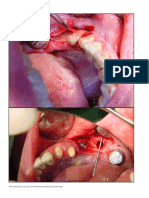
Professional Documents
Culture Documents
2016 - Byk Foto
2016 - Byk Foto
Uploaded by
Syifa IKOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2016 - Byk Foto
2016 - Byk Foto
Uploaded by
Syifa IKCopyright:
Available Formats
_CCMBM 3_2016.
qxp_- 23/01/17 09:39 Pagina 214
Original article
Mesenchymal stem cells in post-surgical cavities
of large maxillary bone lesions
Roberto Bertolai Concerning the literature, the careful enucleation of small lesions
Carlo Catelani plus a scrupulous surgical debridement (uncombined with bio-
Mattia Signorini materials filling of the remaining cavity) is the unanimous proce-
Alessandro Rossi dure to ensure a satisfactory bone regeneration (1-6, 8-22).
Domenico Giannini In regard to post-surgical medium and large cavities (> 3 cm), de-
fined by Horowitz’s 1989 classification (1-7), there isn’t any con-
sensus on treatment procedure; conversely there’re different al-
University of Florence, School of Human Health Sciences, ternative proposals to the only spontaneous regeneration.
Surgery and Translational Medicine Department (DCMT), The techniques about post-surgical enucleated large cavities in-
Head and Neck Unit, Florence, Italy clude the use of biomaterials, autogenous bone and combinations
of these materials. Many studies have shown, by periodical cli-
nical-radiographic follow-ups, that residual large cavities can un-
Address for correspondence: dergo to spontaneous regeneration in the totality of cases. Re-
Roberto Bertolai viewing the literature, there’s not evidence to fill cavity with au-
University of Florence, School of Human Health Sciences, tologous bone grafts or alloplastic materials, which could increase
Surgery and Translational Medicine Department (DCMT) postoperative morbidity and would delay the healing time (8-22).
Head and Neck Unit, After the surgery procedure, the fracture is the complication of lar-
Largo Palagi 1 ge lesions due to the size and the excessive bone fragility. Whe-
Florence, Italy never is possible, to reduce that risk, fixing plates are used intra-
Phone: 0039 055 7948383 operatively and removed after healing.
E-mail: bertolai@unifi.it After the enucleation of an expansive benign bone lesion, newly
formed bone developed from primary clot organization (which spil-
ls from the cavity walls) and progressively fills the residual cavity.
Summary Usually an almost complete cavity ossification is radiographical-
ly visible since 6 to 12 months; however the time range is rela-
Background. Recent studies have highlighted that MSCs ted to cyst initial size, number of intact residual walls and to pa-
are capable of regenerating large bone defects when used tient’s age [young patients heal faster (8, 9, 12, 22, 23)].
in combination with bone substitutes and increasing allo- Concerning large cavities (more than 3 cm in diameter), literatu-
graft osteointegration. We investigated the hypothesis that re indicates this data about the average percentage reduction (9,
autologous MSCs may lead to increased bone regenera- 12, 23):
tion and reduced healing time in post-surgical cavities of • at 6 months: 12-15% reduction
large maxillary bone lesions. • at 12 months: 43-49% reduction
Methods. This study involved 10 patients (TEST GROUP) • at 24 months: 83-91% reduction
(6 males and 4 females). All patients had expansive In the last years, a tissue engineering procedure has been set up:
mandibular lesions larger than 3 cm. From the surgical as a graft material autologous mesenchymal stem cells (MSCs)
point of view, the 10 patients were treated with MSCs are used in combination with an osteoconductive scaffold. MSCs
(withdrawal of the iliac crest bone marrow BMMSs) directly are a population of bone marrow-derived (BMMSCs), or human
into the post-surgical cavity, without the addition of filler. adipose-derived stem cells (hADSCs), non-hematopoietic multi-
Results. Clinical and radiological data, in the postopera- potent cells which can be expanded and in vitro differentiated into
tive, were compared to those of patients who did not re- osteogenic phenotype (40, 41). Recent studies have highlighted
ceive any grafting of MSCs. The 7 patients with mandibular that MSCs are capable of regenerating large bone defects when
lesions showed a rapid and very good healing with an 85- used in combination with bone substitutes (42, 43) and increasing
90% ossification of the major defect at 12 months. allograft osteointegration (44). Another method that has been used
Conclusions. Through the use of stem cells a greater ossi- to improve the outcome of bone grafting is based on autologous
fication of the residual cavity (85-90%) was observed at 12 platelets. Indeed, they are a readily accessible, safe and cheap
months after surgical enucleation in contenitive defects. source of growth factors, such as platelet-derived growth factor
(PDGF), transforming growth factor beta (TGF-b), epidermal growth
KEY WORDS: bone regeneration; maxillary defects; maxillary bone cysts; MSCs;
bone grafts.
factor (EGF), vascular endothelial growth factor (VEGF) and in-
sulin-like growth factor I (IGF-I), which play a key role in tissue ho-
meostasis (45, 46). Platelets are usually concentrated in a small
Introduction volume of plasma called platelet-rich plasma (PRP), which can be
an excellent addition to MSCs and freeze-dried bone grafts. In fact,
Enucleation and surgical debridement represent the most effec- MSCs express receptors for the growth factors contained in the
tive techniques to treat a massive benign expansive bone lesion PRP and in vitro studies have shown that the addition of PRP pro-
of the maxilla. motes MSC proliferation.
