Professional Documents
Culture Documents
06 Handout 1
06 Handout 1
Uploaded by
kjfhghjfggjfCopyright:
Available Formats
You might also like
- Chapter 1-Lesson 1Document4 pagesChapter 1-Lesson 1Bùi Hữu ĐứcNo ratings yet
- Chem U 2 G 11Document23 pagesChem U 2 G 11EApp And AgreementNo ratings yet
- Mahasiswa Mampu Menjelaskan Konsep Kuantisasi Energi: Kompetensi 1Document43 pagesMahasiswa Mampu Menjelaskan Konsep Kuantisasi Energi: Kompetensi 1Imam SyNo ratings yet
- 5) Quantum Mechanics - UpdatedDocument86 pages5) Quantum Mechanics - UpdatedGame 1No ratings yet
- VSS Lesson 1 Early Atomic Theories Chemistry 12Document11 pagesVSS Lesson 1 Early Atomic Theories Chemistry 12Jaideep GillNo ratings yet
- AP Chemistry: Chapter 7 - Atomic Structure & PeriodicityDocument14 pagesAP Chemistry: Chapter 7 - Atomic Structure & PeriodicityS. Green100% (1)
- Written ReportDocument3 pagesWritten ReportFatima Kent DelfinNo ratings yet
- 4) Quantum MechanicsDocument75 pages4) Quantum MechanicsGame 1No ratings yet
- EP - UNIT 2 - Quantum MechanicsDocument34 pagesEP - UNIT 2 - Quantum Mechanicsthetop10nerdNo ratings yet
- Chemistry Group 1 AssignmentDocument29 pagesChemistry Group 1 AssignmentTizu GetachewNo ratings yet
- New Microsoft Word DocumentDocument9 pagesNew Microsoft Word DocumentAritra SahaNo ratings yet
- Electromagnetic Waves, Planck's Quantum Theory, and Photoelectric EffectDocument50 pagesElectromagnetic Waves, Planck's Quantum Theory, and Photoelectric EffectElaine MataNo ratings yet
- Quantum Mechanics - Britannica Online Encyclopedia PDFDocument29 pagesQuantum Mechanics - Britannica Online Encyclopedia PDFMahesh MaheshwariNo ratings yet
- Notes - Quantum Theory of Light and Matter WavesDocument34 pagesNotes - Quantum Theory of Light and Matter Wavesphysics olympiad100% (1)
- Chapter Four: The Electronic Structure of AtomsDocument50 pagesChapter Four: The Electronic Structure of AtomsmajedmajedmajedNo ratings yet
- Electromagnetic WavesDocument7 pagesElectromagnetic Wavesmary ann leddaNo ratings yet
- Dhselect 5Document9 pagesDhselect 5Biswanath Gouda (Biswanath)No ratings yet
- Moderm PhysicsDocument39 pagesModerm Physicsgul zarrinNo ratings yet
- Ecture O.: (The Electronic Structure of Atoms)Document43 pagesEcture O.: (The Electronic Structure of Atoms)Yarisse RivasNo ratings yet
- Nature of Electromagnetic WavesDocument24 pagesNature of Electromagnetic WavesEivol Kimoy ÖNo ratings yet
- AP Physics Chapter 27 Quantum PhysicsDocument78 pagesAP Physics Chapter 27 Quantum PhysicsMythos EntertainmentNo ratings yet
- Unit - I - Quantum Mechanics PhysicsDocument34 pagesUnit - I - Quantum Mechanics Physicsabisheik942No ratings yet
- L1 Nature LightDocument3 pagesL1 Nature Lighttrs smritiNo ratings yet
- Chapter 7 Atomic Structure and PreriodicityDocument87 pagesChapter 7 Atomic Structure and PreriodicitydeemahhwNo ratings yet
- Light As An Electromagnetic WaveDocument11 pagesLight As An Electromagnetic WavejehonieeeNo ratings yet
- Bab 2 Blackbody, PhotoelectricDocument35 pagesBab 2 Blackbody, PhotoelectricFakrurazi NajmiNo ratings yet
- Electronic Structure of AtomsDocument59 pagesElectronic Structure of Atomstalktotiffanycheng100% (1)
