHMe2 NMe2
O O
+H
Miotine (ionised) Miotine (free base)
In order to prevent this happening, the amine could be alkylated to form a quaternary
structure. This cannot lose its positive charge and cannot cross the blood brain barrier
(see also section 22.13.1.2).
Me
MeHN O Me
N
Me
O Me
7) Aciclovir contains several polar functional groups that can participate in hydrogen
bonding. However, polar groups can hinder the passage of a drug across the fatty cell
membranes of the cells lining the gut wall.
HBA
O
HBA
HBD N
HN
HBD
H2N N N
HBA
HBD O
HO
HBA
HBA
One way of solving this problem would be to mask a polar group such that the
molecule is less polar and can cross cell membranes more easily. The group used to
mask the polar group would have to be easily removed by a metabolic reaction in
order to release the active drug once it has been absorbed. There are two accessible
functional groups which could be masked - the primary amine or the alcohol. One
could mask the amine as an amide or the alcohol as an ester. Masking the alcohol is
© Oxford University Press, 2013. All rights reserved.
Patrick, An Introduction to Medicinal Chemistry 5e
Chapter 14 – Drug design: optimizing access to the target
the better option since it is well known that esters are easily cleaved by esterases in
the blood. Esters are also more susceptible to hydrolysis than amides. A variety of
ester prodrugs could be tried out to find the best. It is also worth considering what will
be released from the drug when the ester is hydrolysed. Preferably, this should be a
naturally occurring chemical such as an amino acid (see also section 20.6.1).
O O
N N
HN HN
amine
H2N N N H2N N N
O
alcohol O O
HO R O
Ester prodrug
8) The following diagram shows where metabolism occurs on this molecule. To
prevent this from happening, metabolic blockers could be added to the para positions.
Halogen atoms are often used for this purpose, but SAR results demonstrate that
aromatic substituents are bad for activity. This may be a steric problem if the aromatic
rings are fitting into small hydrophobic pockets in the binding site. Therefore, one
should add fluoro substituents since fluorine is comparable in size with hydrogen.
H H
N N
O O O O
metabolism metabolism
F F
N N N N
H H H H
9) The most likely explanation is that the two analogues lack a group that is easily
metabolised, resulting in excessively long half lives. The methyl group of celecoxib is
susceptible to oxidation which would result in a polar group such as a carboxylic acid.
This could undergo a conjugation reaction resulting in a polar metabolite which would
be rapidly excreted.
10) There are five main positions which are particularly susceptible to metabolism.
More than one metabolic reaction may occur on the one molecule and it has been
predicted that there are over 40 possible metabolites that could be formed based on
these reactions alone.
© Oxford University Press, 2013. All rights reserved.
Patrick, An Introduction to Medicinal Chemistry 5e
Chapter 14 – Drug design: optimizing access to the target
Demethylation
Oxidation OMe
N
O
Oxidation
Ring
SCH 48461 opening OMe
Demethylation
Oxidation of the aromatic ring at the para position could be blocked by introducing a
fluoro-substituent. Demethylation could be prevented by changing the methyl group to
a different alkyl group, or removing the methoxy group entirely. Benzylic oxidation
could be blocked by introducing a substituent at that position to act as a steric shield.
Another possibility might be to place ortho substituents on the aromatic ring to act as
steric shields. The 4-membered lactam ring is susceptible to hydrolysis. Substituents
on the aromatic ring linked to the lactam nitrogen might act as steric shields and help
to make the ring less susceptible to hydrolysis.
It should be noted that metabolic reactions are not always detrimental to activity.
Some may even improve activity. For example, benzylic oxidation and demethylation
of one of the methoxy groups have been shown to be advantageous to activity. Such
studies led to the design of the following structure which has improved activity. Note
that two of the possible metabolic reactions have been blocked and that two groups
introduced by metabolic reactions have been added because they are beneficial to
activity.
© Oxford University Press, 2013. All rights reserved.
Patrick, An Introduction to Medicinal Chemistry 5e
Chapter 14 – Drug design: optimizing access to the target
Methoxy group
replaced with more
Beneficial active phenol
alcohol
introduced
OH
OH
N
F
O
Oxidation
blocked Ring
opening F
Methoxy group removed
Oxidation blocked
© Oxford University Press, 2013. All rights reserved.