214 Clinical Cases in Mineral and Bone Metabolism 2016; 13(3):214-220
_CCMBM 3_2016.qxp_- 23/01/17 09:39 Pagina 215
Mesenchymal stem cells in post-surgical cavities of large maxillary bone lesions
Based on the above grounds, in the present study we test the hy-
pothesis that grafting of autologous BMMSCs and PRP may lead
to increased bone regeneration and reduced healing time.
MSCs
A Mesenchymal Stem Cell (MSC) is a type of adult stem cell,
immature and undifferentiated. It originates from the mesoderm,
the germ of the blastocyst between ectoderm and endoderm. In
recent years, MSCs have attracted a lot of attention thanks to
their versatility. Their action is not limited to the trophic effect alo-
ne, given by the production of various types of cytokines and
growth factors, but they are also able to migrate and differen-
tiate into different types of mesenchymal tissues. In 2006 In-
ternational Society for Cellular Therapy (ISCT) has proposed mi-
nimal criteria to define MSCs: a mesenchymal stem cell can be
defined if it shows adhesion plastic in normal crop and if it has Figure 1 - An occasional radiological finding of follicular cysts in a patient
a fibroblast-like morphology (24-34). Some researchers also ar- of 29 years old with no clinical signs or symptoms.
gue that MSC have the same functions of fibroblasts (35). The
mesenchymal stem cells in culture express surface antigens
CD73, CD90 and CD105, while not expressing CD11b, CD14,
CD19, CD34, CD45, CD79a or HLA-DR (36). Mesenchymal stem
cells have a role in tissue regeneration: they are divided cycli-
cally and the newly formed cells replace those cells that show
sign of aging via membrane receptors or damaged cells. They
are mainly located in the bone marrow: here are on average
0.01% of the total bone marrow cells (35-37). Clinical trials with
MSCs began in 1999 by Horowitz et al. demonstrating that “the
bone marrow derived mesenchymal cells” increase the mineral
content in the whole organism and the subsequent bone formation
in children with osteogenesis imperfect (38). Since then, MSCs
have been applied to patients suffering from osteitis and bone
defects (39).
Figure 2 - Subject 9 years old: radiological appearance of ameloblastic
fibro-odontoma; the 4.6 tooth is pushed by benign tumor at the base of
the jaw.
Materials and methods
This study was conducted between 2013 and 2015 and involved according to our protocol that included new X-rays and clinical vi-
10 patients (TEST GROUP) (6 males and 4 females) aged between sits at 3, 6, 12 and 24 months after surgery.
12 and 75 (average age 50). All possible postoperative complications were considered, paying
Patients presented to our attention in the Department of Maxillofacial particular attention to the absence of infection, pain or neurolo-
Surgery of the University of Florence. In the 80% of cases the le- gical disorders. Bone regeneration was evaluated by X-ray exa-
sion was occasionally detected, while in the remaining part (20%) minations observing the size variations of the operated area. For
there were painful symptoms of inflammatory origin which requi- the feedback and the calculation of residual areas we used the
red a radiographic evaluation. To obtain a greater standardiza- dedicated digital software provided by the radiology center. All this
tion, the surgical procedure was performed by the same surgical data were compared with 10 patients treated without MSCs (con-
team using the same instruments. In the pre-surgical, the proto- trol groups).
col provided for the collection of the patients’ medical history and Surgical procedure with stem cells:
radiographic examinations (CT). All patients showed bone lesions: • withdrawal of the iliac crest bone marrow and stem cell pre-
they were expansive, benign, voluminous maxillary or mandibu- paration
lar lesions with a diameter greater than 3 cm. All lesion were hi- • enucleation of the bone lesion
stologically assessed and the results were 3 keratocysts, 4 folli- • the lesion is fixed in formalin and sent to the Department of Hi-
cular cysts (Figure 1), 2 periapical inflammatory cysts and 1 ame- stology and Pathology for histological analysis
loblastic fibro-odondotama (Figure 2). All patients were opera- • application of mesenchymal stem cells
ted using the same anesthetic technique which involved general • suture.
anesthesia via nasotracheal intubation. They all received pre-ope-
rative antibiotic coverage through the use of a broad-spectrum an-
tibiotic (2 g of amoxicillin and clavulanic acid) administered at lea- Preparation of stem cells, of platelet-rich plasma (PRP) and
st 1 hour before operation and to be continued at the same dose autologous thrombin serum (ATS)
twice a day for 6 days. Then, the oral hygiene protocol provided
the use of mouthwash with chlorhexidine 0.12% three times a day Our protocol involves the use of Regenkit Extracell Glue (RegenLab
for 15 days. From the surgical point of view, the 10 patients were SA Losanne, Swiss) disposable kits, dedicated to the preparation
treated with mesenchymal stem cells and PRP directly into the post- of concentrated autologous stem cells from blood samples of iliac
surgical cavity, without filler addition. Check-ups were carried out crest bone marrow and PRP platelet concentrate complete with
Clinical Cases in Mineral and Bone Metabolism 2016; 13(3):214-220 215
_CCMBM 3_2016.qxp_- 23/01/17 09:39 Pagina 216
R. Bertolai et al.
Figure 3 - Step 1a – The Regenlab procedure: withdrawal of blood marrow (iliac crest) Step 2a – transfer tube Step 3a – centrifugation Step 4a – col-
lection and concentration of stem cells.
autologous thrombin serum ATS. This protocol refers to the Bran- Results
di-Innocenti’ study in which it is shown that the number of MSC’s
per ml was 8 times higher than the control group (47). Only one patient didn’t come to control visits. The 7 patients with
The procedure to obtain the concentrate of stem cells has been mandibular lesions showed a rapid and very good healing with an
already described in our previously published item (48). It consi- 85-90% ossification of the major defect at 12 months (Figures 4,
sts of four steps plus the one of mixing and application (Figure 3). 5). The 2 patients with maxillary bone lesions showed good tis-
sue healing but a large loss of vestibular bone.