- Class #02 - Units, Scale, and The Mole: For Test #1 On ThursdayDocument11 pagesClass #02 - Units, Scale, and The Mole: For Test #1 On ThursdayLina ChitadzeNo ratings yet
- Chapter Five (2) - 2Document68 pagesChapter Five (2) - 2Wengelu evanNo ratings yet
- Che101 Chap 7Document47 pagesChe101 Chap 7David MaranzhyanNo ratings yet
- Chapter Two. EEE & ETI 1204 Material ScienceDocument29 pagesChapter Two. EEE & ETI 1204 Material ScienceKIRAGU PURITY NJERINo ratings yet
- Chemistry 1103: Dr. Jhon Zapata RiveraDocument37 pagesChemistry 1103: Dr. Jhon Zapata RiveraJuliRoGamer YtNo ratings yet
- State When The Emission Spectrum of Hydrogen Was Discovered. Outline The Apparatus and Findings of This ExperimentDocument56 pagesState When The Emission Spectrum of Hydrogen Was Discovered. Outline The Apparatus and Findings of This ExperimentMaryFifiNo ratings yet
- Chem Bonding 1Document66 pagesChem Bonding 1Nikitha AkulaNo ratings yet
- Quantum Mechanics Notes-Part 1Document15 pagesQuantum Mechanics Notes-Part 1aman bhatiaNo ratings yet
- PPT1 and 2Document43 pagesPPT1 and 2Adugnaw BiksNo ratings yet
- GeneralChemistry 1Document50 pagesGeneralChemistry 1peter168205No ratings yet
- Wave Properties of ParticleDocument35 pagesWave Properties of ParticleAnnida ChairatunisaNo ratings yet
- ProjectDocument3 pagesProjectapi-285213472No ratings yet
- Lecture 7Document30 pagesLecture 7Ahmad HassanNo ratings yet
- Atomic StructureDocument49 pagesAtomic StructureFatimaNo ratings yet
- JotterPad - JotterPadDocument21 pagesJotterPad - JotterPadOlajide HeritageNo ratings yet
- Quanta To QuarksDocument32 pagesQuanta To QuarksDaniel Bu100% (5)
- 02 Quantum Theory and Atomic StructuresDocument20 pages02 Quantum Theory and Atomic StructuresChrissa GuicoNo ratings yet
- Quantum Theory & Atomic StructureDocument24 pagesQuantum Theory & Atomic Structurevrejie46No ratings yet
- The Duality of Matter and WavesDocument7 pagesThe Duality of Matter and Wavesbsantrich1760No ratings yet
- B068 PDFDocument17 pagesB068 PDFprashanthNo ratings yet
- MITchem Lec 4Document7 pagesMITchem Lec 4maggiesszzNo ratings yet
- CHM012 - Module 3 (Part 2)Document16 pagesCHM012 - Module 3 (Part 2)haibaalisa00No ratings yet
- Modern Physics 3 PDFDocument38 pagesModern Physics 3 PDFDodi SaputraNo ratings yet
- Week 1Document4 pagesWeek 1jia aganaNo ratings yet
- String TheoryDocument39 pagesString Theoryantarikshsaxena100% (2)
- QuantumDocument388 pagesQuantumyana33No ratings yet
- Class 11 Chemistry Chapter 2 Structure of AtomDocument15 pagesClass 11 Chemistry Chapter 2 Structure of Atomgokul100% (1)
- R22 - Unit-I, 2 Quantum Mechanics and SemiconductorsDocument309 pagesR22 - Unit-I, 2 Quantum Mechanics and Semiconductorsnareshreddyy1984No ratings yet
- Structure of Atom Part IDocument75 pagesStructure of Atom Part IShreyansh SinghNo ratings yet
- 2021 Structure of AtomDocument13 pages2021 Structure of AtomSora RoseNo ratings yet
- Quantum MechanicsDocument35 pagesQuantum MechanicsJonNo ratings yet
- Handout 02Document10 pagesHandout 02JAMESNo ratings yet
- The Enigmatic Electron: Electron Behaviour and How It Influences Our LivesFrom EverandThe Enigmatic Electron: Electron Behaviour and How It Influences Our LivesNo ratings yet
