Professional Documents
Culture Documents
PHOTOSYNTHESIS: Pigments of The Chloroplasts: Naira Mitz Ampuan BS Biology Student
PHOTOSYNTHESIS: Pigments of The Chloroplasts: Naira Mitz Ampuan BS Biology Student
Uploaded by
naira mitzOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
PHOTOSYNTHESIS: Pigments of The Chloroplasts: Naira Mitz Ampuan BS Biology Student
PHOTOSYNTHESIS: Pigments of The Chloroplasts: Naira Mitz Ampuan BS Biology Student
Uploaded by
naira mitzCopyright:
Available Formats
EXPERIMENT 9
PHOTOSYNTHESIS: Pigments of the Chloroplasts
Photosynthesis uses energy absorbed by several photosynthetic pigments. The light
energy used for photosynthesis is not absorbed by just a single type of pigment. Instead,
several different photosynthetic pigments with different absorption spectra absorb the energy
that is eventually used for photosynthesis. In photosynthetic organisms including plants, protists,
and bacteria, these photosynthetic pigments include chlorophylls, carotenoids, and phycobilins.
Each of these absorbs light at different parts of the visible light spectrum and has a different
function in the plant.
Chlorophylls: In plants photosynthetic pigments two chlorophylls predominate: chlorophyll a
and chlorophyll b. These two molecules differ only slightly in their molecular structure. Both have
a complex ring structure similar to that of the heme group of hemoglobin. In the center of each
chlorophyll ring is a magnesium atom, and attached at a peripheral location on the ring is a long
hydrocarbon “tail,” which can adhere the chlorophyll molecule to proteins in the hydrophobic
portion of the thylakoid membrane. They are polar (water-soluble) pigments that act as electron-
transporters in photosynthesis and provide green colors to plants. Chlorophyll a produces a
bright green to blue-green band, and chlorophyll b produces a dull olive to yellow-green band.
Chlorophyll a is more soluble than chlorophyll b and will travel farther up the paper with the
solvent (carbon tetrachloride) used in the experiment.
Carotenes: Carotenes are non-polar (fat-soluble) hydrocarbons. This class of pigments ranges
in color from yellow to red. They are also essential as antennal pigments in photosynthesis.
Carotene itself produces a yellow-orange band.
It is possible to separate these pigments from one another by the use of paper
chromatography. Paper chromatography is a laboratory technique that separates components
within a mixture by using the differential affinities of the components for a solvent and for the
paper through which they pass.
Principle of Paper Chromatography:
• Capillary Action – the movement of liquid within the spaces of a porous material due to
the forces of adhesion, cohesion, and surface tension. The liquid is able to move up
the filter paper because the cohesive forces (attraction between the liquid molecules),
Naira Mitz Ampuan BS Biology student bitchy_princess24@yahoo.com
together with adhesive forces (attraction between the liquid and the paper) are
stronger than the force of gravity.
• Solubility – the degree to which a material (solute) dissolves into a solvent. Solutes
dissolve into solvents that have similar properties (Like dissolves like).This allows
different solutes to be separated by different combinations of solvents. Separation of
components depends on both their solubility in the mobile phase and their differential
affinity to the mobile phase and the stationary phase.
Because pigments have unique attributes that define them, several color bands would be
expected if there is more than one present. Based on the bands formed on the filter paper, the
retention factor, or Rf, value can be calculated for each pigment. This is done by dividing the
distance the pigment traveled by the distance the solvent traveled.
Eqn. 1 Rf = distance pigment traveled
distance solvent traveled
MATERIALS:
Papaya leaf tissue (Carica papaya)
chromatography paper
chromatography solvent (carbon
tetrachloride)
chromatography chamber with lid
ruler
mortar and pestle
capillary pipette
calcium carbonate (added during the
leaf extraction process to prevent
the chlorophyll from degrading to
pheophytin)
anhydrous sodium sulfate
Naira Mitz Ampuan BS Biology student bitchy_princess24@yahoo.com
METHODOLOGY:
1. (Preparation of pigment solution) In a mortar, the following were added: 1g of papaya
leaf tissue, and 15mL acetone. Using the pestle, the mixture is ground for 5 minutes or
until the solution is dark in color. This step is to disrupt the plant cell walls.
2. (Application of the pigments) Using the capillary pipette, a line of the pigment solution
was applied onto the chromatography paper. After dying the stain, the procedure is
repeated. It was first ensured that the pigment stain is TOTALLY dry before running the
chromatogram.