A. Withdrawals of bone were performed, eight months after surgery,
Step 1a – withdrawal of blood marrow (iliac crest) for a histological evaluation. Histological examination showed a
Step 2a – transfer tube complete new bone formation (Figure 6).
Step 3a – centrifugation No complications were observed.
Step 4a – collection and concentration of stem cells
B. Discussion and conclusion
Step 1b – collection of peripheral whole blood
Step 2b – centrifugation Radiographic controls of test group showed a faster bone healing
Step 3b – preparation of serum thrombin than the control group (patients without use of MSCs) (Figure 7)
Step 4b – collection of concentrates and the one described in the literature concerning spontaneous
Step 5 –mixing and application bone healing after enucleation.
In sterile field the PRP was placed in a provided container. Then, For large cysts the literature reports a time value of 24 months to
the stem cell concentrate was added together with the autologous obtain an ossification of about 85-90% of the residual cavity.
thrombin serum to activate coagulation. All this was placed insi- By the use of MCs, it was observed an ossification of 85-90% of
de the residual bone cavity after enucleation and surgical debri- the residual bone cavity at only 12 months from the surgery, hal-
dement. After that the flap was sutured. ving, in fact, the time.
216 Clinical Cases in Mineral and Bone Metabolism 2016; 13(3):214-220
_CCMBM 3_2016.qxp_- 23/01/17 09:39 Pagina 217
Mesenchymal stem cells in post-surgical cavities of large maxillary bone lesions
Figure 6 A, B - Same case as in Figure 2; A) ameloblastic fibro-odontoma:
8 months after surgery a huge new bone regeneration; B) histological fea-
tures, hematoxylin-eosin 400 x, compact lamellar cortical bone, with
alive osteocytes.
Figure 4 A-C - Same case as in Figure 2; A) ameloblastic fibro odontoma;
B) radiographic check after surgery in C) radiographic check at 6 months
after surgery; note the growth of the tooth 4.6.
Figure 5 - Cheratocyst in a 31 y.o. man: radiological check after the surgical therapy at 3, 6 and 12 months. Note the good bone healing.
Clinical Cases in Mineral and Bone Metabolism 2016; 13(3):214-220 217
_CCMBM 3_2016.qxp_- 23/01/17 09:39 Pagina 218
R. Bertolai et al.
Figure 7 A, B - A) keratocysts after surgery with the use of MSCs, and X-ray control to 6 months; the good bone healing and faster when compared
with a surgical cavity (B dentigerous cyst) in which are not used MSCs.
Figure 8 A, B - A) bone cavity after sinus lifts procedure; the vascularization is given only by raised upward mucosa and by outside palatine mucosa;
B) a post surgical cavity where it is evident a large blood supply coming from bony walls.
Comparing the results of this study with those of our previously 8). A well-vascularized post surgical cavity appears to be a
published work, concerning the use of the MSCs in sinus lift more favorable environment for the activity of MSCs in order
procedure (48), we have been able to see different times of to exploit their full potential. The excellent results obtained seem
osteogenesis. In fact, the bone healing times between the dou- to be due to the receiving site, which is ideal for the clot sta-
ble sinus lift and the ones obtained without the use of stem bility and vascularization. In fact, very good results have been
cells are fully comparable. Instead, the use of stem cells in the achieved in cases where the bone defect to be regenerated
post-surgical cavity seems to speed up significantly the bone was contenitive (residual bone cavities with three walls), whe-
healing times. This seems to be related to the vascular envi- reas in patients treated with absence of vestibular wall sup-
ronment of the post surgical cavity because different from the port, there were no striking results from the bone regenera-
cavities after sinus lift, owing to a lack of vascularity (Figure tion point of view.
218 Clinical Cases in Mineral and Bone Metabolism 2016; 13(3):214-220
_CCMBM 3_2016.qxp_- 23/01/17 09:39 Pagina 219
Mesenchymal stem cells in post-surgical cavities of large maxillary bone lesions
Since the aim of MSCs using is reaching a faster “restitutio ad in- 22. Ettl T, Gosau M, et al. Jaw cysts. Filling or no filling after enucleation?
tegrum”, the result considerably reduces the incidence of post-ope- A review. Journal of Cranio-Maxillo-FacialSurgery. 2012;40:485e493.
rative complications and an easier operative outcome. 23. Carini F, Lomartire G, et al. Valutazioni radiografiche della guarigio-
Further studies will be needed to confirm our results and the en- ne ossea spontanea di lesioni cistiche mascellari e mandibolari dopo
couraging evidences encountered so far. enucleazione con tecniche piezoelettriche. Il Dentista Moderno.