- Elephant's Toothpaste : Presented To Mr. Jefferson R. Borillo Lpt. Physics Teacher/AdviserDocument13 pagesElephant's Toothpaste : Presented To Mr. Jefferson R. Borillo Lpt. Physics Teacher/AdviserkjfhghjfggjfNo ratings yet
- LESSON 33: Elephant Toothpaste: - Description - MaterialsDocument13 pagesLESSON 33: Elephant Toothpaste: - Description - MaterialskjfhghjfggjfNo ratings yet
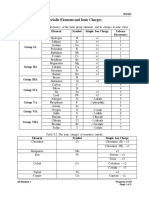
- Periodic Elements and Ionic ChargesDocument2 pagesPeriodic Elements and Ionic ChargeskjfhghjfggjfNo ratings yet
- Elemental Properties and Periodic TrendsDocument1 pageElemental Properties and Periodic TrendskjfhghjfggjfNo ratings yet
- XII PhysicsDocument55 pagesXII PhysicsCharu BhanotNo ratings yet
- Assignment em WavesDocument2 pagesAssignment em WavesCHANDERBATISH MOHANNo ratings yet
- Satellite Antenna TestingDocument47 pagesSatellite Antenna Testingsailee_gharatNo ratings yet
- NEET Physics WeightageDocument4 pagesNEET Physics WeightagevishNo ratings yet
- Phy101 Assignment No. 2Document3 pagesPhy101 Assignment No. 2Asim Ihsan (Apna)No ratings yet
- User Manual OCTOPUS-OFFICE SHIP MOTION ANALYSIS SOFTWAREDocument69 pagesUser Manual OCTOPUS-OFFICE SHIP MOTION ANALYSIS SOFTWAREMiruna ClinciuNo ratings yet
- Practice Set 1Document1 pagePractice Set 1Lalit Ranjan SahuNo ratings yet
- List of Scientific Instruments & Their Uses PDF For UPSC, Banking & SSC ExamsDocument8 pagesList of Scientific Instruments & Their Uses PDF For UPSC, Banking & SSC ExamsYashNo ratings yet
- Advanced Well-Log Analysis in Geothermal PDFDocument4 pagesAdvanced Well-Log Analysis in Geothermal PDFEdyson DeniltonyNo ratings yet
- Otc 18587 MS PDocument15 pagesOtc 18587 MS PSiul Otrebla AtelavazNo ratings yet
- PHYSICAL SCIENCE MODULE 15-EditedDocument24 pagesPHYSICAL SCIENCE MODULE 15-EditedLove Joy JumawanNo ratings yet
- Light Answer Key: Red, Orange, Yellow, Green, Blue, Indigo, VioletDocument4 pagesLight Answer Key: Red, Orange, Yellow, Green, Blue, Indigo, VioletHayiath QureshiNo ratings yet
- Engineering Physics Notes - 2Document117 pagesEngineering Physics Notes - 2Maaran APECNo ratings yet
- BBC Engineering Training Manual - Microphones (Robertson)Document380 pagesBBC Engineering Training Manual - Microphones (Robertson)Nino Rios100% (1)
- B.Tech - Civil-SyllabusDocument126 pagesB.Tech - Civil-SyllabusSrinivas JupalliNo ratings yet
- Iit/Aiims 2020-Screeningcum Scholarship Exam: Date: 00 October 2018Document11 pagesIit/Aiims 2020-Screeningcum Scholarship Exam: Date: 00 October 2018LukewarmNo ratings yet
- Zenith Tutorials Phy 102 PDF SeriesDocument24 pagesZenith Tutorials Phy 102 PDF SeriesPhake CodedNo ratings yet
- Polsarpro TutorialDocument173 pagesPolsarpro Tutorialapi-3854701No ratings yet
- IR SpectrometryDocument46 pagesIR Spectrometryortizan8No ratings yet
- Wave Motion PDFDocument23 pagesWave Motion PDFFRANCES VISAYA100% (1)
- A Detailed Lesson Plan in Science 4 - SOUNDSDocument5 pagesA Detailed Lesson Plan in Science 4 - SOUNDSLourdes ManansalaNo ratings yet
- Building Utilities 3 - Acoustics and Lighting SystemsDocument2 pagesBuilding Utilities 3 - Acoustics and Lighting SystemsNICOLE ADRIENNE RIVERANo ratings yet
- 1000 Electromagnetic Theory MCQsDocument369 pages1000 Electromagnetic Theory MCQskibrom atsbha50% (14)