You might also like
- Answers To Chapter 5: (In-Text & Asterisked Problems)Document10 pagesAnswers To Chapter 5: (In-Text & Asterisked Problems)平野健太朗No ratings yet
- 001 Introduction To Medicine - Microbiology - Virology 6 7 2023Document57 pages001 Introduction To Medicine - Microbiology - Virology 6 7 2023Yawen MekuriaNo ratings yet
- Lecture Notes Chem 51A S. King: O H OH H HO HO H H H CH OHDocument41 pagesLecture Notes Chem 51A S. King: O H OH H HO HO H H H CH OHTamiaNo ratings yet
- New StrcuresDocument5 pagesNew StrcuresYogesh SuryawanshiNo ratings yet
- Synthetic Approaches To The 2010-2014 New AgrochemicalsDocument65 pagesSynthetic Approaches To The 2010-2014 New AgrochemicalsprashantNo ratings yet
- GattacaDocument22 pagesGattacatimletran1234No ratings yet
- Aurora Kinase Inhibitors As Anticancer AgentsDocument17 pagesAurora Kinase Inhibitors As Anticancer Agentsrohitgautam25No ratings yet
- X910 - ALBiology - Assignment - 01 V2Document14 pagesX910 - ALBiology - Assignment - 01 V2Mikila GittensNo ratings yet
- Tetrodotoxin: TTX: BackgroundDocument12 pagesTetrodotoxin: TTX: BackgroundMarrauNo ratings yet
- Only MethodologyDocument6 pagesOnly MethodologySidu BhosleNo ratings yet
- Pro. Dr. Muhammad Ashraf Chairman Department of Pharmacology and Toxicology, UVAS, LahoreDocument29 pagesPro. Dr. Muhammad Ashraf Chairman Department of Pharmacology and Toxicology, UVAS, LahoreMuhammad Shahid BilalNo ratings yet
- Antibiotik Betha LaktamDocument18 pagesAntibiotik Betha LaktamdmujahidinNo ratings yet
- 4-5 Biokimia Bahan Hayati LautDocument33 pages4-5 Biokimia Bahan Hayati LautSofyan ZenNo ratings yet
- 2023-03-24 Printed As PDF Presi Pt09Document2 pages2023-03-24 Printed As PDF Presi Pt09sauronsauronNo ratings yet
- Nucleac Acid Mid Bio IIDocument47 pagesNucleac Acid Mid Bio IIseada JemalNo ratings yet
- Problem Set 2Document1 pageProblem Set 2aNo ratings yet
- ArunimaDocument21 pagesArunimaarunimabiswas90No ratings yet
- (Aqa A Level Science) Teresa Quigg - Aqa A Level Chemistry Studentbook 2 (Aqa A Level Science) - Hodder Education (2015)Document3 pages(Aqa A Level Science) Teresa Quigg - Aqa A Level Chemistry Studentbook 2 (Aqa A Level Science) - Hodder Education (2015)Dr kamruzzaman EyeNo ratings yet
- Heterocycle AnswersDocument6 pagesHeterocycle AnswersChris WongNo ratings yet
- 2023-03-24 Printed As PDF Presi Pt04Document2 pages2023-03-24 Printed As PDF Presi Pt04sauronsauronNo ratings yet
- 1Document2 pages1Stien DwinyNo ratings yet
- DNA ReplicationDocument20 pagesDNA ReplicationFrie An PanteNo ratings yet
- MBMB 1236C Lecture 04 Purines and Pyrimidines DegradationDocument13 pagesMBMB 1236C Lecture 04 Purines and Pyrimidines DegradationLarry TongNo ratings yet
- DNA PPSXDocument19 pagesDNA PPSXTintin Brusola SalenNo ratings yet
- Biology 30 Molecular Genetics Notes Review 2021Document15 pagesBiology 30 Molecular Genetics Notes Review 2021sherlynmamac98No ratings yet
- Seiple Oct 06Document13 pagesSeiple Oct 06Sơn Nguyễn KimNo ratings yet
- 2023-03-24 Printed As PDF Presi Pt07Document2 pages2023-03-24 Printed As PDF Presi Pt07sauronsauronNo ratings yet
- Unit 3 QuestionsDocument18 pagesUnit 3 QuestionsLynd TaylorNo ratings yet
- Ejercicos Piridinas 2Document3 pagesEjercicos Piridinas 2Sebastian Codoceo AlfaroNo ratings yet
- Polyamide Selection-NylonDocument24 pagesPolyamide Selection-NylonShivaranjani Kuruparan100% (1)
- Diels-Alder ExperimentDocument7 pagesDiels-Alder ExperimentSimranjit Kaur100% (3)