2008.
24. Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the
References right spot with mesenchymal stromal cells. Stem cells. 2010;28(8):1446-
55.
1. Barone R, Chiapasco M, Clauser C. Chirurgia Orale. Manuale Atlan- 25. Korbling M, Estrov Z. Adult stem cells for tissue repair – a new the-
te. 1st ed. Firenze: Timeo Ed., 1999. rapeutic concept? The New England journal of medicine.
2. Chiapasco M. Manuale illustrato di Chirurgia Orale, 2a ed. Milano: Mas- 2003;349(6):570-82.
son Elsevier, 2006. 26. Pittenger MF, Mackay AM, Beck SC, et al. Multi- lineage potential of
3. Kramer IRH, Pindborg JJ, Shear M. The WHO Histological Typing of adult human mesenchymal stem cells. Science. 1999;284(5411):143-
Odontogenetic Tumours, a commentary on the Second Edition. Can- 7.
cer. 1992;70(12):2988-2994. 27. Weissman, IL, Shiruzu JA. The origins of the identification and isola-
4. Philipsen HP, Reichart PA. Classification of odontogenictumours. A tion of hemato poietic stem cells, and their compatibility to induced do-
historical review, J Oral Pathol Med. 2006;35:525-9. nor-specific transplantation tolerance and treat autoimmune diseases.
5. Koseoglu BG, Atalay B, Erdem MA. Odontogenic cyst: a clinical study Blood. 2008;112(9):3543-53.
of 90 cases. J Oral Sci. 2004;46(4):253-257. 28. Denzwa M, Ishikawa H, Itokazu Y, et al. Bone marrow stromal cells
6. Ficarra G. Manuale di Patologia e Medicina Orale, 3a ed. Milano: Mc- generated muscle cells and repair muscle degeneration. Science.
Graw-Hill, 2006. 2005;309(5732):314-7.
7. Horowitz I, Bodner L. Use of xenograft bone with aspirated marrow 29. Dezawa M, Kanno H, Hoshino M, et al. Specific induction of neuro-
for treatment of cystic defect of the jaws. Head & Neck. 1989;11:516- nal cells from bone marrow stromal cells and application for autolo-
523. gous transplantation. The journal of clinical investigation.
8. Santamaria J, Garcia AM, De Vincente JC, Landa S, Lopez-Arranz 2004;113(12):1701-10.
JS. Bone regeneration after radicular cyst removal with and without 30. Dezawa M, Takahashi D, Esaki M, et al. Sciatic nerve regeneration
guided bone regeneration. Int J Oral MaxillofacSurg. 1998;27(2):118- in rats induced by transplantation of in vitro differentiated bone mar-
20. row stromal cells. The European journal of neuroscience,
9. Chiapasco M, Rossi A, Motta JJ, Crescentini M. Spontaneous bone 2001.14(11):p.1771-6.
regeneration after enucleation of large mandibular cysts: a radiographic 31. D'Ippolito G, Diabira S, Howard GA, et al. Marrow isolated adult mul-
computer analysis of 27 consecutive cases. J OralMaxillofacSurg. tilineare inducible (MIAMI) cells, a unique population of postnatal young
2000;58(9):942-8. and old human cells with extensive expansion and differentiation po-
10. Chiapasco M. Manuale illustrato di chirurgia orale. Milano: Masson, tential. Journal of cell science, 2004; 117(pt14):2971-81.
2002. 32. Kucia M, Reca R, Campbell FR, et al. A population of a very small em-
11. Stoelinga PJW, Bronkhorst FB. The incidence, multiple presentation bryonic-like CXCR4(+) SSEA-1(+) Oct-4+ stem cells identifield in adult
and recurrence of aggressive cyst o jaws. J CranioMaxillofacSurg. bone marrow. Leukemia: official journal of the leukemia society of Ame-
1987;15:184-195. rica, leukemia research fund, U.K. 2006;20(5):857-69.
12. Stoelinga PJW. Long-term follow-up on keratocysts treated according 33. Wojakowski W, Kucia, M, Zuba E, et al. Very small embryonic-like stem
to a defined protocol. Int J Oral MaxillofacSurg. 2001;30:14-25. cells in cardiovascular repair. Pharmacology e therapeutics.
13. Pippi R, Vitolo D. A clinical radiographic and histologic revaluation of 2011;129(1):21-8.
a 10 years’ sample of surgically treated cysts of the jaws, with spe- 34. Kuroda Y, et al. Unique multipotent cells in adult human mesenchy-
cial emphasis on keratocysts. Min Stomatol. 2004;53(5):251-61. mal cell populations. Proceedings of the National Academy of Scien-
14. Moskow B, Gold, Gottsegen R. Effects of sclera collagen upon the hea- ces of the U.S.A. 2010;107(19):8639-43.
ling of experimental osseous wounds. J Oral MaxillofacSurg. 35. Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mi-
1976;47:596-602. staken identity? In Cytotherapy. 2012;14(5):516-21.