- ALi Physics Notes 10thDocument27 pagesALi Physics Notes 10thKhawajaAbdulmoeed gamerNo ratings yet
- Jee Main and Advanced Nurture Detailed SyllabusDocument1 pageJee Main and Advanced Nurture Detailed SyllabusDivyansh SinghNo ratings yet
- TOFD PresentationDocument29 pagesTOFD Presentationnirmalmthp100% (6)
- EXPERIMENTDocument2 pagesEXPERIMENTGracilla ElmidoNo ratings yet
- Content Overview: Candidates For Cambridge International AS Level Physics Study The Following TopicsDocument11 pagesContent Overview: Candidates For Cambridge International AS Level Physics Study The Following TopicsAreeba EjazNo ratings yet
- SMAS - Paper - Negative - Stiffness HoneycombDocument23 pagesSMAS - Paper - Negative - Stiffness Honeycombraniya.zouaghiNo ratings yet
- Beat Phenomenon: O, RespectivelyDocument5 pagesBeat Phenomenon: O, RespectivelyPradip GuptaNo ratings yet
06 Handout 1
06 Handout 1
Uploaded by
kjfhghjfggjfOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
06 Handout 1
06 Handout 1
Uploaded by
kjfhghjfggjfCopyright:
Available Formats
SH1684
Quantum Mechanical Model of Atom
"We must be clear that when it comes to atoms, language can only be used as in poetry." —Niels Bohr
The key scientists to consider for this lesson are:
1. Sir Joseph John Thomson – Known for his early research studies in atomic structure, the
Plum-Pudding Atomic Model.
2. Ernest Rutherford – Discovered the nucleus of the atom and developed the planetary
model of the first structure of the atom.
3. Erwin Schrödinger – Developed the equations that define the wave-like traits of atomic
behavior as deduced from spectral analysis.
4. Werner Heisenberg – Devised the uncertainty principle which defined mathematica lly
that any measurement of a particle's position and momentum cannot be determined
concurrently. Matter can either be described as a wave or a particle, but not both
simultaneously.
5. Niels Bohr – Perceived the problem with Rutherford's planetary model of the atom, and
used Planck's quantum theory to describe the phenomenon of electron movement. He used
Heisenberg's uncertainty principle to redefine further electron motion in the atom. His
interpretation is called "complementarity," as it used the mutually exclusive concepts of
wave particle to reach the maximum possible understanding of the quantum model of the
atomic theory.
6. Max Planck – Able to deduce the relationship between the energy and the frequency of
radiation.
7. Albert Einstein – Responsible for the theory of relativity which redefined mass, energy,
motion, space, and time. When this theory was joined with theories and knowledge of the
atom, a new age in chemistry and physics dawned.
General Properties of all electromagnetic radiation:
1. Electromagnetic radiation can travel through empty space. Most other types of waves must
travel through some sort of substance.
2. The speed of light is always a constant. (Speed of light: 2.99792458 x 108 m/s
or 3.00x108 m/s)
3. Wavelengths are measured between the distances of either crests or troughs. It is usually
characterized by the Greek symbol λ (lambda).
Waves and their Characteristics:
1. Amplitude – the distance from the maximum vertical displacement of the wave to the
middle of the wave, height of the wave.