3. (Developing the chromatograms) The stained paper was placed into the developing
chamber. The lid was placed. The solvent was allowed to migrate upward until the
solvent front is 1cm from the top of the paper. Then immediately, the paper was removed
and the solvent front was marked.
4. (Obtaining Rf values) After allowing the chromatogram to dry, the distances traveled by
each pigment band were measured. After which, Rf values were computed using the
above stated equation.
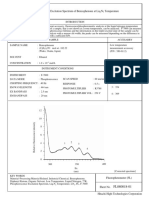
RESULTS AND DISCUSSIONS:
Band Distances and Rf values
Band Distance Band Color Pigment Rf
(cm)
1 6.8 Yellow orange carotene 0.97
2 5.4 Blue green Chlorophyll a 0.77
3 3.6 Yellow green Chlorophyll b 0.51
Solvent front (in cm) = 7.0
It is noted that there is a trend in the movement of the color bands, representing the
characteristic polarity of the pigment. Using a nonpolar solvent (carbon tetrachloride), the
nonpolar color bands (pigments) move farther. That is to say, carotenes (nonpolar) travels
further the two polar chlorophylls. Such that,
Continuum on Distances Traveled:
Naira Mitz Ampuan BS Biology student bitchy_princess24@yahoo.com
Carotene > Chl a > Chl b
Polarity Continuum:
Carotene < Chl a < Chl b
Moreover, as the Rf value increases, the degree of polarity decreases, such that those
located at the top of the chromatogram is the most nonpolar. It is to be noted that the above
explained phenomenon is only applicable for experiments utilizing a nonpolar solvent. For the
case of a polar solvent, an opposite outcome is expected. That is, the pigment band that travel
the farthest, the one with the highest Rf value, is the most polar.
GUIDE QUESTIONS
Q: Define Fluorescence.
A: Fluorescence is a process in which an atom or molecule emits radiation in the course of a
transition from a higher to a lower electronic state. A more restricted definition, applicable
particularly to atomic processes, excludes the special case, known as resonance radiation, in
which the wavelength of the emitted radiation is the same as that of the exciting radiation. The
term fluorescence is further restricted to phenomena in which the time interval between the
acts of excitation and emission is small, of excitation and emission is small, of the order of
10-8 –10-3 second. This distinguishes fluorescence from phosphorescence, where the
time interval between absorption and emission may extend from 10-3 second to several
hours. The phenomenon of fluorescence was known by the middle of the century. It was the
British scientist Stokes who first made the observation that the fluorescing light has longer
wavelengths than the excitation light, a phenomenon that has become to be known as Stokes
shift.
One of the most prominent processes is the chlorophyll fluorescence, which is readily
observed when a solution of chlorophyll (green leaves) is illuminated with a strong source of
continuum radiation. The red colour characteristics of fluorescence readily appear in a sample
cuvette. Light energy is absorbed by chlorophyll within plant tissues and used to drive
photochemistry of photosynthesis and thus become chemical energy available to the plant for
growth. Light in the waveband 400-700 nm is absorbed by chlorophyll and used for
photochemistry. This light is termed photosynthetic ally Active Radiation (PAR). Although
fluorescence emission from whole leaf system is too weak to be viewed with the naked eye, it
can be observed from the illuminated extracts of a chlorophyll solution. Peak fluorescence
occurs in the red region of the spectrum (685 nm) and extends into the infrared region to around
Naira Mitz Ampuan BS Biology student bitchy_princess24@yahoo.com
800nm. The fluorescence from chlorophyll has been used extensively in the past to characterize
and investigate agricultural plants.
Q: Define Phosphorescence.
A: Phosphorescence is a specific type of photoilluminescence related to fluorescence. It
corresponds to light emissions accompanying radiative electronic transitions from triplet to
ground states of pigment molecules. Unlike fluorescence, a phosphorescent material does not
immediately reemit the radiation it absorbs. The slower time scales of the reemission are related
with “forbidden” energy state transitions in quantum mechanics. As these transmissions occur
less often in certain materials, absorbed radiation may be reemitted at a lower intensity for up to
several hours.
In simpler terms, phosphorescence is a process in which energy absorbed is released
relatively slowly in the form of light. This is in some cases the mechanism used for glow-in-the-
dark materials which are charged by exposure to light. Unlike the relatively swift reactions in a
common fluorescent tube, phosphorescent materials used for these materials absorb the energy
and store it for longer time as the subatomic reactions required to reemit the light occur less
often.
CONCLUSIONS:
Results of the paper chromatography demonstrate that the chloroplasts of a Carica
papaya leaf contains of three types of plant pigments- chlorophyll a, chlorophyll b, and carotene.
Researchers also conclude that among the three pigments, carotenes are the most non polar.