15. Voorsmit RA, Stoelinga PJ, van Haelst UJ. The management of ke- 36. Dominici M, Le Blanc K, Mueller, et al. Minimal criteria for defining mul-
ratocyst. J Maxillofacial Surg. 1981Nov. tipotent mesenchymal stromal cells. The International Society for Cel-
16. Mitchell R. An evaluation of bone healing in cavities in the jaws im- lular Therapy position statement in Cytotherapy. 2006;8(4):315-317.
planted with a collagen matrix. Br J Oral MaxillofacSurg. 1992;30:180- 37. Beyer N, Nardi L. da Silva M. Mesenchymal Stem Cells: Isolation, In
182. Vitro Expansion and Characterization. In Anna M. Wobus, Kenneth
17. Meiselman F. Surgical management of the odontogenic keratocyst: Boheler, Stem Cells, Handbook of experimental pharmacology.
conservative approach. J Oral MaxillofacSurg. 1994;52:960-963. 2006;174:249-82.
18. Kawai T, Murakami S, Hiranuma H, Sakuda M. Healing after remo- 38. Horwits EM, et al. Transplantability and therapeutic effect of bone mar-
val of benign cysts and tumors of the jaws. A radiologic appraisal. Oral row-derived mesenchymal cells in children with osteogenesis imper-
Surg Oral Med Oral Pathol. 1995;79:517-525. fect. Nature Medicin. 1999;5(3):309-13.
19. Abdulwassie H, Dhanrajani PJ. Prosthodontic implant rehabilitation af- 39. Niedzwiedzki T, et al. Bone healing after bone marrow stromal cell tran-
ter the treatment of a pathologic lesion in the mandible: a case report. splantation to the bone defect. Biomaterials. 1993;14(2):115-21.
Implant Dent. 2001;10(3):178-81. 40. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of
20. Bodner L. Effect of decalcified freeze-dried bone allograft on the hea- fibroblast colonies in monolayer cultures of guinea-pig bone marrow
ling of jaw defects after cyst enucleation. J Oral MaxillofacSurg. and spleen cells. Cell and tissue kinetics. 1970;3:393-403.
1996;54:1282-1286. 41. Bruder SP, Kraus KH, Goldberg VM, Kadiyala S. The effect of implants
21. Bodner L, Bar-Ziv J. Characteristics of bone formation following mar- loaded with autologous mesenchymal stem cells on the healing of ca-
supialization of jaw cysts. Dentomaxillofac Radiology. 1998; 27:166- nine segmental bone defects. The Journal of bone and joint surgery.
171. American volume. 1998;80:985-96.
Clinical Cases in Mineral and Bone Metabolism 2016; 13(3):214-220 219
_CCMBM 3_2016.qxp_- 23/01/17 09:39 Pagina 220
R. Bertolai et al.
42. De Kok IJ, Drapeau SJ, Young R, Cooper LF. Evaluation of mesen- fadi FF, Wikesjo UM. Analysis of rat calvaria defects implanted with
chymal stem cells following implantation in alveolar sockets: a cani- a platelet-rich plasma preparation: histologic and histometric obser-
ne safety study. The International journal of oral & maxillofacial im- vations. Journal of clinical periodontology. 2005;32:966-72.
plants. 2005;20:511-8. 46. Christgau M, Moder D, Hiller KA, Dada A, Schmitz G, Schmalz G.
43. Dallari D, Fini M, Stagni C, Torricelli P, NicoliAldini N, Giavaresi G, Growth factors and cytokines in autologous platelet concentrate and
Cenni E, Baldini N, Cenacchi A, Bassi A, Giardino R, Fornasari PM, their correlation to periodontal regeneration outcomes. Journal of cli-
Giunti A. In vivo study on the healing of bone defects treated with nical periodontology. 2006;33:837-45.
bone marrow stromal cells, platelet-rich plasma, and freeze-dried bone 47. Ciuffi, S, Fabbri R, Zonefrati C, Carulli F, Matassi D, Chicon Paez, Ci-
allografts, alone and in combination. Journal of orthopaedic research: vinini R, Innocenti M, Brandi ML. Humanmesenchymalstem cells: iso-
official publication of the Orthopaedic Research Society. 2006;24:877- lation and concentrationfrom bone marrow using the regen kit devi-
88. ce. Clinical Cases in Mineral and Bone Metabolism. 2010;7(3):207-
44. Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Ven- 240.
to AM, Meotti C, Bertoja AZ, Giardino R, Fornasari PM, Mercuri M, Pic- 48. Bertolai R, Catelani C, Aversa A, Rossi A, Giannini D, Bani D. Bone
ci P. Platelet-derived growth factors enhance proliferation of human graft and mesenchymal stem cells: clinical observations and histolo-
stromal stem cells. Biomaterials. 2003; 24:3095-100. gical analysis. Clinical Cases in Mineral and Bone Metabolism.