2. Wavelength (λ) – the distance of one full cycle of the oscillation. Longer wavelength
waves carry low energy while shorter wavelengths carry high energy. Relationship with
the speed of light:
𝒄𝒄
𝛌𝛌 =
𝒗𝒗
Assumption: Shorter wavelength = greater frequency = higher energy
3. Frequency (ν) – defined as the number of cycles per second, and is expressed as Hertz
(Hz). Frequency is directly proportional to energy and can be express as:
E= hν
06 Handout 1 *Property of STI
Page 1 of 2
SH1684
4. Period (T) – the amount of time a wave takes to travel one wavelength; it is measured in
seconds (s).
5. Velocity (c) – the constant speed of electromagnetic wave in vacuum, 3.00 x 10-8 m/s.
c=λν
where:
• c is the speed of light (c= 3.00 x 10-8 m/s)
• λ is wavelength
• ν is frequency
• E is energy
• h is Planck's constant, (h= 6.62607 x 10-34 𝐽𝐽 ∙ 𝑠𝑠)
Planck’s Quantum Theory
• Proposed by Max Planck during the 19th century.
• Energy is always emitted in integral multiples of hv, postulated that the energy of a
particular quantum of radiant energy could be described by the equation:
𝐜𝐜
𝐄𝐄 = 𝐡𝐡
𝛌𝛌
Photoelectric Effect
• Contributed by Albert Einstein, five years after Planck presented his Quantum Theory.
• A phenomenon in which electrons are ejected from the surface of certain metals exposed
to light of at least a certain minimum frequency.
• Particles of light are called photons which possess energy.
E= hν
References:
Indiana University Northwest. (n.d.). Modern Atomic Theory. Retrieved from
http://www.iun.edu/~cpanhd/C101webnotes/modern-atomic-theory/rutherford- model.html on
March 23, 2017.
Khan Academy. The Quantum Mechanical Model of the Atom. Retrieved from
https://www.khanacademy.org/science/physics/quantum-physics/quantum-numbers-
and-orbitals/a/the-quantum- mechanical-model-of-the-atom on March 23, 2017.
Nobel Media AB. 2014. "J.J. Thomson -Biographical". Retrieved on March 23, 2017.
http://www.nobelprize.org/nobel_prizes/physics/laureates/1906/thomson-bio.html.
06 Handout 1 *Property of STI
Page 2 of 2
You might also like
- Chapter 1-Lesson 1Document4 pagesChapter 1-Lesson 1Bùi Hữu ĐứcNo ratings yet
- Chem U 2 G 11Document23 pagesChem U 2 G 11EApp And AgreementNo ratings yet
- Mahasiswa Mampu Menjelaskan Konsep Kuantisasi Energi: Kompetensi 1Document43 pagesMahasiswa Mampu Menjelaskan Konsep Kuantisasi Energi: Kompetensi 1Imam SyNo ratings yet
- 5) Quantum Mechanics - UpdatedDocument86 pages5) Quantum Mechanics - UpdatedGame 1No ratings yet
- VSS Lesson 1 Early Atomic Theories Chemistry 12Document11 pagesVSS Lesson 1 Early Atomic Theories Chemistry 12Jaideep GillNo ratings yet
- AP Chemistry: Chapter 7 - Atomic Structure & PeriodicityDocument14 pagesAP Chemistry: Chapter 7 - Atomic Structure & PeriodicityS. Green100% (1)
- Written ReportDocument3 pagesWritten ReportFatima Kent DelfinNo ratings yet
- 4) Quantum MechanicsDocument75 pages4) Quantum MechanicsGame 1No ratings yet
- EP - UNIT 2 - Quantum MechanicsDocument34 pagesEP - UNIT 2 - Quantum Mechanicsthetop10nerdNo ratings yet
- Chemistry Group 1 AssignmentDocument29 pagesChemistry Group 1 AssignmentTizu GetachewNo ratings yet