- Allantoin BenzocaineDocument1 pageAllantoin BenzocaineDea PertiwiNo ratings yet
- Practical FCHG412 Prodrugs: Module Outcomes For The PracticalDocument14 pagesPractical FCHG412 Prodrugs: Module Outcomes For The PracticalGreg RalphNo ratings yet
- 2023-03-24 Printed As PDF Presi Pt03Document2 pages2023-03-24 Printed As PDF Presi Pt03sauronsauronNo ratings yet
- Opoid AnalgesicsDocument48 pagesOpoid AnalgesicsPratik KhanalNo ratings yet
- Biochemistry (Digestion) (269-272)Document4 pagesBiochemistry (Digestion) (269-272)Handika SaputraNo ratings yet
- Patrick Ch22 p3Document12 pagesPatrick Ch22 p3Oxy GenNo ratings yet
- Formation of A Polynucleotide: A Condensation Reaction Takes PlaceDocument3 pagesFormation of A Polynucleotide: A Condensation Reaction Takes PlaceKate TaylorNo ratings yet
- Rumus Gugus KimiaDocument1 pageRumus Gugus KimiaBagus WijayaNo ratings yet
- Antineoplastics 22Document50 pagesAntineoplastics 22ahmed montaserNo ratings yet
- The β - D Glucose Scaffold as a β- Turn Mimetic: Saidulu DaraDocument19 pagesThe β - D Glucose Scaffold as a β- Turn Mimetic: Saidulu Daraglreddy09No ratings yet
- Sintesis de IndolesDocument14 pagesSintesis de IndolesLili AmadorNo ratings yet
- Total Synthesis - 2 CDXDocument6 pagesTotal Synthesis - 2 CDXNguyễn Đức DuyNo ratings yet
- NH HN NH O HO O OH Exact Mass: 1198,62 Molecular Weight: 1199,38Document1 pageNH HN NH O HO O OH Exact Mass: 1198,62 Molecular Weight: 1199,38Rigel_TNo ratings yet
- Figure 1-NITROSAMINES - CONTAMINATIONDocument1 pageFigure 1-NITROSAMINES - CONTAMINATIONد. وليد الشريفNo ratings yet
- 2006-CHM6108 - L5L6 SlidesDocument52 pages2006-CHM6108 - L5L6 Slidesaidar.seralinNo ratings yet
- Anti Cancer DrugDocument37 pagesAnti Cancer DrugUmashankar SharmaNo ratings yet
- Chemistry SynopsisDocument7 pagesChemistry SynopsisDr Prashant Shihora100% (1)
- 2006-CHM6108 - L5L6 HandoutDocument9 pages2006-CHM6108 - L5L6 Handoutaidar.seralinNo ratings yet
- Basics of Human BiologyDocument21 pagesBasics of Human Biologysarthak doomraNo ratings yet
- Gutekunst Jun 09 PDFDocument13 pagesGutekunst Jun 09 PDFMadame MoseilleNo ratings yet
- Proline PDFDocument52 pagesProline PDFRathinNo ratings yet
- Chemistry NoteDocument27 pagesChemistry NoteUmar M abubakarNo ratings yet
- Repaglinide: Chemical Name: (S) - (Document4 pagesRepaglinide: Chemical Name: (S) - (Rhenso Victor Albites CondoriNo ratings yet
- Keys To The Problems (2021/02/13) : Blue LEDDocument4 pagesKeys To The Problems (2021/02/13) : Blue LEDHuân TrầnNo ratings yet
- Organ Sharing WRDocument3 pagesOrgan Sharing WRDivakar DhandeNo ratings yet
- Single-Molecule Visualization of DNA G-Quadruplex Formation in Live CellsDocument20 pagesSingle-Molecule Visualization of DNA G-Quadruplex Formation in Live CellsDharanibalan PNo ratings yet
- 10 1039@c9pp00182dDocument10 pages10 1039@c9pp00182dluizdrNo ratings yet
- Intraligand Lowest Excited States in Tricarbon Ylhalobis (Styrylpyridine) Rhenium (ComplexesDocument7 pagesIntraligand Lowest Excited States in Tricarbon Ylhalobis (Styrylpyridine) Rhenium (ComplexesluizdrNo ratings yet
- The Nature T Ricarbonylchloro-1,10-Phenanthrolinerhenium (: Lowest Excited inDocument6 pagesThe Nature T Ricarbonylchloro-1,10-Phenanthrolinerhenium (: Lowest Excited inluizdrNo ratings yet
- An Amine Linker Group Modulates Luminescent Properties in A Rhenium (I)Document9 pagesAn Amine Linker Group Modulates Luminescent Properties in A Rhenium (I)luizdrNo ratings yet