45. Pryor ME, Polimeni G, Koo KT, Hartman MJ, Gross H, April M, Sa- 2015;12(2)184-188.
220 Clinical Cases in Mineral and Bone Metabolism 2016; 13(3):214-220
You might also like
- Crisis Communication Plan TemplateDocument36 pagesCrisis Communication Plan TemplateJgjbfhbbgb100% (1)
- Heart Disease Prediction Using Machine Learning: Aftab Alam Khan Alka Joshi Amit Rai Gaurav KumarDocument44 pagesHeart Disease Prediction Using Machine Learning: Aftab Alam Khan Alka Joshi Amit Rai Gaurav KumarAmit RaiNo ratings yet
- Histologia MeniniDocument10 pagesHistologia Meninijuanita enriquezNo ratings yet
- SCAFFOLDS COMBINED WITH STEM CELLS AND GROWTH FACTORS IN RECONSTRUCTION OF LARGE BONE DEFECTS. Rodolfo Capanna, Pietro de BiaseDocument7 pagesSCAFFOLDS COMBINED WITH STEM CELLS AND GROWTH FACTORS IN RECONSTRUCTION OF LARGE BONE DEFECTS. Rodolfo Capanna, Pietro de BiaseNuno Craveiro LopesNo ratings yet
- Rasperini 2013Document9 pagesRasperini 2013Alexa LoyaNo ratings yet
- Regeneration and Repair of Periodontal TissuesDocument11 pagesRegeneration and Repair of Periodontal TissuesAna Maria Montoya GomezNo ratings yet
- 1 s2.0 S2212440323000457 MainDocument7 pages1 s2.0 S2212440323000457 MaindreneanastriNo ratings yet
- Mandibular Reconstruction With Tissue EngineeringDocument5 pagesMandibular Reconstruction With Tissue EngineeringtamisatnamNo ratings yet
- Periosteal Pocket Flap For Horizontal Bone RegenerDocument10 pagesPeriosteal Pocket Flap For Horizontal Bone RegenerViorel Ion100% (1)
- Journal of Orthopaedic Translation: Lin Xu, Hao Qin, Jia Tan, Zhilin Cheng, Xiang Luo, Haitao Tan, Wenhua HuangDocument7 pagesJournal of Orthopaedic Translation: Lin Xu, Hao Qin, Jia Tan, Zhilin Cheng, Xiang Luo, Haitao Tan, Wenhua HuangLeiNo ratings yet
- Surgical Management of Odontogenic CystsDocument4 pagesSurgical Management of Odontogenic CystsHana FauziyahNo ratings yet
- (22857079 - Acta Medica Transilvanica) Management and Prevention of Complications of Guided Bone RegenerationDocument3 pages(22857079 - Acta Medica Transilvanica) Management and Prevention of Complications of Guided Bone RegenerationGabriela ArgeseanuNo ratings yet
- Stem Cell and Research in Plastic Surgery: ReviewDocument3 pagesStem Cell and Research in Plastic Surgery: ReviewhajarhaniyahNo ratings yet
- Techniques On Vertical Ridge Augmentation IndicatiDocument30 pagesTechniques On Vertical Ridge Augmentation IndicatiFelix SchweppeNo ratings yet
- Fresh Osteochondral Allograft Transplantation For.7Document13 pagesFresh Osteochondral Allograft Transplantation For.7cooperorthopaedicsNo ratings yet
- 13b Collagen 1Document7 pages13b Collagen 1María Fernanda EstévezNo ratings yet
- Evaluation of Healing Rates and Safety With A Bioinductive Collagen Patch For Large and Massive Rotator Cuff TearsDocument8 pagesEvaluation of Healing Rates and Safety With A Bioinductive Collagen Patch For Large and Massive Rotator Cuff TearsGuiss TlacotiaNo ratings yet
- Free Flaps MaxillaDocument7 pagesFree Flaps MaxillaFahad QiamNo ratings yet
- Clinical Application of Scaffolds For Cartilage Tissue EngineeringDocument17 pagesClinical Application of Scaffolds For Cartilage Tissue EngineeringikulNo ratings yet
- Endoscopic Endonasal Reconstruction of Anterior Skull Base Defects What Factors Really Affect The OutcomesDocument8 pagesEndoscopic Endonasal Reconstruction of Anterior Skull Base Defects What Factors Really Affect The OutcomesSanooj SeyedNo ratings yet
- Charbonnier2019 PDFDocument9 pagesCharbonnier2019 PDFRENATO SUDATINo ratings yet
- 1 s2.0 S1877056812000400 MainDocument8 pages1 s2.0 S1877056812000400 Maindaniel Montaño gomezNo ratings yet
- Bone GraftDocument4 pagesBone Graftsmansa123No ratings yet
- Controversiesin Traditionaloraland MaxillofacialreconstructionDocument13 pagesControversiesin Traditionaloraland MaxillofacialreconstructionSheetal HNo ratings yet
- Masquelet InjuryDocument8 pagesMasquelet Injurydrets70No ratings yet
- Laino Et Al. - 2021 - Dental Pulp Stem Cells On Implant Surface An in VDocument12 pagesLaino Et Al. - 2021 - Dental Pulp Stem Cells On Implant Surface An in VEbaycustomer number01No ratings yet
- Experimental Posterolateral Spinal Fusion With Porous Ceramics and Mesenchymal Stem CellsDocument8 pagesExperimental Posterolateral Spinal Fusion With Porous Ceramics and Mesenchymal Stem CellsccsloureiroNo ratings yet
- Aps 39 345Document9 pagesAps 39 345isabelNo ratings yet