- New Microsoft Word DocumentDocument9 pagesNew Microsoft Word DocumentAritra SahaNo ratings yet
- Electromagnetic Waves, Planck's Quantum Theory, and Photoelectric EffectDocument50 pagesElectromagnetic Waves, Planck's Quantum Theory, and Photoelectric EffectElaine MataNo ratings yet
- Quantum Mechanics - Britannica Online Encyclopedia PDFDocument29 pagesQuantum Mechanics - Britannica Online Encyclopedia PDFMahesh MaheshwariNo ratings yet
- Notes - Quantum Theory of Light and Matter WavesDocument34 pagesNotes - Quantum Theory of Light and Matter Wavesphysics olympiad100% (1)
- Chapter Four: The Electronic Structure of AtomsDocument50 pagesChapter Four: The Electronic Structure of AtomsmajedmajedmajedNo ratings yet
- Electromagnetic WavesDocument7 pagesElectromagnetic Wavesmary ann leddaNo ratings yet
- Dhselect 5Document9 pagesDhselect 5Biswanath Gouda (Biswanath)No ratings yet
- Moderm PhysicsDocument39 pagesModerm Physicsgul zarrinNo ratings yet
- Ecture O.: (The Electronic Structure of Atoms)Document43 pagesEcture O.: (The Electronic Structure of Atoms)Yarisse RivasNo ratings yet
- Nature of Electromagnetic WavesDocument24 pagesNature of Electromagnetic WavesEivol Kimoy ÖNo ratings yet
- AP Physics Chapter 27 Quantum PhysicsDocument78 pagesAP Physics Chapter 27 Quantum PhysicsMythos EntertainmentNo ratings yet
- Unit - I - Quantum Mechanics PhysicsDocument34 pagesUnit - I - Quantum Mechanics Physicsabisheik942No ratings yet
- L1 Nature LightDocument3 pagesL1 Nature Lighttrs smritiNo ratings yet
- Chapter 7 Atomic Structure and PreriodicityDocument87 pagesChapter 7 Atomic Structure and PreriodicitydeemahhwNo ratings yet
- Light As An Electromagnetic WaveDocument11 pagesLight As An Electromagnetic WavejehonieeeNo ratings yet
- Bab 2 Blackbody, PhotoelectricDocument35 pagesBab 2 Blackbody, PhotoelectricFakrurazi NajmiNo ratings yet
- Electronic Structure of AtomsDocument59 pagesElectronic Structure of Atomstalktotiffanycheng100% (1)
- Class #02 - Units, Scale, and The Mole: For Test #1 On ThursdayDocument11 pagesClass #02 - Units, Scale, and The Mole: For Test #1 On ThursdayLina ChitadzeNo ratings yet
- Chapter Five (2) - 2Document68 pagesChapter Five (2) - 2Wengelu evanNo ratings yet
- Che101 Chap 7Document47 pagesChe101 Chap 7David MaranzhyanNo ratings yet
- Chapter Two. EEE & ETI 1204 Material ScienceDocument29 pagesChapter Two. EEE & ETI 1204 Material ScienceKIRAGU PURITY NJERINo ratings yet
- Chemistry 1103: Dr. Jhon Zapata RiveraDocument37 pagesChemistry 1103: Dr. Jhon Zapata RiveraJuliRoGamer YtNo ratings yet
- State When The Emission Spectrum of Hydrogen Was Discovered. Outline The Apparatus and Findings of This ExperimentDocument56 pagesState When The Emission Spectrum of Hydrogen Was Discovered. Outline The Apparatus and Findings of This ExperimentMaryFifiNo ratings yet
- Chem Bonding 1Document66 pagesChem Bonding 1Nikitha AkulaNo ratings yet
- Quantum Mechanics Notes-Part 1Document15 pagesQuantum Mechanics Notes-Part 1aman bhatiaNo ratings yet
- PPT1 and 2Document43 pagesPPT1 and 2Adugnaw BiksNo ratings yet