Between the two chlorophylls, although they have similar polarity (attributed to their similar
structures) their solubility varies. Chlorophyll b is less soluble than chlorophyll a.
REFERENCES:
G.H.Krause and E Weis. Ann. Rev. plant. physiol. plant. Mol. Biol. Vol 42, 313-349 (1991).
WL Buller and Kitajima. Biochim. Biophys. Acta. Vol 396, 72-85 (1975).
E Weis and J A Berry. Biochim. Biophys. Acta.Vol 894,198-208 (1940).
E W Chappelle, J E McMurtrey III and M S Kim. Remote. Sens. Environ.Vol 36, 213-218
(1991).
A Rosema and H Zahn. Remote Sen. Environ.Vol.62, 101-108 (1997).
Naira Mitz Ampuan BS Biology student bitchy_princess24@yahoo.com
You might also like
- Sta108 - Group Project AssignmentDocument8 pagesSta108 - Group Project Assignmentnurain fasihahNo ratings yet
- Answer Scheme Quiz CSC126 - Okt2021Document7 pagesAnswer Scheme Quiz CSC126 - Okt2021Haziq GenjiNo ratings yet
- Reactions of Aliphatic Alcohols and PhenolDocument9 pagesReactions of Aliphatic Alcohols and Phenolmoon star angelNo ratings yet
- Bio320 Lab 2Document4 pagesBio320 Lab 2Mirza KarmilaNo ratings yet
- HPLC Assignment ProblemsDocument3 pagesHPLC Assignment ProblemsIla Ainaa100% (1)
- Jared Diamond - Taiwan's Gift To The WorldDocument2 pagesJared Diamond - Taiwan's Gift To The WorldJojo Malig100% (1)
- Time Resolved Pulsed Laser Photolysis Study of The Rate Constant and Reaction Mechanism For Ru (Bpy) 32+ Phosphorescence Quenching by O2Document10 pagesTime Resolved Pulsed Laser Photolysis Study of The Rate Constant and Reaction Mechanism For Ru (Bpy) 32+ Phosphorescence Quenching by O2kjg51163824No ratings yet
- Mitosis ReportDocument18 pagesMitosis ReportFarhan Zolkeflee0% (1)
- PHY110 Chapter 3Document45 pagesPHY110 Chapter 3Nur SyahiraNo ratings yet
- Lab Report BIO330Document11 pagesLab Report BIO330Oh SehunNo ratings yet
- Lab Report Upsi SKT1013 Diploma Science Experiment 2Document7 pagesLab Report Upsi SKT1013 Diploma Science Experiment 2Nur Wanyz SyazwanieNo ratings yet
- CHM 131 Chapter 3Document90 pagesCHM 131 Chapter 3Syalin NorainNo ratings yet
- Lab Report skt1013Document6 pagesLab Report skt1013Nur Wanyz SyazwanieNo ratings yet
- Capillary Electrophoresis: Topic 5Document88 pagesCapillary Electrophoresis: Topic 5Johan DaniyalNo ratings yet
- A Survey About Online and Distance Learning (Odl) On Uitm Tapah StudentsDocument23 pagesA Survey About Online and Distance Learning (Odl) On Uitm Tapah StudentsAzdy HaiqalNo ratings yet
- Basic Analytical Chemistry CHM 256: Laboratory Case Study Report (Chromatography)Document3 pagesBasic Analytical Chemistry CHM 256: Laboratory Case Study Report (Chromatography)ShafikaNo ratings yet
- STA108 - Tutorial 2 (With Answers)Document4 pagesSTA108 - Tutorial 2 (With Answers)sofiya fatiniNo ratings yet
- BIO122 Chapter 2-1Document55 pagesBIO122 Chapter 2-1Mohd AsrulNo ratings yet
- Lab SBL Exp 2Document9 pagesLab SBL Exp 2api-384057570No ratings yet
- PHY150 Elctricity and Magnetism Experiment 5Document7 pagesPHY150 Elctricity and Magnetism Experiment 5hfzfrdNo ratings yet
- INTRODUCTION CHM 361 Case StudyDocument3 pagesINTRODUCTION CHM 361 Case StudyAnnisaNo ratings yet
- Faculty OF Applied Sciences Diploma in Science (AS120) : CapacitenceDocument10 pagesFaculty OF Applied Sciences Diploma in Science (AS120) : CapacitenceMirza KarmilaNo ratings yet
- Photosynthesis Lab ReportDocument3 pagesPhotosynthesis Lab ReportDevourekZurgenskinNo ratings yet