- Articulo en Inglés 2Document8 pagesArticulo en Inglés 2Luis Ramirez BenaventeNo ratings yet
- Gholobova Et Al. - Biofabrication - 2020 - Functional Evaluation of Prevascularization in One-Stage Versus Two-Stage Tissue EngineeriDocument19 pagesGholobova Et Al. - Biofabrication - 2020 - Functional Evaluation of Prevascularization in One-Stage Versus Two-Stage Tissue EngineeriLievenNo ratings yet
- Vangsness2014 MesnchymalDocument9 pagesVangsness2014 MesnchymalbuddybbuddyNo ratings yet
- Brun Svold 1993Document12 pagesBrun Svold 1993Marihana ValdezNo ratings yet
- Development of An Engineering Autologous Palatal Mucosa-Like Tissue For Potential Clinical ApplicationsDocument8 pagesDevelopment of An Engineering Autologous Palatal Mucosa-Like Tissue For Potential Clinical ApplicationsTahir AliNo ratings yet
- Article PDFDocument5 pagesArticle PDFLauren LimNo ratings yet
- Omad 061Document3 pagesOmad 061youssef ibneloualidNo ratings yet
- Knee Stem CellsDocument25 pagesKnee Stem CellsKN TMNo ratings yet
- Basic Arthroplasty - Unit 4 - Bone Loss BacksteinDocument9 pagesBasic Arthroplasty - Unit 4 - Bone Loss BacksteinShu Yang HuNo ratings yet
- He 2019Document10 pagesHe 2019Rashif AkmalNo ratings yet
- TRIGONOMETRIADocument7 pagesTRIGONOMETRIACatalina RodríguezNo ratings yet
- Ic Rehabilitation of The Edentulous Patient Maxillectomy UiriDocument5 pagesIc Rehabilitation of The Edentulous Patient Maxillectomy Uiriayush srivastavaNo ratings yet
- Cel MM Derivadas de Almohadilla de Grasa Infrapatelar para Tto de Osteoartritis de RodillaDocument6 pagesCel MM Derivadas de Almohadilla de Grasa Infrapatelar para Tto de Osteoartritis de RodillaDIEGONo ratings yet
- Intra-Articular Delivery of Umbilical Cord-Derived Mesenchymal Stem Cells Temporarily Retard The Progression of Osteoarthritis in A Rat ModelDocument10 pagesIntra-Articular Delivery of Umbilical Cord-Derived Mesenchymal Stem Cells Temporarily Retard The Progression of Osteoarthritis in A Rat ModelNorman Enrique Brenes CorderoNo ratings yet
- Guided Tissue RegenerationDocument131 pagesGuided Tissue Regenerationt sNo ratings yet
- Fulco Et Al 2014Document10 pagesFulco Et Al 2014Alex PerryNo ratings yet
- Korn 2017Document14 pagesKorn 2017Aris SetyawanNo ratings yet
- 1 s2.0 S1877056823003262 MainDocument6 pages1 s2.0 S1877056823003262 MainlucianomedufmgNo ratings yet
- Aneurysmal Bone CystDocument5 pagesAneurysmal Bone CystAustine OsaweNo ratings yet
- GCT PDFDocument6 pagesGCT PDFsandeep nemaNo ratings yet
- Pnas200905439 3299..3304Document6 pagesPnas200905439 3299..3304Mohamed TaabouzNo ratings yet
- Tuberosidad HistologiaDocument10 pagesTuberosidad HistologiaMaximiliano Jara ContrerasNo ratings yet
- The Meniscus Is A Fibercartilagenous Structure, Which Plays A Significant Role in MaintainingDocument7 pagesThe Meniscus Is A Fibercartilagenous Structure, Which Plays A Significant Role in MaintainingStefan StefNo ratings yet
- Analysis of Risk Factors For New Vertebral Fracture After Percutaneous VertebroplastyDocument7 pagesAnalysis of Risk Factors For New Vertebral Fracture After Percutaneous VertebroplastyCecilia Suarez HerreraNo ratings yet
- 10 1111@cid 12748Document8 pages10 1111@cid 12748Behnam TaghaviNo ratings yet
- Expansion of The Zone of Keratinised Tissue For Healthy Implant Abutment Interface Using Deepithelized Amnion:Chorion AllograftDocument6 pagesExpansion of The Zone of Keratinised Tissue For Healthy Implant Abutment Interface Using Deepithelized Amnion:Chorion AllograftzethyhanumNo ratings yet
- Soleus Muscle Flap For The Coverage of Pre-Tibial Defect of Middle Third of LegDocument6 pagesSoleus Muscle Flap For The Coverage of Pre-Tibial Defect of Middle Third of LegHassan KhanNo ratings yet
- Three-Dimensional Vertical Alveolar Ridge Augmentation in The Posterior Maxilla: A 10-Year Clinical StudyDocument10 pagesThree-Dimensional Vertical Alveolar Ridge Augmentation in The Posterior Maxilla: A 10-Year Clinical StudyFabian Sanabria100% (1)
- RetrieveDocument10 pagesRetrieveMaria CorenaNo ratings yet
- Eramo2017 RegenrationDocument15 pagesEramo2017 Regenrationrasagna reddyNo ratings yet
- Injertos Aumento RebordeDocument13 pagesInjertos Aumento Rebordejose miguel perez rodriguez100% (1)
- Buccal-Lingual Bone Remodeling in Immediately Loaded Fresh Socket Implants A Cone Beam Computed Tomography StudyDocument8 pagesBuccal-Lingual Bone Remodeling in Immediately Loaded Fresh Socket Implants A Cone Beam Computed Tomography StudyYuki MuraNo ratings yet