- GeneralChemistry 1Document50 pagesGeneralChemistry 1peter168205No ratings yet
- Wave Properties of ParticleDocument35 pagesWave Properties of ParticleAnnida ChairatunisaNo ratings yet
- ProjectDocument3 pagesProjectapi-285213472No ratings yet
- Lecture 7Document30 pagesLecture 7Ahmad HassanNo ratings yet
- Atomic StructureDocument49 pagesAtomic StructureFatimaNo ratings yet
- JotterPad - JotterPadDocument21 pagesJotterPad - JotterPadOlajide HeritageNo ratings yet
- Quanta To QuarksDocument32 pagesQuanta To QuarksDaniel Bu100% (5)
- 02 Quantum Theory and Atomic StructuresDocument20 pages02 Quantum Theory and Atomic StructuresChrissa GuicoNo ratings yet
- Quantum Theory & Atomic StructureDocument24 pagesQuantum Theory & Atomic Structurevrejie46No ratings yet
- The Duality of Matter and WavesDocument7 pagesThe Duality of Matter and Wavesbsantrich1760No ratings yet
- B068 PDFDocument17 pagesB068 PDFprashanthNo ratings yet
- MITchem Lec 4Document7 pagesMITchem Lec 4maggiesszzNo ratings yet
- CHM012 - Module 3 (Part 2)Document16 pagesCHM012 - Module 3 (Part 2)haibaalisa00No ratings yet
- Modern Physics 3 PDFDocument38 pagesModern Physics 3 PDFDodi SaputraNo ratings yet
- Week 1Document4 pagesWeek 1jia aganaNo ratings yet
- String TheoryDocument39 pagesString Theoryantarikshsaxena100% (2)
- QuantumDocument388 pagesQuantumyana33No ratings yet
- Class 11 Chemistry Chapter 2 Structure of AtomDocument15 pagesClass 11 Chemistry Chapter 2 Structure of Atomgokul100% (1)
- R22 - Unit-I, 2 Quantum Mechanics and SemiconductorsDocument309 pagesR22 - Unit-I, 2 Quantum Mechanics and Semiconductorsnareshreddyy1984No ratings yet
- Structure of Atom Part IDocument75 pagesStructure of Atom Part IShreyansh SinghNo ratings yet
- 2021 Structure of AtomDocument13 pages2021 Structure of AtomSora RoseNo ratings yet
- Quantum MechanicsDocument35 pagesQuantum MechanicsJonNo ratings yet
- Handout 02Document10 pagesHandout 02JAMESNo ratings yet
- The Enigmatic Electron: Electron Behaviour and How It Influences Our LivesFrom EverandThe Enigmatic Electron: Electron Behaviour and How It Influences Our LivesNo ratings yet
- Elephant's Toothpaste : Presented To Mr. Jefferson R. Borillo Lpt. Physics Teacher/AdviserDocument13 pagesElephant's Toothpaste : Presented To Mr. Jefferson R. Borillo Lpt. Physics Teacher/AdviserkjfhghjfggjfNo ratings yet
- LESSON 33: Elephant Toothpaste: - Description - MaterialsDocument13 pagesLESSON 33: Elephant Toothpaste: - Description - MaterialskjfhghjfggjfNo ratings yet
- Periodic Elements and Ionic ChargesDocument2 pagesPeriodic Elements and Ionic ChargeskjfhghjfggjfNo ratings yet
- Elemental Properties and Periodic TrendsDocument1 pageElemental Properties and Periodic TrendskjfhghjfggjfNo ratings yet
- XII PhysicsDocument55 pagesXII PhysicsCharu BhanotNo ratings yet
- Assignment em WavesDocument2 pagesAssignment em WavesCHANDERBATISH MOHANNo ratings yet
- Satellite Antenna TestingDocument47 pagesSatellite Antenna Testingsailee_gharatNo ratings yet
- NEET Physics WeightageDocument4 pagesNEET Physics WeightagevishNo ratings yet
- Phy101 Assignment No. 2Document3 pagesPhy101 Assignment No. 2Asim Ihsan (Apna)No ratings yet