- CODE: PHY260: Lab Report Course: Optics and WavesDocument10 pagesCODE: PHY260: Lab Report Course: Optics and WavesCassy100% (1)
- Tutorial Chapter 6Document3 pagesTutorial Chapter 6malzNo ratings yet
- Bio270 Lab Report StarchDocument13 pagesBio270 Lab Report StarchFathiah Nh100% (1)
- CHM301 Lab Report 2Document14 pagesCHM301 Lab Report 2Nurul Adira FaziraNo ratings yet
- CHM 3402 Experment 3Document9 pagesCHM 3402 Experment 3Luqman HakimNo ratings yet
- Lab 1 CHM 510 Complete 2011Document20 pagesLab 1 CHM 510 Complete 2011Nor Hasliza100% (1)
- Bio310 Lab 1Document19 pagesBio310 Lab 1nursyahirahNo ratings yet
- Organic Chemistry Lab IiDocument7 pagesOrganic Chemistry Lab IiShahizatul Annurizzah Saprudin100% (1)
- BIO122 Chapter 4Document174 pagesBIO122 Chapter 4Miss KillerNo ratings yet
- Lab Report CHM As1202aDocument12 pagesLab Report CHM As1202aNURUL AINUN MUHAMMAD NORNo ratings yet
- Workbook: Laboratory BIO320: Universiti Teknologi Mara (Uitm)Document4 pagesWorkbook: Laboratory BIO320: Universiti Teknologi Mara (Uitm)Mirza KarmilaNo ratings yet
- PHY110 Mechanics I: Lab ManualDocument28 pagesPHY110 Mechanics I: Lab ManualHana The Pencil KnightNo ratings yet
- Intro N Theory AASDocument14 pagesIntro N Theory AASMc JaeNo ratings yet
- Elc550 Test Brain Drain May 2021Document5 pagesElc550 Test Brain Drain May 2021Nurul IzzatiNo ratings yet
- Experiment 2 (Measurement of Equivalent Radius, Bulk Density and Solid Density of GranularDocument8 pagesExperiment 2 (Measurement of Equivalent Radius, Bulk Density and Solid Density of GranularMuhd Syahmi0% (1)
- Lab Report 2 AOTWDocument14 pagesLab Report 2 AOTWFatihah AlhataNo ratings yet
- LAB REPORT 6 - StudentDocument8 pagesLAB REPORT 6 - StudentVeshal RameshNo ratings yet
- Light Intensity and TranspirationDocument1 pageLight Intensity and TranspirationJordan ZhengNo ratings yet
- Course Outline AS231Document2 pagesCourse Outline AS231fara carneaNo ratings yet
- Case Study FSGDocument15 pagesCase Study FSGmeklin0% (1)
- Experiment of Gas ChromatographyDocument10 pagesExperiment of Gas Chromatographyadda93% (15)
- Assignment 1 Phy 340Document5 pagesAssignment 1 Phy 340Afiq IskandarNo ratings yet
- Experiment 2Document5 pagesExperiment 2Alya HaifaNo ratings yet
- Course OutlineDocument4 pagesCourse OutlineNabilah HarisNo ratings yet
- SBL 1023 Exp 3Document7 pagesSBL 1023 Exp 3api-383623349No ratings yet
- Lab Report Bio 330Document4 pagesLab Report Bio 330hyunjeans booNo ratings yet
- STA108 Project 1Document27 pagesSTA108 Project 1moon star angel100% (3)
- Bio 270 Lab Part I - As120Document8 pagesBio 270 Lab Part I - As120QhairunnissaNo ratings yet
- Chem ch6Document18 pagesChem ch6Chandler100% (1)
- Lab Report Bio320 WorklabDocument14 pagesLab Report Bio320 WorklabNik aininNo ratings yet
- Lab Report 6 Plant PhysiologyDocument7 pagesLab Report 6 Plant Physiologyapi-384857069No ratings yet
- MIC481 Lab ReportDocument6 pagesMIC481 Lab ReportAbg Khairul Hannan Bin Abg AbdillahNo ratings yet
- Project Sta108 (Finalized) (Lasttt)Document55 pagesProject Sta108 (Finalized) (Lasttt)daniaNo ratings yet
- CHM 213 - Exp 5Document9 pagesCHM 213 - Exp 5hafiqahNo ratings yet
- Lab Manual BIO330 PDFDocument28 pagesLab Manual BIO330 PDFSyuhadah HasbollahNo ratings yet
- Test 1 CHM256 - Question Paper - 231105 - 121107Document7 pagesTest 1 CHM256 - Question Paper - 231105 - 121107Aqilah NajwaNo ratings yet
- Group Project STA 108Document18 pagesGroup Project STA 108Cassy0% (1)