- Tollemar2016 PDFDocument50 pagesTollemar2016 PDFWara Samsarga GedeNo ratings yet
- Joint Function Preservation: A Focus on the Osteochondral UnitFrom EverandJoint Function Preservation: A Focus on the Osteochondral UnitNo ratings yet
- 2016 - Diagnostic Features and Management Strategy of A Refractory Case of Radionecrosis of The MandibleDocument12 pages2016 - Diagnostic Features and Management Strategy of A Refractory Case of Radionecrosis of The MandibleSyifa IKNo ratings yet
- 2018 - Banyak Foto - Oral Rehabilitation of The Cancer PatientDocument18 pages2018 - Banyak Foto - Oral Rehabilitation of The Cancer PatientSyifa IKNo ratings yet
- 2017 - Ameloblastic Fibroodontoma Uncommon Case Presentation in A 6 Year Old ChildDocument4 pages2017 - Ameloblastic Fibroodontoma Uncommon Case Presentation in A 6 Year Old ChildSyifa IKNo ratings yet
- 2017 CBCTDocument4 pages2017 CBCTSyifa IKNo ratings yet
- Shallow and Flat Anterior ChamberDocument8 pagesShallow and Flat Anterior Chamberjudah samuelNo ratings yet
- Tab9 PDFDocument7 pagesTab9 PDFGokul GoNo ratings yet
- Night Sex (Men Version)Document4 pagesNight Sex (Men Version)Sean LiamNo ratings yet
- Defence of Insanity in India and England: Comparative Legal ParadigmDocument8 pagesDefence of Insanity in India and England: Comparative Legal ParadigmMukund PandeyNo ratings yet
- Companies - Chemicals, Pharmaceuticals & Plastics - Saudi Arabia - Kompass Business Directory1Document28 pagesCompanies - Chemicals, Pharmaceuticals & Plastics - Saudi Arabia - Kompass Business Directory1Afshan NaikNo ratings yet
- Order of DrawDocument8 pagesOrder of DrawVincent ReyesNo ratings yet
- Work Related Stress-A Literature ReviewDocument7 pagesWork Related Stress-A Literature ReviewDorin TriffNo ratings yet
- Metabolic and Complicated Cataract: DR - Ajai Agrawal Additional Professor Department of Ophthalmology AIIMS, RishikeshDocument31 pagesMetabolic and Complicated Cataract: DR - Ajai Agrawal Additional Professor Department of Ophthalmology AIIMS, Rishikeshsai shantanu100% (1)
- 12.2 Guided ReadingDocument2 pages12.2 Guided ReadingGrant Hasleton100% (1)
- Activity Checklist Lecture 240307 110843Document76 pagesActivity Checklist Lecture 240307 110843ALI-A ALINo ratings yet
- Lily Gives BirthDocument6 pagesLily Gives Birthmoorditya moordiNo ratings yet
- Planning CommissionDocument3 pagesPlanning CommissionmddhanaNo ratings yet
- Enclosure No. 2 To Deped Order No. 89, S. 2012: RemarksDocument2 pagesEnclosure No. 2 To Deped Order No. 89, S. 2012: RemarksJessa BurgosNo ratings yet
- The Analysis and Reflection On That Sugar FilmDocument2 pagesThe Analysis and Reflection On That Sugar FilmkkkkNo ratings yet
- Binge Anxiety Killer Cheat Sheet: InstructionsDocument4 pagesBinge Anxiety Killer Cheat Sheet: Instructionsdoppler_No ratings yet
- Impeach Mayorkas ResolutionDocument9 pagesImpeach Mayorkas ResolutionHayden SparksNo ratings yet
- ProstataDocument26 pagesProstatasidney souzaNo ratings yet
- Developmental AssessmentDocument5 pagesDevelopmental AssessmentSamwel KangyNo ratings yet
- Knowledge On Breastfeeding InterpretationDocument7 pagesKnowledge On Breastfeeding InterpretationHope SerquiñaNo ratings yet
- Hand - Arm Vibration: Training GuideDocument5 pagesHand - Arm Vibration: Training GuideSaddem HadfiNo ratings yet
- IV Therapy QuestionsDocument20 pagesIV Therapy QuestionsClaudina CariasoNo ratings yet
- Drug StudyDocument5 pagesDrug StudyEliza PejanNo ratings yet
- DM No. 265, S. 202 - Reiteration of Participation To The 4th Quarter Nationwide Simultaneous Earthquake Drill (Nsed)Document7 pagesDM No. 265, S. 202 - Reiteration of Participation To The 4th Quarter Nationwide Simultaneous Earthquake Drill (Nsed)ace yuntingNo ratings yet
- Price List September 2022Document61 pagesPrice List September 2022Ade IrawanNo ratings yet
- Air Water Land Noise Pollution Information and PicsDocument10 pagesAir Water Land Noise Pollution Information and PicsHarendra Yadav100% (3)
- Pre-Test BLSDocument4 pagesPre-Test BLSXen Oconnor Eileen SimbranNo ratings yet
- Exercises Present ProgressiveDocument3 pagesExercises Present ProgressiveMiluska LagosNo ratings yet
- 12 Advantages and Disadvantages of Animal Testing On CosmeticsDocument3 pages12 Advantages and Disadvantages of Animal Testing On CosmeticsElo RubioNo ratings yet