- User Manual OCTOPUS-OFFICE SHIP MOTION ANALYSIS SOFTWAREDocument69 pagesUser Manual OCTOPUS-OFFICE SHIP MOTION ANALYSIS SOFTWAREMiruna ClinciuNo ratings yet
- Practice Set 1Document1 pagePractice Set 1Lalit Ranjan SahuNo ratings yet
- List of Scientific Instruments & Their Uses PDF For UPSC, Banking & SSC ExamsDocument8 pagesList of Scientific Instruments & Their Uses PDF For UPSC, Banking & SSC ExamsYashNo ratings yet
- Advanced Well-Log Analysis in Geothermal PDFDocument4 pagesAdvanced Well-Log Analysis in Geothermal PDFEdyson DeniltonyNo ratings yet
- Otc 18587 MS PDocument15 pagesOtc 18587 MS PSiul Otrebla AtelavazNo ratings yet
- PHYSICAL SCIENCE MODULE 15-EditedDocument24 pagesPHYSICAL SCIENCE MODULE 15-EditedLove Joy JumawanNo ratings yet
- Light Answer Key: Red, Orange, Yellow, Green, Blue, Indigo, VioletDocument4 pagesLight Answer Key: Red, Orange, Yellow, Green, Blue, Indigo, VioletHayiath QureshiNo ratings yet
- Engineering Physics Notes - 2Document117 pagesEngineering Physics Notes - 2Maaran APECNo ratings yet
- BBC Engineering Training Manual - Microphones (Robertson)Document380 pagesBBC Engineering Training Manual - Microphones (Robertson)Nino Rios100% (1)
- B.Tech - Civil-SyllabusDocument126 pagesB.Tech - Civil-SyllabusSrinivas JupalliNo ratings yet
- Iit/Aiims 2020-Screeningcum Scholarship Exam: Date: 00 October 2018Document11 pagesIit/Aiims 2020-Screeningcum Scholarship Exam: Date: 00 October 2018LukewarmNo ratings yet
- Zenith Tutorials Phy 102 PDF SeriesDocument24 pagesZenith Tutorials Phy 102 PDF SeriesPhake CodedNo ratings yet
- Polsarpro TutorialDocument173 pagesPolsarpro Tutorialapi-3854701No ratings yet
- IR SpectrometryDocument46 pagesIR Spectrometryortizan8No ratings yet
- Wave Motion PDFDocument23 pagesWave Motion PDFFRANCES VISAYA100% (1)
- A Detailed Lesson Plan in Science 4 - SOUNDSDocument5 pagesA Detailed Lesson Plan in Science 4 - SOUNDSLourdes ManansalaNo ratings yet
- Building Utilities 3 - Acoustics and Lighting SystemsDocument2 pagesBuilding Utilities 3 - Acoustics and Lighting SystemsNICOLE ADRIENNE RIVERANo ratings yet
- 1000 Electromagnetic Theory MCQsDocument369 pages1000 Electromagnetic Theory MCQskibrom atsbha50% (14)
- ALi Physics Notes 10thDocument27 pagesALi Physics Notes 10thKhawajaAbdulmoeed gamerNo ratings yet
- Jee Main and Advanced Nurture Detailed SyllabusDocument1 pageJee Main and Advanced Nurture Detailed SyllabusDivyansh SinghNo ratings yet
- TOFD PresentationDocument29 pagesTOFD Presentationnirmalmthp100% (6)
- EXPERIMENTDocument2 pagesEXPERIMENTGracilla ElmidoNo ratings yet
- Content Overview: Candidates For Cambridge International AS Level Physics Study The Following TopicsDocument11 pagesContent Overview: Candidates For Cambridge International AS Level Physics Study The Following TopicsAreeba EjazNo ratings yet
- SMAS - Paper - Negative - Stiffness HoneycombDocument23 pagesSMAS - Paper - Negative - Stiffness Honeycombraniya.zouaghiNo ratings yet
- Beat Phenomenon: O, RespectivelyDocument5 pagesBeat Phenomenon: O, RespectivelyPradip GuptaNo ratings yet