- Activity 5 Lab ReportDocument5 pagesActivity 5 Lab ReportJay MarcosNo ratings yet
- Bio Study Sheet PhotosynthesisDocument42 pagesBio Study Sheet PhotosynthesisSalim RihaniNo ratings yet
- Fluorescence and PhosphorescenceDocument15 pagesFluorescence and PhosphorescencePalupi DarmantiNo ratings yet
- Luminescenta Combinatiilor Complexe Ala LantanidelorDocument92 pagesLuminescenta Combinatiilor Complexe Ala LantanidelorMaria Luiza CraciunNo ratings yet
- Color-Control of Long-Lasting Phosphorescence (LLP) Through Rare Earth Ion-Doped Cadmium Metasilicate PhosphorsDocument7 pagesColor-Control of Long-Lasting Phosphorescence (LLP) Through Rare Earth Ion-Doped Cadmium Metasilicate PhosphorsIlse Guadalupe EncinasNo ratings yet
- Fluorescence IntroductionDocument7 pagesFluorescence Introductionprakush_prakushNo ratings yet
- Photochemistry 2Document9 pagesPhotochemistry 2SkywalkerNo ratings yet
- Fluorescence Spectrophotometry: The Electronic Excited StateDocument4 pagesFluorescence Spectrophotometry: The Electronic Excited Stateadriana_obrNo ratings yet
- Phosphorescence Excitation Spectrum of Benzophenone at Liq.N TemperatureDocument5 pagesPhosphorescence Excitation Spectrum of Benzophenone at Liq.N TemperatureNisar Ali Mphil-Chem ANo ratings yet
- Molecular Luminescence Spectroscopy: Prof. Dr. Ayman H. KamelDocument27 pagesMolecular Luminescence Spectroscopy: Prof. Dr. Ayman H. KamelFatima AhmedNo ratings yet
- Himanshu Shara 2010B2A2196P: Prepared byDocument24 pagesHimanshu Shara 2010B2A2196P: Prepared byHimanshu SharaNo ratings yet
- Photochemistry-Ppt 7422144 PowerpointDocument10 pagesPhotochemistry-Ppt 7422144 PowerpointAranga100% (1)
- PhotochemistryDocument20 pagesPhotochemistryAranga100% (1)
- Fluorescence N PhosphorescenceDocument14 pagesFluorescence N Phosphorescenceanon_543130923No ratings yet
- 1 MaterialsDocument54 pages1 MaterialsscreenameNo ratings yet
- Molecular Luminescence SpectrosDocument19 pagesMolecular Luminescence Spectrosedwedq100% (1)
- Fluorescence and PhosphorescenceDocument24 pagesFluorescence and Phosphorescencehumera0% (1)
- Phosphor H2NDocument1 pagePhosphor H2NdjedrezaNo ratings yet
- Atomic Fluorescence SpectrosDocument15 pagesAtomic Fluorescence SpectrosSubh Ki PEyli KiranNo ratings yet
- PhotoluminescenceDocument28 pagesPhotoluminescenceLiêu LyNo ratings yet
- Chapter 26/27: Molecular Absorption SpectrometryDocument14 pagesChapter 26/27: Molecular Absorption SpectrometryS. MartinezNo ratings yet
- Fluorescence KineticsDocument10 pagesFluorescence KineticsDan McNo ratings yet
- 9 Science Experiments About Light For KidsDocument7 pages9 Science Experiments About Light For KidsgaylebugayongNo ratings yet
- FluorophoreDocument17 pagesFluorophoreBasab BijayeeNo ratings yet
- Linkbelt Rough Terrain Crane RTC 8025 II RTC 8030 II Service ManualDocument22 pagesLinkbelt Rough Terrain Crane RTC 8025 II RTC 8030 II Service Manualjilljames090502dze100% (112)
- Photoluminescence Spectroscopy and Its Applications 2Document11 pagesPhotoluminescence Spectroscopy and Its Applications 2Rohith100% (2)
- Study Smart Road With Glowing LinesDocument6 pagesStudy Smart Road With Glowing LinesIJRASETPublicationsNo ratings yet
- Chapter 1 Introduction-CorrectedDocument56 pagesChapter 1 Introduction-CorrectedSushruti Richaa KashyapNo ratings yet
- Summary of EXP3Document9 pagesSummary of EXP3Tany TurkiNo ratings yet
- 01 Smart and Modern MaterialsDocument22 pages01 Smart and Modern MaterialsKriti KumariNo ratings yet